Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
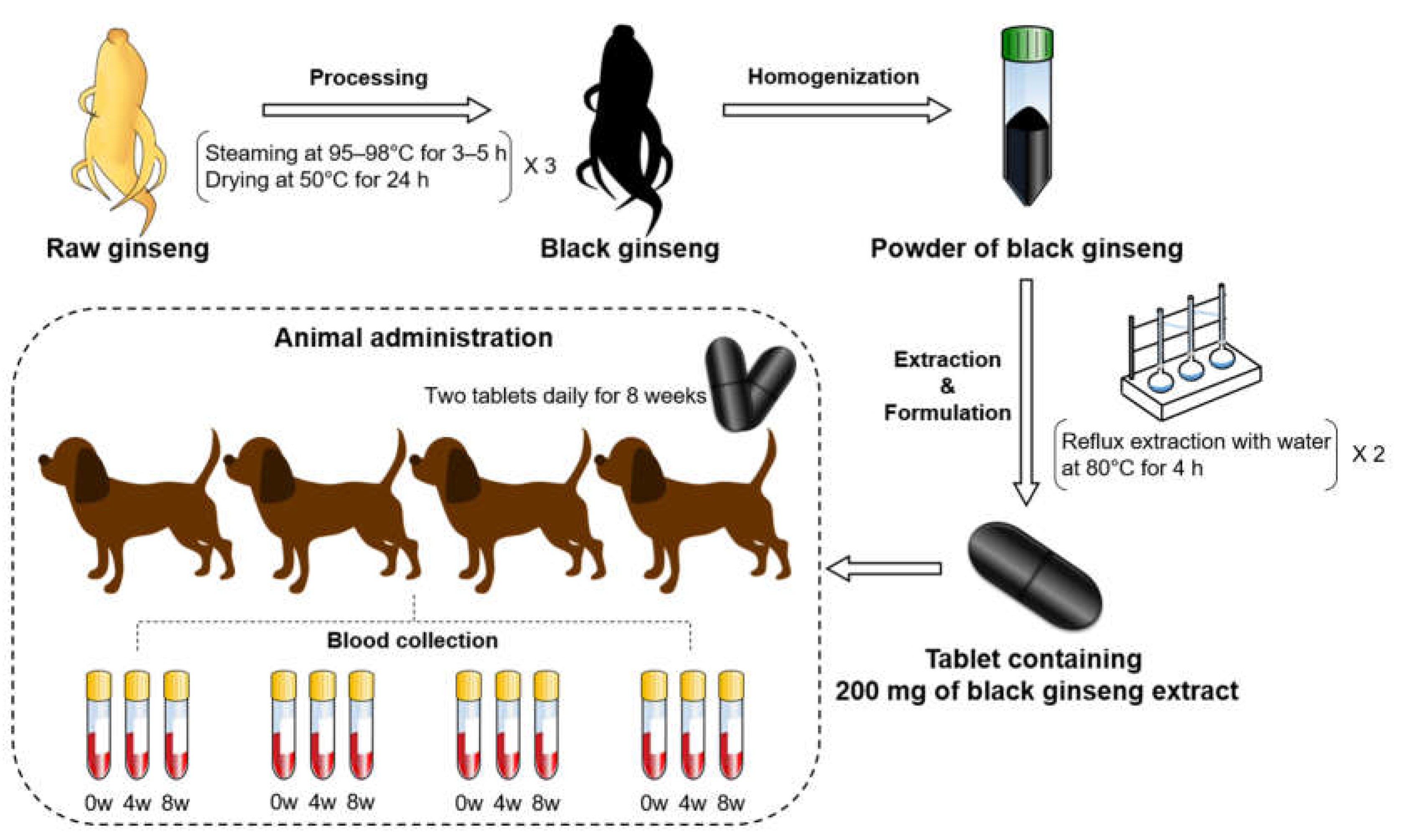
4.1. Preparation of Black Ginseng
4.2. Animal Adminstration and Sample Collection
4.3. HR-MAS NMR Measurement
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Pan, J.Y.; Xiao, X.Y.; Lin, R.C.; Cheng, Y.Y. Simultaneous determination of ginsenosides in Panax ginseng with di erent growth ages using high-performance liquid chromatography–mass spectrometry. Phytochem. Anal. 2006, 17, 424–430. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, S.-H.; Choi, B.-R.; Choi, D.J.; Lee, Y.-G.; Kim, H.-G.; Kim, G.-S.; Kim, K.; Lee, Y.-H.; Baek, N.-I.; et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, J.H.; Cho, S.H.; Shen, G.N.; Jin, L.G.; Myung, C.S.; Kim, D.H.; Yun, J.D.; Roh, S.S.; Park, Y.J.; et al. Preparation of black panax ginseng by new methods and its antitumor activity. Korea J. Herbol. 2008, 23, 85–92. [Google Scholar]
- Chen, G.; Li, H.; Gao, Y.; Zhang, L.; Zhao, Y. Flavored black ginseng exhibited antitumor activity via improving immune function and inducing apoptosis. Food Funct. 2017, 8, 1880–1889. [Google Scholar] [CrossRef]
- Kang, S.J.; Han, J.S.; Kim, A.J. Ameliorate Effect of Black Ginseng on HepG2 Cell transplanted in BALB/c Nude Mice. Korean J. Food Nutr. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Song, G.Y.; Chung, K.J.; Shin, Y.J.; Lee, G.W.; Lee, S.Y.; Seo, Y.B. Study on antiangiogenic effect of black ginseng radix. Korea J. Herbol. 2011, 26, 83–90. [Google Scholar]
- Lee, S.R.; Kim, M.R.; Yon, J.M.; Baek, I.J.; Park, C.G.; Lee, B.J.; Yun, Y.W.; Nam, S.Y. Black ginseng inhibits ethanol-induced teratogenesis in cultured mouse embryos through its effects on antioxidant activity. Toxicol. In Vitro 2009, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, D.H.; Woo, W.H. Antioxidant activity of black Panax Ginseng. J. Physiol. Pathol. Korean Med. 2011, 25, 115–121. [Google Scholar] [CrossRef]
- Choudhry, Q.N.; Kim, J.H.; Cho, H.T.; Heo, W.; Lee, J.J.; Lee, J.H.; Kim, Y.J. Ameliorative effect of black ginseng extract against oxidative stress-induced cellular damages in mouse hepatocytes. J. Ginseng Res. 2019, 43, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Lee, M.R.; Oh, C.J.; Cho, J.H.; Wang, C.Y.; Gu, L.J.; Mo, E.K.; Sung, C.K. Characterization of black ginseng extract with acetyl-and butyrylcholin-esterase inhibitory and antioxidant activities. J. Ginseng Res. 2010, 34, 348–354. [Google Scholar] [CrossRef]
- Kim, S.N.; Kang, S.J. Effects of black ginseng (9 times-steaming ginseng) on hypoglycemic action and changes in the composition of ginsenosides on the steaming process. Korean J. Food Sci. Technol. 2009, 41, 77–81. [Google Scholar]
- Seo, Y.S.; Shon, M.Y.; Kong, R.; Kang, O.H.; Zhou, T.; Kim, D.Y.; Kwon, D.Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2016, 190, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Oh, H.J.; Roh, S.S.; Seo, Y.B.; Park, Y.J.; Myung, C.S. Effect of black ginseng on body weight and lipid profiles in male rats fed normal diets. Yakhak Hoeji 2006, 50, 381–385. [Google Scholar]
- Lee, M.R.; Kim, B.C.; Kim, R.; Oh, H.I.; Kim, H.K.; Choi, K.J.; Sung, C.K. Anti-obesity effects of black ginseng extract in high fat diet-fed mice. J. Ginseng Res. 2013, 37, 308–349. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, A.J.; Cheon, Y.P.; Lee, M. Anti-obesity effects of water and ethanol extracts of black Ginseng. J. Korean Soc. Food Sci. Nutr. 2015, 44, 314–323. [Google Scholar] [CrossRef]
- Saba, E.; Jeon, B.R.; Jeong, D.H.; Lee, K.; Goo, Y.K.; Kim, S.H.; Sung, C.K.; Roh, S.S.; Kim, S.D.; Kim, H.K.; et al. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng Res. 2016, 40, 160–168. [Google Scholar] [CrossRef]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS ONE 2016, 11, e0146843. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, J.H.; Hong, H.D.; Kwon, J.; Lee, E.J.; Jang, M.; Lee, S.Y.; Han, A.R.; Nam, T.G.; Hong, S.K.; et al. Ginsenosides Rg5 and Rk1, the skin-whitening agents in black Ginseng. J. Funct. Foods 2018, 45, 67–74. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, A.J.; Lee, M.S.; Lee, Y.H. Anti-wrinkle effect of oriental medicine cosmetics containing black Ginseng. J. Korea Acad. Industr. Coop. Soc. 2010, 11, 3325–3329. [Google Scholar] [CrossRef]
- Carlos, G.; Dos Santos, F.P.; Fröehlich, P.E. Canine metabolomics advances. Metabolomics 2020, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.; Enot, D.P.; Overy, D.P.; Scott, I.M.; Jones, P.G.; Allaway, D.; Draper, J. Metabolite fingerprinting of urine suggests breed-specific dietary metabolism differences in domestic dogs. Br. J. Nutr. 2010, 103, 1127–1138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viant, M.R.; Ludwig, C.; Rhodes, S.; Günther, U.L.; Allaway, D. Validation of a urine metabolome fingerprint in dog for phenotypic classification. Metabolomics 2007, 3, 453–463. [Google Scholar] [CrossRef]
- Allaway, D.; Kamlage, B.; Gilham, M.S.; Hewson-Hughes, A.K.; Wiemer, J.C.; Colyer, A.; Rein, D. Effects of dietary glucose supplementation on the fasted plasma metabolome in cats and dogs. Metabolomics 2013, 9, 1096–1108. [Google Scholar] [CrossRef]
- Hall, J.A.; Brockman, J.A.; Jewell, D.E. Dietary fish oil alters the lysophospholipid metabolomic profile and decreases urinary 11-dehydro thromboxane B2 concentration in healthy Beagles. Vet. Immunol. Immunop. 2011, 144, 355–365. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.I.; Cardin, S.; Wait, R.; Chung, Y.L.; Vijayakumar, M.; Maguy, A.; Camm, A.J.; Nattel, S. Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. J. Mol. Cell Cardiol. 2010, 49, 851–863. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Recent and potential developments of biofluid analyses in metabolomics. J. Proteom. 2012, 75, 1079–1088. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, S.; Liu, L.; Nagana Gowda, G.A.; Bonney, P.; Stewart, J.; Knapp, D.W.; Raftery, D. NMR-based metabolomics study of canine bladder cancer. BBA Mol. Basis Dis. 2012, 1822, 1807–1814. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, D.Y.; Park, H.E.; Yoon, D.; Lee, B.; Kim, J.G.; Im, K.H.; Lee, Y.S.; Lee, W.K.; Kim, J.K. Serum Metabolic Profiling Reveals Potential Anti-Inflammatory Effects of the Intake of Black Ginseng Extracts in Beagle Dogs. Molecules 2020, 25, 3759. [Google Scholar] [CrossRef]
- Yoon, D.; Lee, M.; Kim, S.; Kim, S. Applications of NMR spectroscopy based metabolomics: A review. J. Korean Magn. Reson. Soc. 2013, 17, 1–10. [Google Scholar] [CrossRef]
- Santos, A.D.C.; Fonseca, F.A.; Lião, L.M.; Alcantara, G.B.; Barison, A. High-resolution magic angle spinning nuclear magnetic resonance in foodstuff analysis. TrAC Trends Anal. Chem. 2015, 73, 10–18. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Lee, J.; Park, S.Y.; Song, S.Y.; Na, J.; Kim, S.W.; Kim, S.J.; Nou, I.S.; Lee, Y.H.; et al. Metabolic differentiation of diamondback moth (Plutella xylostella (L.)) resistance in cabbage (Brassica oleracea L. ssp. capitata). J. Agric. Food Chem. 2013, 61, 11222–11230. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Jenkins, J.E. HR-MAS NMR spectroscopy in material science. Advanced Aspects of Spectroscopy; Intech Open: London, UK, 2012; pp. 279–306. [Google Scholar]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, e364976. [Google Scholar] [CrossRef]
- Jose, D.G.; Good, R.A. Quantitative effects of nutritional essential amino acid deficiency upon immune responses to tumors in mice. J. Exp. Med. 1973, 137, 1–9. [Google Scholar] [CrossRef]
- Tsukishiro, T.; Shimizu, Y.; Higuchi, K.; Watanabe, A. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J. Gastroenterol. Hepatol. 2000, 15, 849–859. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, K.J.; Choi, S.Y.; Koh, E.J.; Park, J.; Lee, B.Y. Korean ginseng extract ameliorates abnormal immune response through the regulation of inflammatory constituents in Sprague Dawley rat subjected to environmental heat stress. J. Ginseng Res. 2019, 43, 252–260. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.W.; Park, S.J.; Kim, S.; Yu, K.M.; Kim, S.G.; Lee, S.H.; Seo, Y.K.; Cho, N.H.; Kang, K.; et al. Greater efficacy of black ginseng (CJ EnerG) over red ginseng against lethal influenza a virus infection. Nutrients 2019, 11, 1879. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Jones, M.R.; Kopple, J.D. Histidine, an essential amino acid for adult dogs. J. Nutr. 1981, 111, 1074–1084. [Google Scholar] [CrossRef]
- Li, X.T.; Chen, R.; Jin, L.M.; Chen, H.Y. Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am. J. Chin. Med. 2009, 37, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-H.; Lee, H.; Yoon, D.; Kim, B.-S.; Kim, M.-B.; Kim, S. Discrimination of Basal Cell Carcinoma from Normal Skin Tissue Using High-Resolution Magic Angle Spinning 1H NMR Spectroscopy. PLoS ONE 2016, 11, e0150328. [Google Scholar] [CrossRef] [PubMed]





| Compounds | Chemical Shifts (Multiplicities) (ppm) | 0 Week | 4 Weeks | 8 Weeks |
|---|---|---|---|---|
| (Mean (%) ± Standard Deviation) | ||||
| Acetate | 1.93 (s) | 0.211 ± 0.064 | 0.244 ± 0.066 | 0.189 ± 0.062 |
| Alanine | 1.49 (d), 3.79 (q) | 5.180 ± 0.946 | 7.031 ± 1.095 a | 6.531 ± 2.079 |
| Citrate | 2.54 (d), 2.69 (d) | 0.878 ± 0.644 | 0.576 ± 0.126 | 0.734 ± 0.322 |
| Creatine | 3.05 (s), 3.94 (s) | 0.403 ± 0.199 | 0.411 ± 0.181 | 0.374 ± 0.166 |
| Formate | 8.46 (s) | 0.660 ± 0.109 | 0.430 ± 0.098 a | 0.490 ± 0.102 a |
| Glucose | 3.26 (dd), 3.40–3.45 (m), 3.47–3.54 (m), 3.55 (dd), 3.76–3.80 (m), 3.86–3.91 (m), 4.66 (d), 5.23 (d) | 41.627 ± 4.529 | 42.265 ± 7.364 | 44.514 ± 4.092 |
| Glutamate | 2.13–2.05 (m), 2.33–2.36 (m) | 0.839 ± 0.295 | 1.000 ± 0.170 | 0.936 ± 0.139 |
| Glutamine | 2.12–2.15 (m), 2.44–2.48 (m), 3.77 (t) | 6.494 ± 0.155 | 7.483 ± 0.856 | 8.851 ± 1.061 b |
| Glycerol | 3.57 (dd), 3.67 (dd), 3.76 (m) | 0.734 ± 0.104 | 0.649 ± 0.078 | 0.568 ± 0.164 |
| Glycine | 3.57 (s) | 2.800 ± 0.457 | 3.011 ± 0.280 | 2.947 ± 0.619 |
| Histidine | 7.06(s), 7.77 (s) | 1.073 ± 0.187 | 1.329 ± 0.327 | 1.431 ± 0.099 b |
| Isoleucine | 0.95 (t), 1.02 (d), 1.25 (m), 1.46 (m), 1.97 (m), 3.67 (d) | 0.415 ± 0.150 | 0.572 ± 0.099 | 0.530 ± 0.105 |
| Lactate | 1.34 (d), 4.16 (q) | 25.640 ± 8.500 | 24.010 ± 8.663 | 20.714 ± 9.561 |
| Leucine | 0.97 (t), 1.68–1.74 (m), 3.73 (m) | 1.032 ± 0.204 | 1.284 ± 0.274 | 1.328 ± 0.326 |
| Methionine | 2.15 (s), 2.16 (m), 2.65 (t) | 0.531 ± 0.097 | 0.461 ± 0.064 | 0.470 ± 0.066 |
| Proline | 1.99–2.07 (m), 2.36 (m), 3.35 (q), 3.43 (q), 4.14 (dd) | 3.325 ± 0.915 | 2.247 ± 0.330 | 2.425 ± 0.412 |
| Pyruvate | 2.38 (s) | 0.758 ± 0.252 | 0.518 ± 0.116 | 0.409 ± 0.110 |
| Serine | 3.85 (dd), 3.99 (s) | 2.892 ± 0.690 | 2.129 ± 0.336 | 2.366 ± 0.469 |
| Succinate | 2.41 (s) | 0.085 ± 0.010 | 0.075 ± 0.001 | 0.072 ± 0.007 |
| Threonine | 1.33 (d), 3.58 (d), 4.26 (m) | 3.364 ± 1.148 | 2.850 ± 0.556 | 2.663 ± 0.668 |
| Valine | 1.00 (d), 1.05 (d), 2.27 (m), 3.62 (d) | 1.060 ± 0.260 | 1.444 ± 0.323 a | 1.458 ± 0.409 a |
| Name | AUC | p-Value | Log2FC | Name | AUC | p-Value | Log2FC |
|---|---|---|---|---|---|---|---|
| Valine | 0.969 | 0.005 | −0.255 | Serine | 0.844 | 0.066 | 0.287 |
| Formate | 0.938 | 0.009 | 0.155 | Alanine | 0.813 | 0.041 | −0.357 |
| Glutamine | 0.938 | 0.008 | −0.310 | Glutamate | 0.813 | 0.112 | −0.097 |
| Histidine | 0.906 | 0.025 | −0.204 | Glycine | 0.797 | 0.136 | −0.076 |
| Isoleucine | 0.906 | 0.021 | −0.107 | Glycerol | 0.781 | 0.216 | 0.096 |
| Leucine | 0.906 | 0.028 | −0.182 | Pyruvate | 0.781 | 0.047 | 0.223 |
| Proline | 0.906 | 0.033 | 0.407 | Methionine | 0.750 | 0.107 | 0.051 |
| Glucose | 0.719 | 0.299 | −0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, D.; Kim, Y.J.; Lee, W.K.; Choi, B.R.; Oh, S.M.; Lee, Y.S.; Kim, J.K.; Lee, D.Y. Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng. Metabolites 2020, 10, 517. https://doi.org/10.3390/metabo10120517
Yoon D, Kim YJ, Lee WK, Choi BR, Oh SM, Lee YS, Kim JK, Lee DY. Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng. Metabolites. 2020; 10(12):517. https://doi.org/10.3390/metabo10120517
Chicago/Turabian StyleYoon, Dahye, Ye Jin Kim, Wan Kyu Lee, Bo Ram Choi, Seon Min Oh, Young Seob Lee, Jae Kwang Kim, and Dae Young Lee. 2020. "Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng" Metabolites 10, no. 12: 517. https://doi.org/10.3390/metabo10120517
APA StyleYoon, D., Kim, Y. J., Lee, W. K., Choi, B. R., Oh, S. M., Lee, Y. S., Kim, J. K., & Lee, D. Y. (2020). Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng. Metabolites, 10(12), 517. https://doi.org/10.3390/metabo10120517






