Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake
Abstract
1. Introduction
2. Results
2.1. Preparation of Spice Extracts
2.2. Effects of Spice Extracts On HepG2 Cell Viability and Morphology
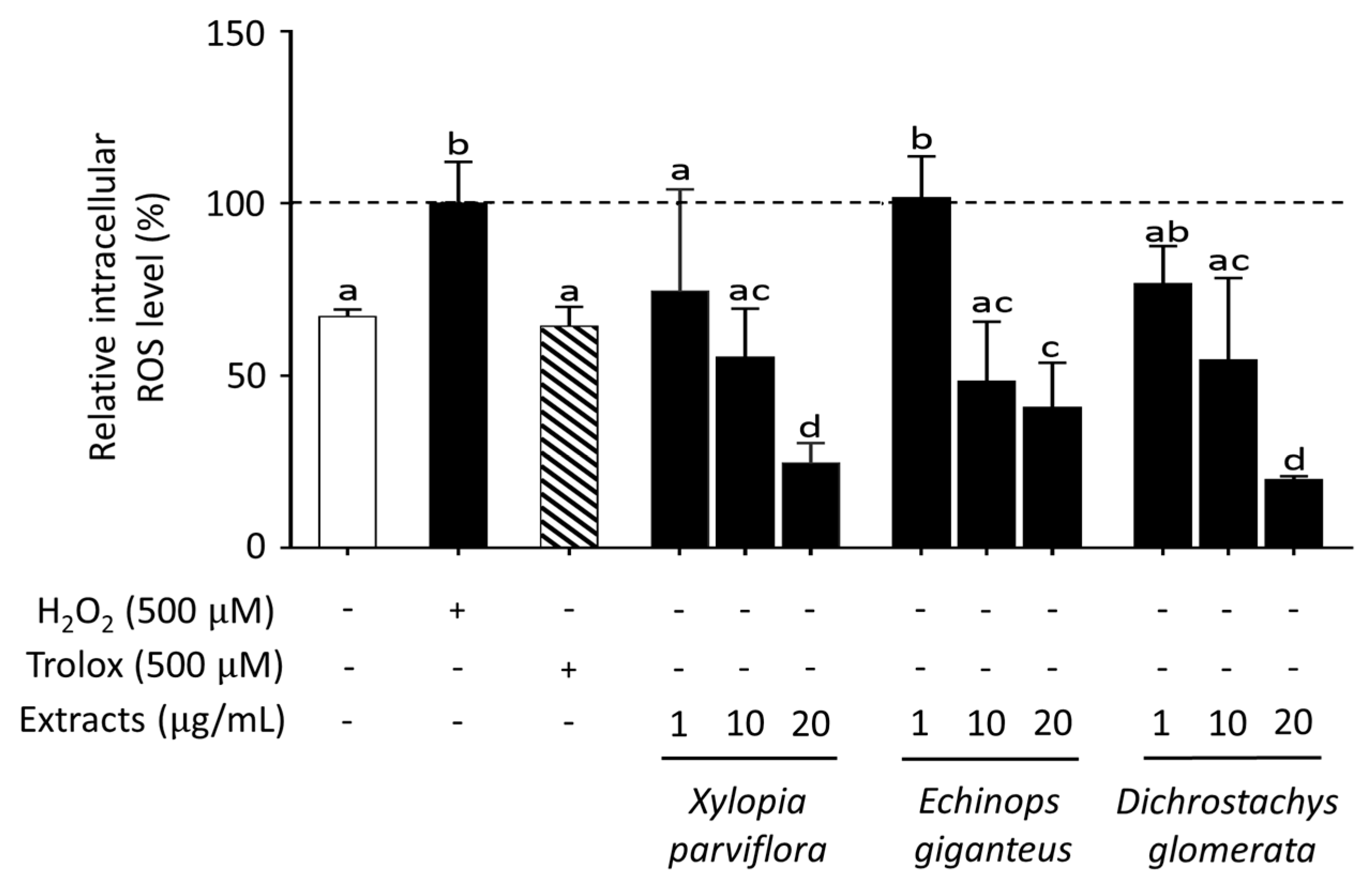
2.3. Effect of Spice Extracts on ROS Production in HepG2 Cells
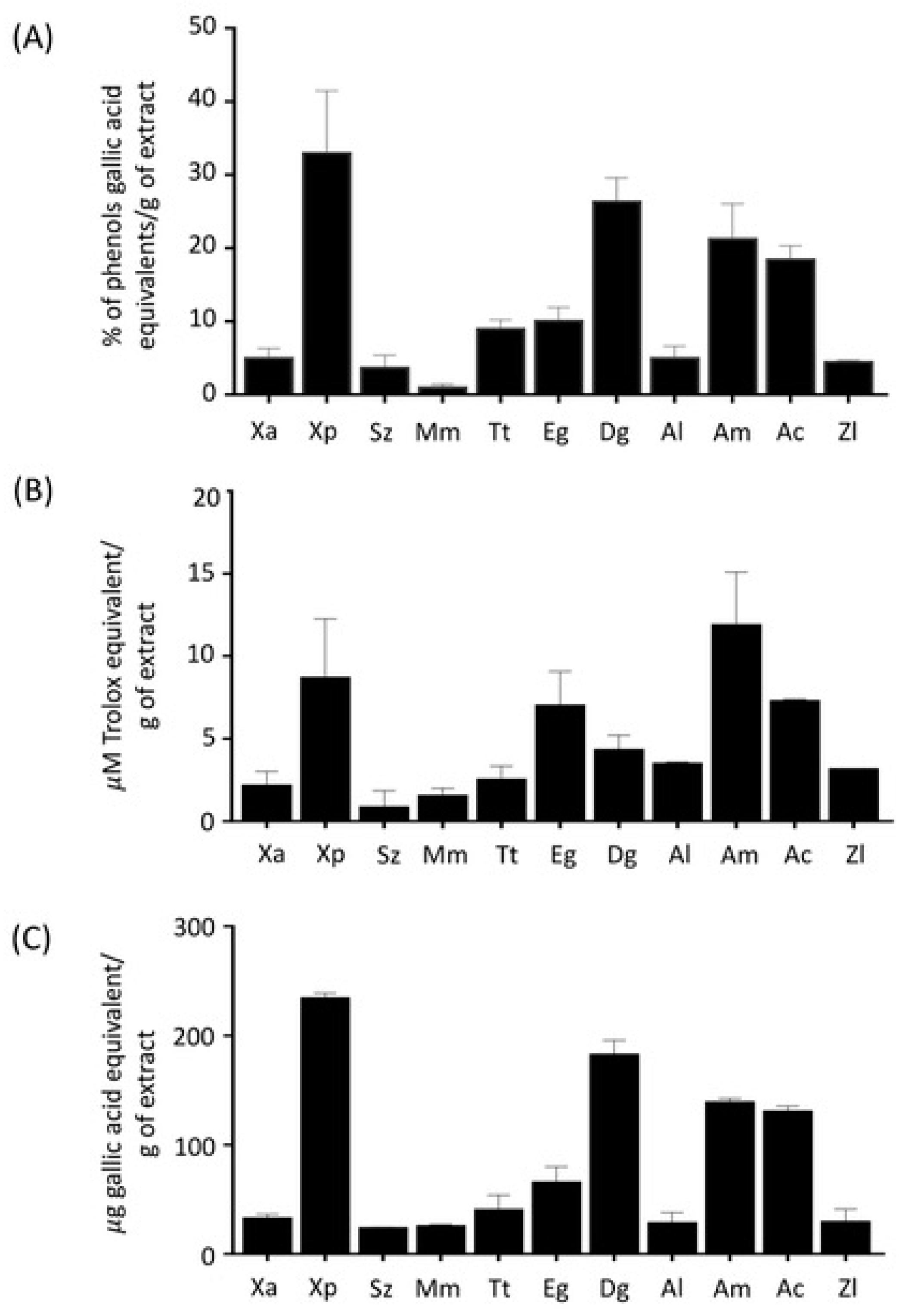
2.4. Evaluation of Antioxidant Ability of Spice Extracts
2.5. Nuclear Translocation of Nrf2
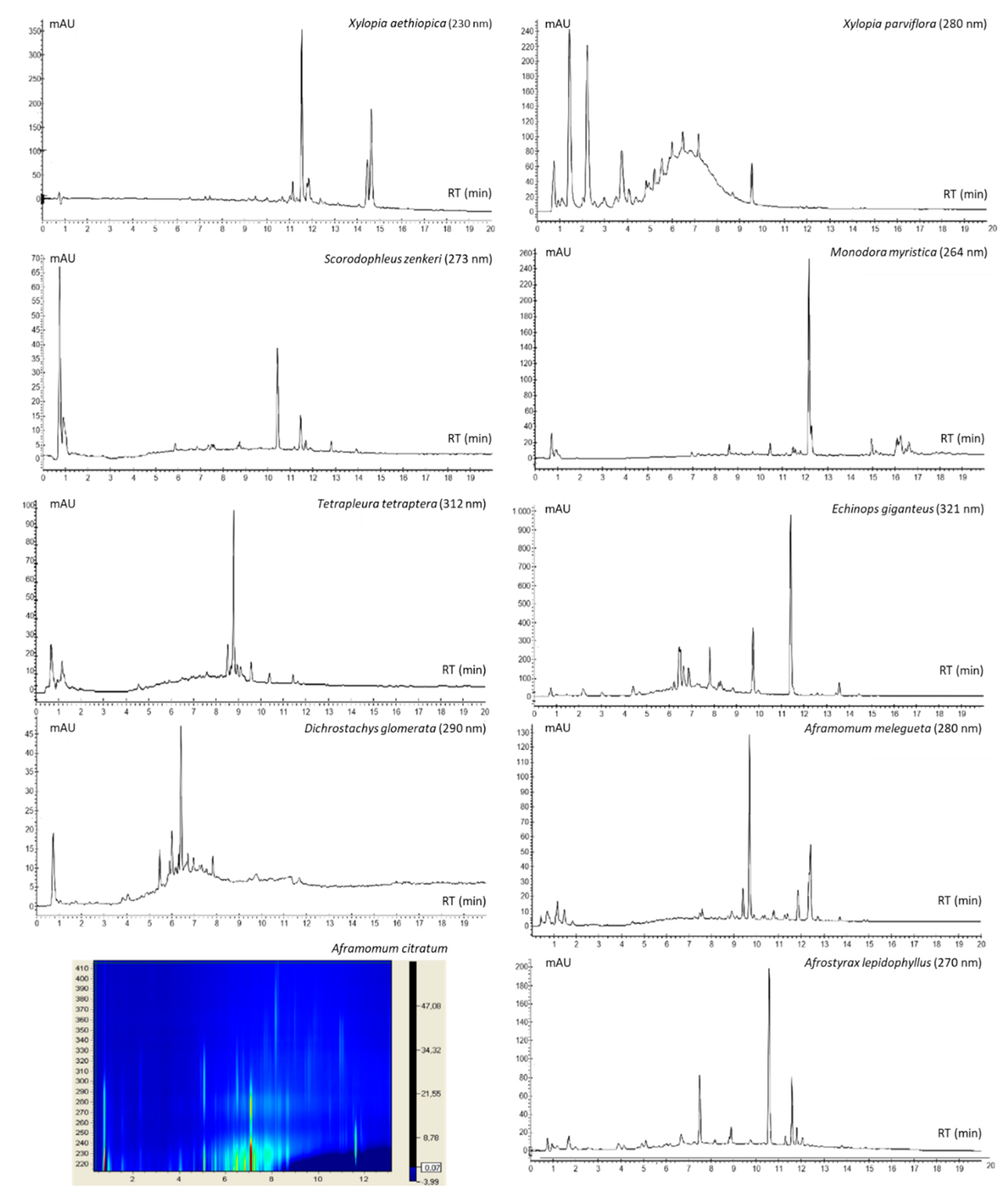
2.6. Fingerprinting of Spice Extracts
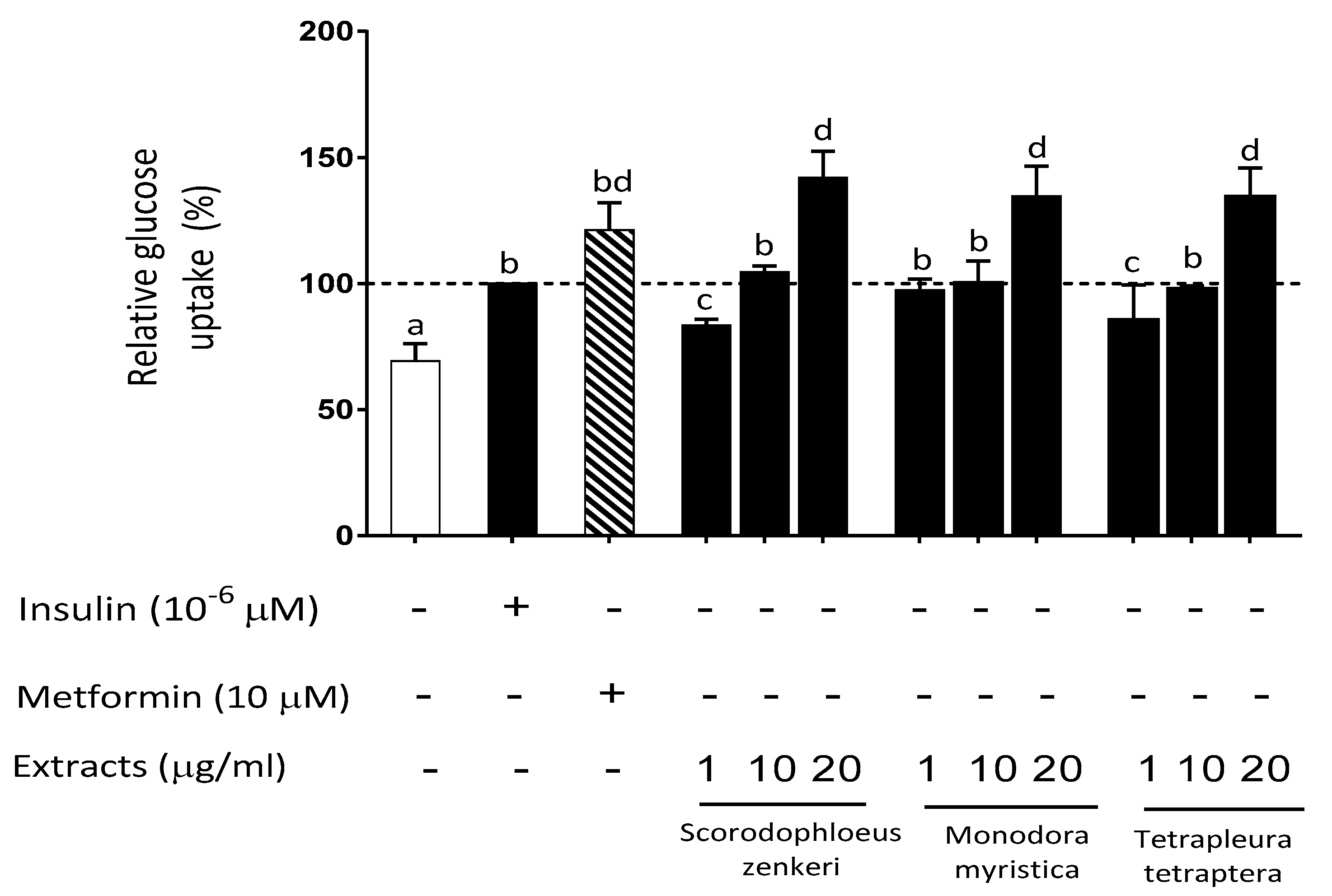
2.7. Effect of Spice Extracts on Oxidative-Stress-Modulated Glucose Uptake in HepG2 cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Spice Extracts Preparation
4.3. Cell Cultures
4.4. MTS Cell Viability Assay
4.5. Morphological Analysis
4.6. ROS Modulation Analysis in Cells
4.7. Determination of the Total Phenolic Content of the Extracts
4.8. Oxygen Radical Absorbance Capacity Assay
4.9. Ferric-Reducing Antioxidant Power Assay
4.10. Nrf2 Immunofluorescence Assay
4.11. HPLC-UV-DAD Analysis
4.12. Glucose Uptake Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Cahova, M.; Palenickova, E.; Dankova, H.; Sticova, E.; Burian, M.; Drahota, Z.; Červinková, Z.; Kučera, O.; Gladkova, C.; Stopka, P.; et al. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am. J. Physiol. Liver Physiol. 2015, 309, G100–G111. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Traditional Medicine WHO African Region. Available online: https://www.afro.who.int/health-topics/traditional-medicine.2020 (accessed on 22 March 2020).
- Kasole, R.; Martin, H.D.; Kimiywe, J. Traditional Medicine and Its Role in the Management of Diabetes Mellitus: “Patients’ and Herbalists’ Perspectives”. Evid.-Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Fennell, C.; Lindsey, K.; McGaw, L.J.; Sparg, S.; Stafford, G.I.; Elgorashi, E.; Grace, O.; Van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Agbor, G.; Oben, J.E.; Ngogang, J.Y.; Xinxing, C.; Vinson, J.A. Antioxidant Capacity of Some Herbs/Spices from Cameroon: A Comparative Study of Two Methods. J. Agric. Food Chem. 2005, 53, 6819–6824. [Google Scholar] [CrossRef]
- Agbor, G.; Moumbegna, P.; Oluwasola, E.; Nwosu, L.; Njoku, R.-C.C.; Kanu, S.; Emekabasi, E.; Akin, F.; Obasi, A.; Abudei, F. Antioxidant capacity of some plants foods and beverages consumed in the eastern region of Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 362–369. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.; Zhang, C.; Guan, Y.; Wu, Y.; Ling, F.; Niu, Y.; Li, Y. Epigallocatechin gallate improves insulin resistance in HepG2 cells through alleviating inflammation and lipotoxicity. Diabetes Res. Clin. Pr. 2018, 142, 363–373. [Google Scholar] [CrossRef]
- Nwakiban, A.P.A.; Sokeng, A.J.; Dell’Agli, M.; Bossi, L.; Beretta, G.; Gelmini, F.; Tchamgoue, A.D.; Agbor, G.A.; Kuiaté, J.-R.; Daglia, M.; et al. Hydroethanolic plant extracts from Cameroon positively modulate enzymes relevant to carbohydrate/lipid digestion and cardio-metabolic diseases. Food Funct. 2019, 10, 6533–6542. [Google Scholar] [CrossRef] [PubMed]
- INTERNATIONAL STANDARD Biological evaluation of medical devices—Part 5:Tests for in vitro cytotoxicity. Available online: http://nhiso.com/wp-content/uploads/2018/05/ISO-10993-5-2009.pdf.2009 (accessed on 22 March 2020).
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxidants Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2012, 109, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Magni, P.; Krogh, V.; Ruscica, M.; Dozio, E.; Testolin, G.; Battezzati, A. Is ghrelin a signal of decreased fat-free mass in elderly subjects? Eur. J. Endocrinol. 2006, 155, 321–330. [Google Scholar] [CrossRef][Green Version]
- Pavanello, C.; Lammi, C.; Ruscica, M.; Bosisio, R.; Mombelli, G.; Zanoni, C.; Calabresi, L.; Sirtori, C.R.; Magni, P.; Arnoldi, A. Effects of a lupin protein concentrate on lipids, blood pressure and insulin resistance in moderately dyslipidaemic patients: A randomised controlled trial. J. Funct. Foods 2017, 37, 8–15. [Google Scholar] [CrossRef]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Gomaraschi, M.; Vitali, C.; Macchi, C.; Morlotti, B.; Aiello, G.; Bosisio, R.; Calabresi, L.; et al. Effect of soy on metabolic syndrome and cardiovascular risk factors: A randomized controlled trial. Eur. J. Nutr. 2016, 57, 499–511. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Proietti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical approach for the management of cardiovascular risk A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018, 9, 841. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Liu, Z.; Fan, R.; Yin, Z.; Mi, Y.; Ren, B.; Liu, X. Athyrium multidentatum (Doll.) Ching extract induce apoptosis via mitochondrial dysfunction and oxidative stress in HepG2 cells. Sci. Rep. 2017, 7, 2275. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Abdou Bouba, A.; Njintang, Y.N.; Scher, J.; Mbofung, C.M.F. Phenolic compounds and radical scavenging potential of twenty Cameroonian spices. J. Agric. Food Chem. 2010, 1, 213–224. [Google Scholar] [CrossRef]
- Etoundi, C.B.; Kuaté, D.; Ngondi, J.L.; Oben, J. Anti-amylase, anti-lipase and antioxidant effects of aqueous extracts of some Cameroonian spices. J. Nat. Prod. 2010, 3, 165–171. [Google Scholar]
- Ene-Obong, H.; Onuoha, N.; Aburime, L.; Mbah, O. Chemical composition and antioxidant activities of some indigenous spices consumed in Nigeria. Food Chem. 2018, 238, 58–64. [Google Scholar] [CrossRef]
- Somanah, J.; Bourdon, E.; Bahorun, T. Extracts of Mauritian Carica papaya (var. solo) protect SW872 and HepG2 cells against hydrogen peroxide induced oxidative stress. J. Food Sci. Technol. 2017, 54, 1917–1927. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Boil. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Caporaso, N.; Fogliano, V. Dietary Antioxidant Compounds and Liver Health. Crit. Rev. Food Sci. Nutr. 2005, 44, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. DIETARYFLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2007, 582, 117–131. [Google Scholar] [CrossRef]
- Park, Y.S.; Uddin, J.; Piao, L.; Hwang, I.; Lee, J.H.; Ha, H. Novel Role of Endogenous Catalase in Macrophage Polarization in Adipose Tissue. Mediat. Inflamm. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Kuate, D.; Kengne, A.P.N.; Dakam, W.; Etoundi, B.C.O.; Paka, G.D.; Ngondi, J.L.; Oben, J.E. Effectiveness of Dichrostachys glomerata Spice Phenolics in Reduction of Oxidative Stress Associated with Obesity and Type 2 Diabetes; a Randomized, Double-Blind Placebo-Controlled Clinical Trial. J. Food Res. 2013, 2, 1. [Google Scholar] [CrossRef]
- Kuate, D.; Kengne, A.-P.; Biapa, P.C.N.; Azantsa, B.G.K.; Muda, W.A.M.W. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015, 14, 50. [Google Scholar] [CrossRef]
- Mohammed, A.; Koorbanally, N.A.; Islam, S. Ethyl acetate fraction of Aframomum melegueta fruit ameliorates pancreatic β-cell dysfunction and major diabetes-related parameters in a type 2 diabetes model of rats. J. Ethnopharmacol. 2015, 175, 518–527. [Google Scholar] [CrossRef]
- Kuate, D.; Etoundi, B.C.; Ngondi, J.L.; Muda, W.; Oben, J.E. Anti-inflammatory, anthropometric and lipomodulatory effects Dyglomera® (aqueous extract of Dichrostachys glomerata) in obese patients with metabolic syndrome. Funct. Foods Health Dis. 2013, 3, 416. [Google Scholar] [CrossRef]
- Adefegha, A.; Oboh, G. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spices. Pharm. Boil. 2012, 50, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jang, M.S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-alpha/beta and PPAR-gamma. Cell Mol Biol (Noisy-le-grand) 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Anim, M.T.; Larbie, C.; Appiah-Opong, R.; Tuffour, I.; Owusu, K.B.A.; Aning, A. PHYTOCHEMICAL, ANTIOXIDANT AND CYTOTOXICITY OF HYDROETHANOLIC EXTRACTS OF CROTALARIA RETUSA L. World. J. Pharm. Res. 2016, 5990, 162–179. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]


| Names of Plants | Family | Herbarium Voucher Number | Part Used | Extract Aspect | Extract Color | Extraction Yield (%) |
|---|---|---|---|---|---|---|
| Xylopia aethiopica (Dunal) A. Rich | Annonaceae | 16419/SRF-Cam** | Fruits | Powder | Brown-strand | 23.9 |
| Xylopia parviflora (A. Rich.) Benth | Annonaceae | 6431/ SRF-Cam | Seeds | Powder | Brown-beige | 20.5 |
| Scorodophloeus zenkeri Harms | Fabaceae | 44803/HNC* | Seeds | Crystal | Brown-auburn | 16 |
| Monodora myristica (Graertm.) Dunal | Annonaceae | 2949/ SRF-Cam | Seeds | Oil | Yellow-saffron | 27.9 |
| Tetrapleura tetraptera (Schum. & Thonn.)Taub | Fabaceae | 12117/SR-Cam | Fruits | Powder | Brown-bistra | 49.2 |
| Echinops giganteus Var Lellyi C. D. Adams | Asteraceae | 23647/SRF-Cam | Roots | Powder | Yellow-topaz | 13.1 |
| Afrostyrax lepidophyllus Mildbr | Huaceae | 44853/HNC | Seeds | Crystal | Yellow-amber | 7.1 |
| Dichrostachys glomerata (Forssk.) Hutch | Fabaceae | 15220/SRF-Cam | Seeds | Crystal | Brown-coffee | 27.7 |
| Aframomum melegueta (Roscoe) K.Schum | Zingiberaceae | 39065/HNC | Fruits | Powder | Brown-acajou | 11.5 |
| Aframomum citratum (Pereira ex Oliv. and Hanb) K. Shum. | Zingiberaceae | 37736/HNC | Fruits | Powder | Beige | 6.4 |
| Zanthoxylum leprieurii Guill. Et Perr. | Rutaceae | 37632/HNC | Seeds | Powder | Brown-bistra | 32.7 |
| H2O2 (500 µM) | Intracellular ROS Level (% of H2O2-treated cells) | |
|---|---|---|
| Control | - | 49.1±18.2a |
| H2O2 (500 µM) | + | 100b |
| Trolox (500 µM) | + | 69.0±15.0c |
| Xylopia aethiopica | + | 35.4±14.0d |
| Xylopia parviflora | + | 37.8±8.3d |
| Scorodophloeus zenkeri | + | 73.2±15.0ab |
| Monodora myristica | + | 82.6±11.4ab |
| Tetrapleura tetraptera | + | 66.2±17.5c |
| Echinops giganteus | + | 50.9±16.3ac |
| Afrostyrax lepidophyllus | + | 105.8±17.6b |
| Dichrostachys glomerata | + | 43.5±8.3a |
| Aframomum melegueta | + | 56.6±9.0ac |
| Aframomum citratum | + | 37.2±15.4d |
| Zanthoxylum leprieurii | + | 52.3±19.5c |
| Glucose Uptake (% of Insulin) | |
|---|---|
| Control | 52.31±6.55a |
| Insulin (10 µM) | 100b |
| Metformin (10 µM) | 124.81±3.10c |
| Xylopia aethiopica | 68.15±8.64ad |
| Xylopia parviflora | 57.64±6.54a |
| Scorodophloeus zenkeri | 104.59±1.39cd |
| Monodora myristica | 100.55±7.62cd |
| Tetrapleura tetraptera | 98.05±1.54cd |
| Echinops giganteus | 69.03±4.54ad |
| Afrostyrax lepidophyllus | 55.55±3.49a |
| Dichrostachys glomerata | 60.29±5.76a |
| Aframomum melegueta | 70.15±6.37d |
| Aframomum citratum | 58.82±8.56a |
| Zanthoxylum leprieurii | 56.84±7.11a |
| H2O2 (500 µM) | Glucose Uptake (% of Control) | |
|---|---|---|
| Control | - | 100±7.2a |
| H2O2 (500 µM) | + | 77.7± 2.6b |
| Trolox (500 µM) | + | 98.9±12.6a |
| Xylopia aethiopica | + | 100.5±10.1a |
| Xylopia parviflora | + | 102.6±25.0a |
| Scorodophloeus zenkeri | + | 100.4±14.7a |
| Monodora myristica | + | 100.5±2.8a |
| Tetrapleura tetraptera | + | 90.0±16.9a |
| Echinops giganteus | + | 89.3±20.3a |
| Afrostyrax lepidophyllus | + | 91.0±20.3a |
| Dichrostachys glomerata | + | 98.6±20.9a |
| Aframomum melegueta | + | 95.1±16.1a |
| Aframomum citratum | + | 87.1±14.7a |
| Zanthoxylum leprieurii | + | 91.7±12.3a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchan Nwakiban, A.P.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Deutou Tchamgoue, A.; Agbor, G.A.; et al. Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake. Metabolites 2020, 10, 182. https://doi.org/10.3390/metabo10050182
Atchan Nwakiban AP, Cicolari S, Piazza S, Gelmini F, Sangiovanni E, Martinelli G, Bossi L, Carpentier-Maguire E, Deutou Tchamgoue A, Agbor GA, et al. Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake. Metabolites. 2020; 10(5):182. https://doi.org/10.3390/metabo10050182
Chicago/Turabian StyleAtchan Nwakiban, Achille Parfait, Stefania Cicolari, Stefano Piazza, Fabrizio Gelmini, Enrico Sangiovanni, Giulia Martinelli, Lorenzo Bossi, Eugénie Carpentier-Maguire, Armelle Deutou Tchamgoue, Gabriel A. Agbor, and et al. 2020. "Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake" Metabolites 10, no. 5: 182. https://doi.org/10.3390/metabo10050182
APA StyleAtchan Nwakiban, A. P., Cicolari, S., Piazza, S., Gelmini, F., Sangiovanni, E., Martinelli, G., Bossi, L., Carpentier-Maguire, E., Deutou Tchamgoue, A., Agbor, G. A., Kuiaté, J.-R., Beretta, G., Dell’Agli, M., & Magni, P. (2020). Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake. Metabolites, 10(5), 182. https://doi.org/10.3390/metabo10050182









