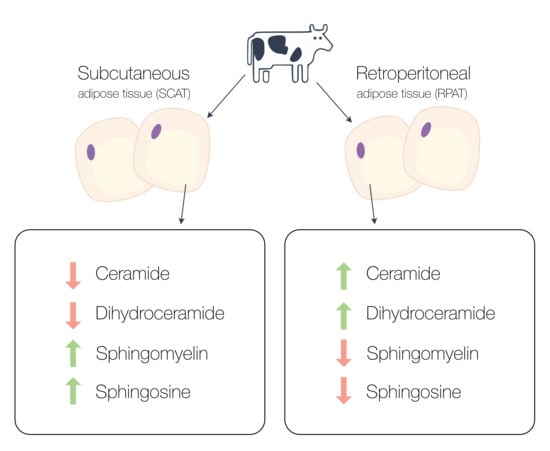
Sphingolipid Profiling Reveals Different Extent of Ceramide Accumulation in Bovine Retroperitoneal and Subcutaneous Adipose Tissues
Abstract
1. Introduction
2. Results
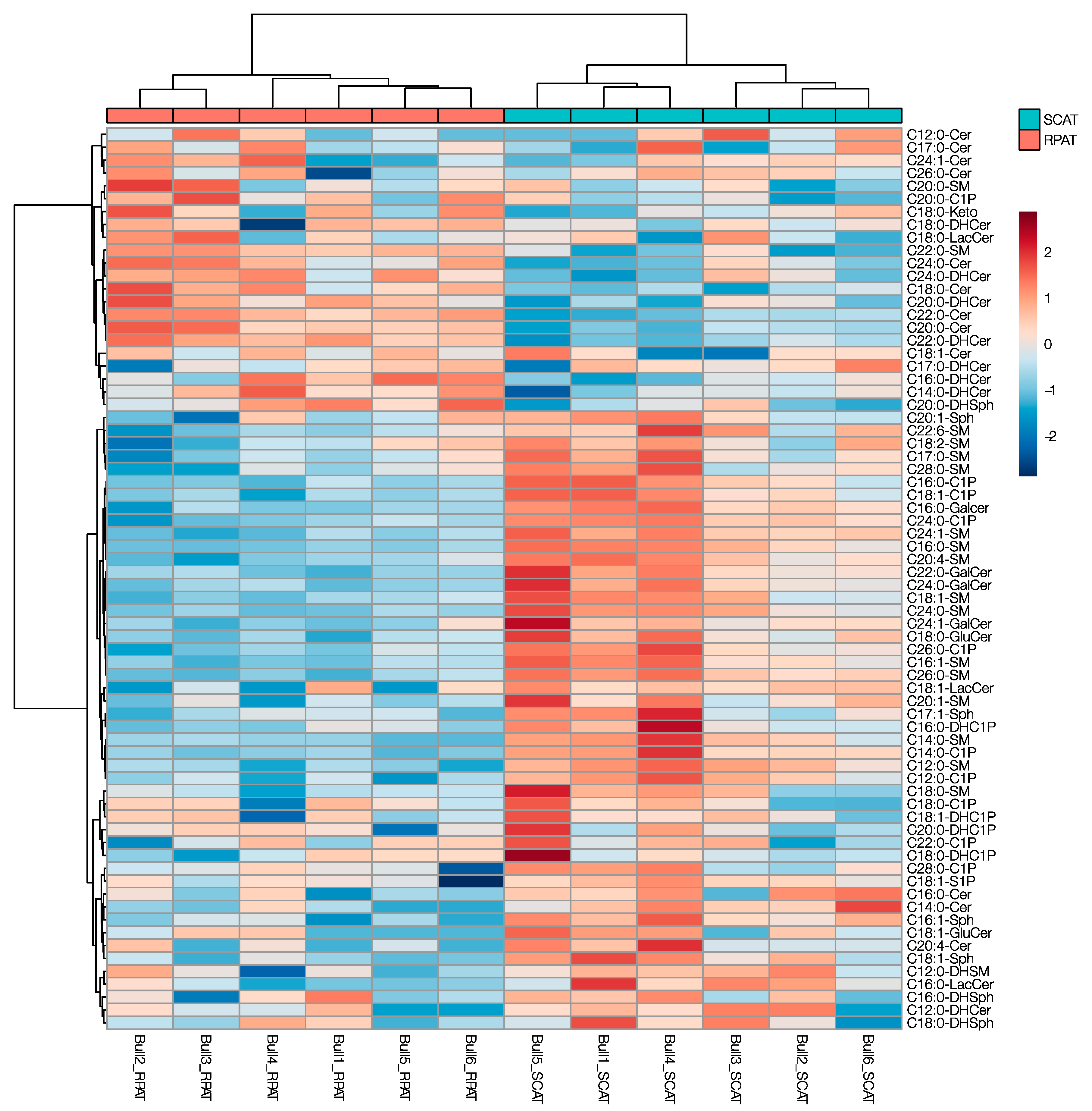
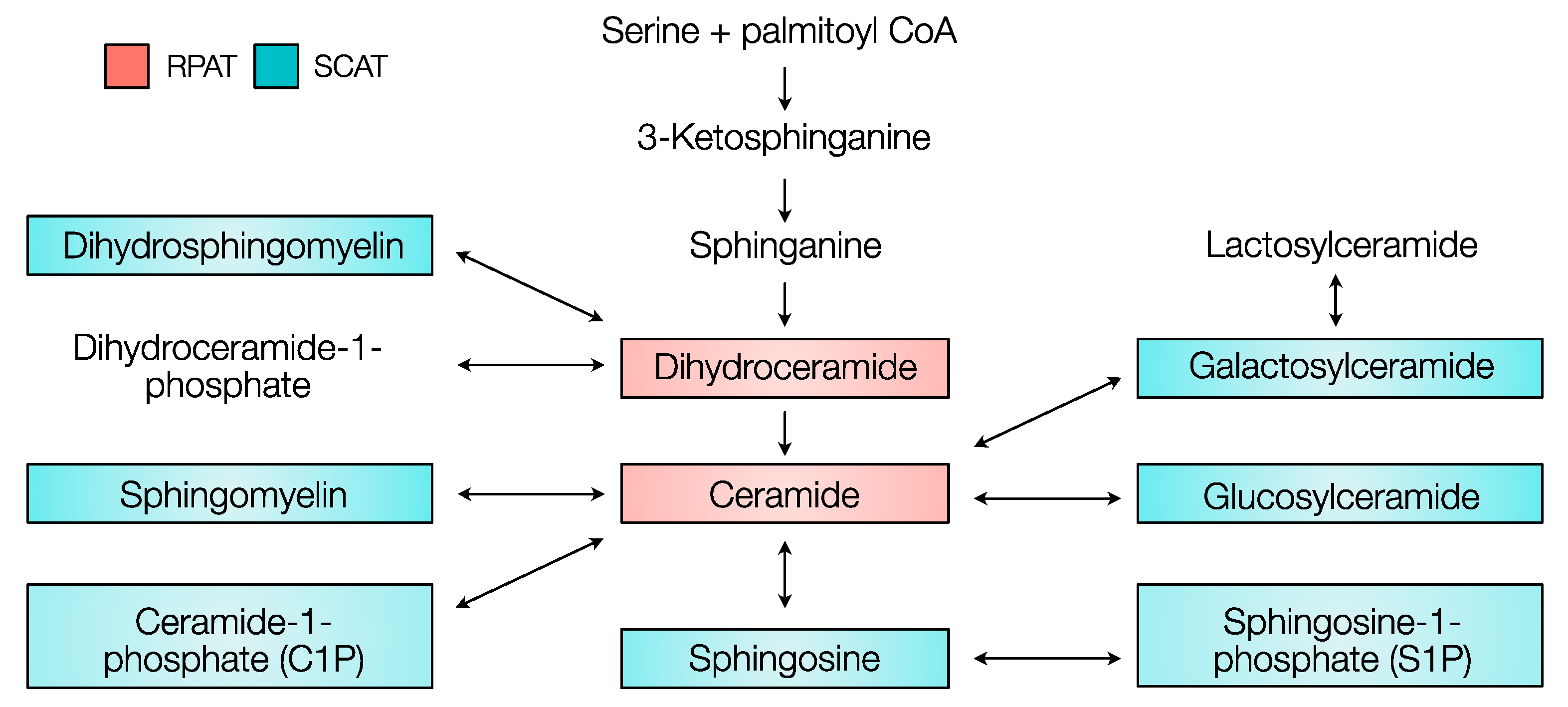
2.1. Comparative Sphingolipid Distribution in SCAT and RPAT
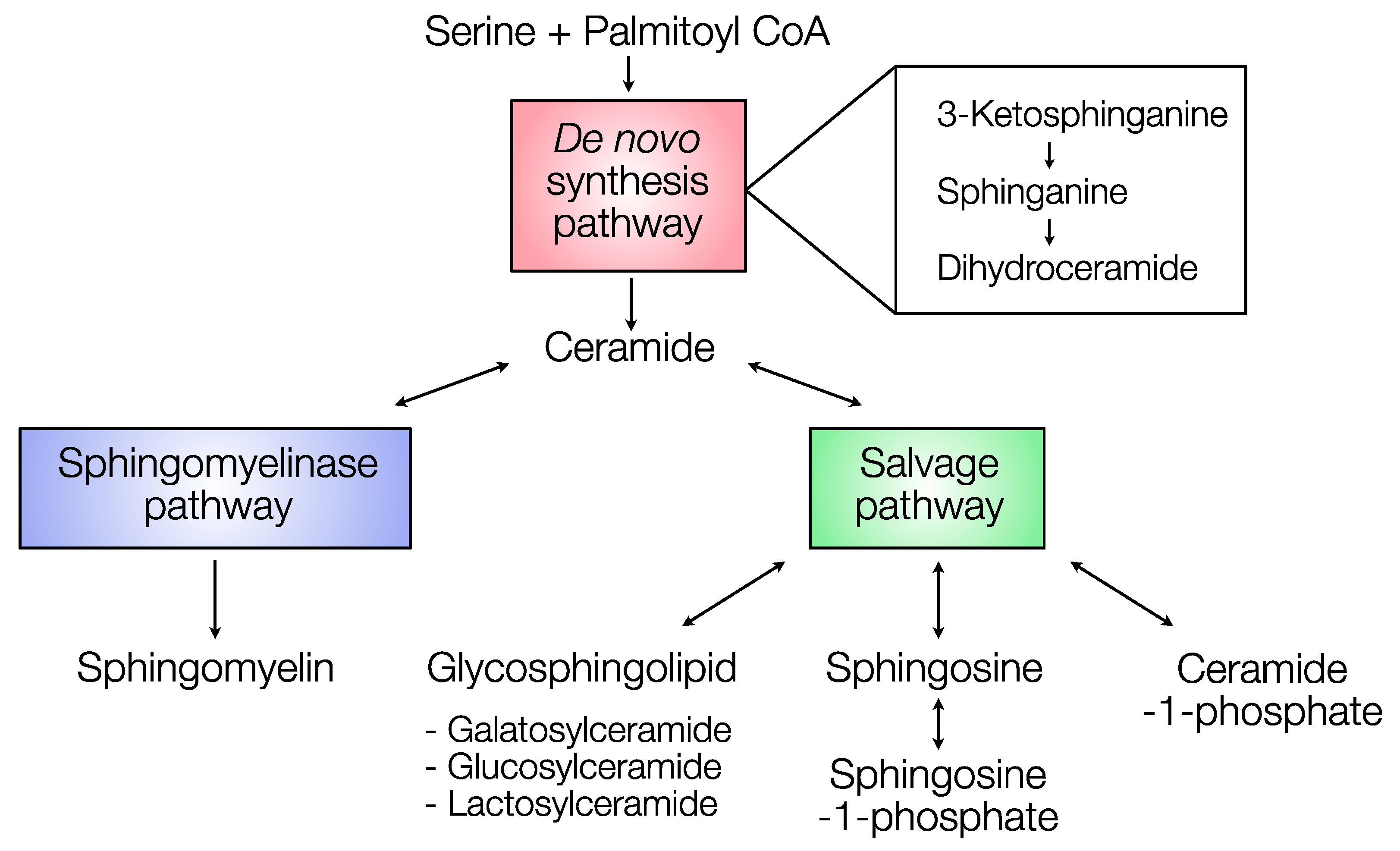
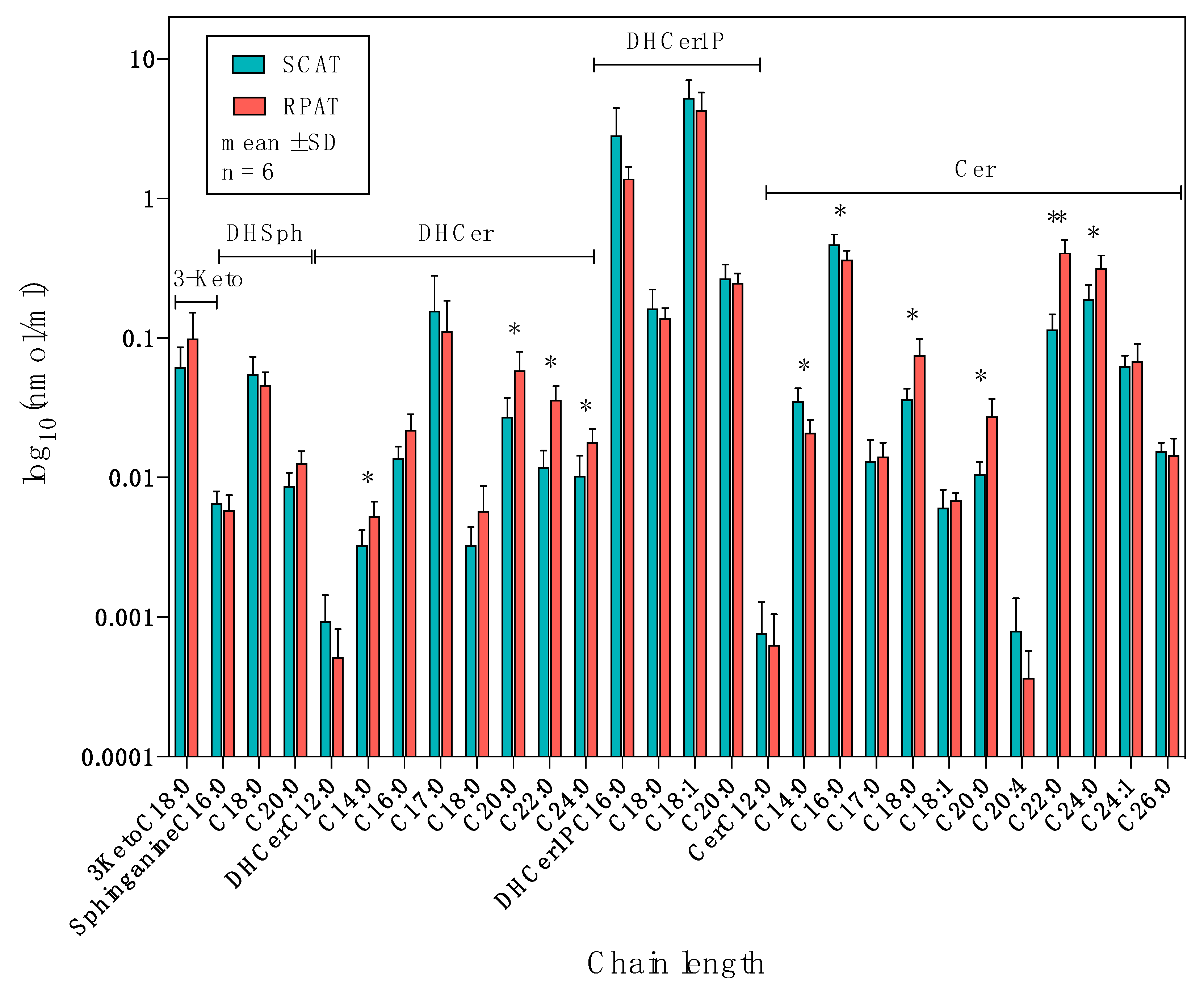
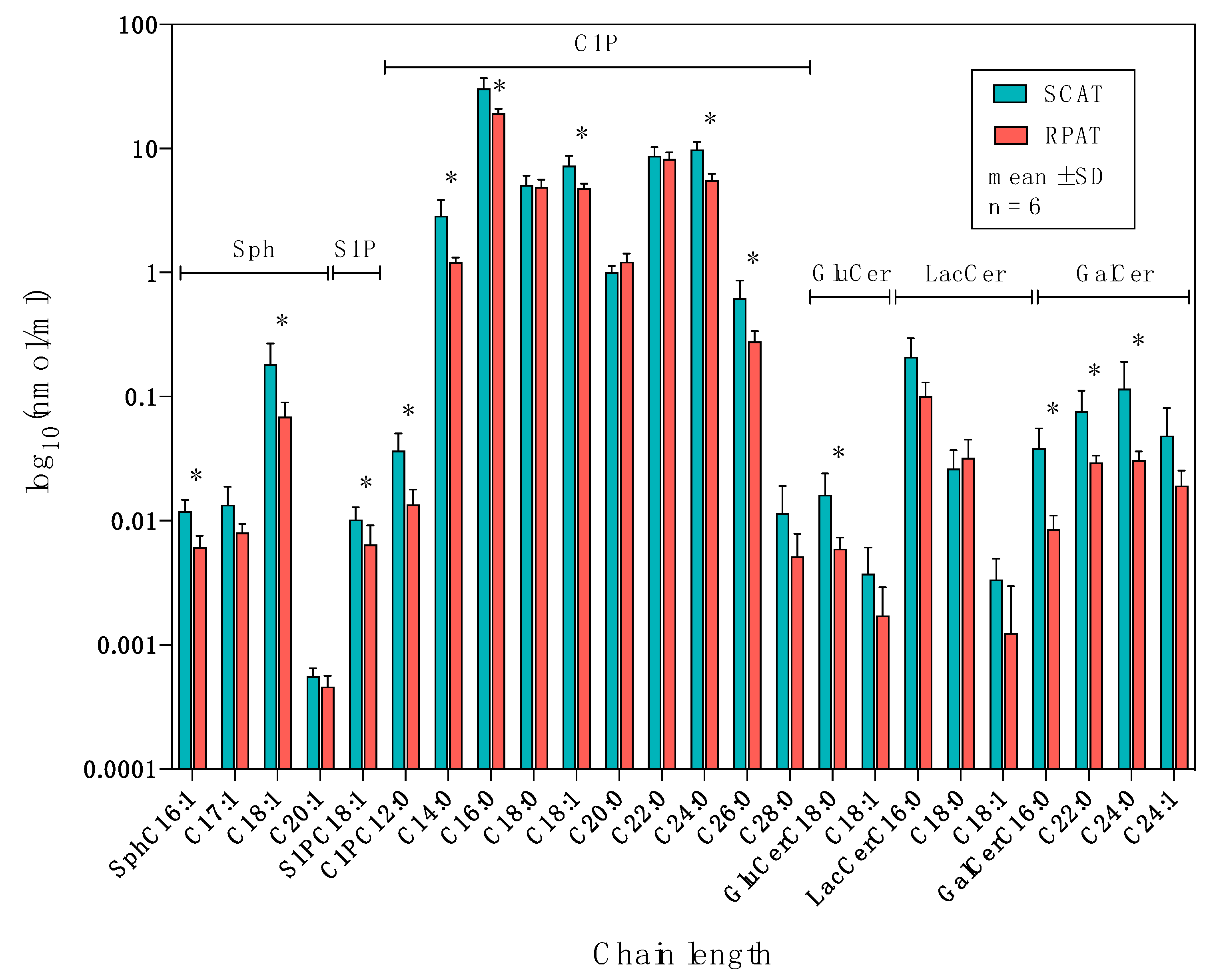
2.2. Distribution of Sphingolipids in the De Novo Synthesis Pathway
2.3. Distribution of Sphingolipids in the Salvage Pathway
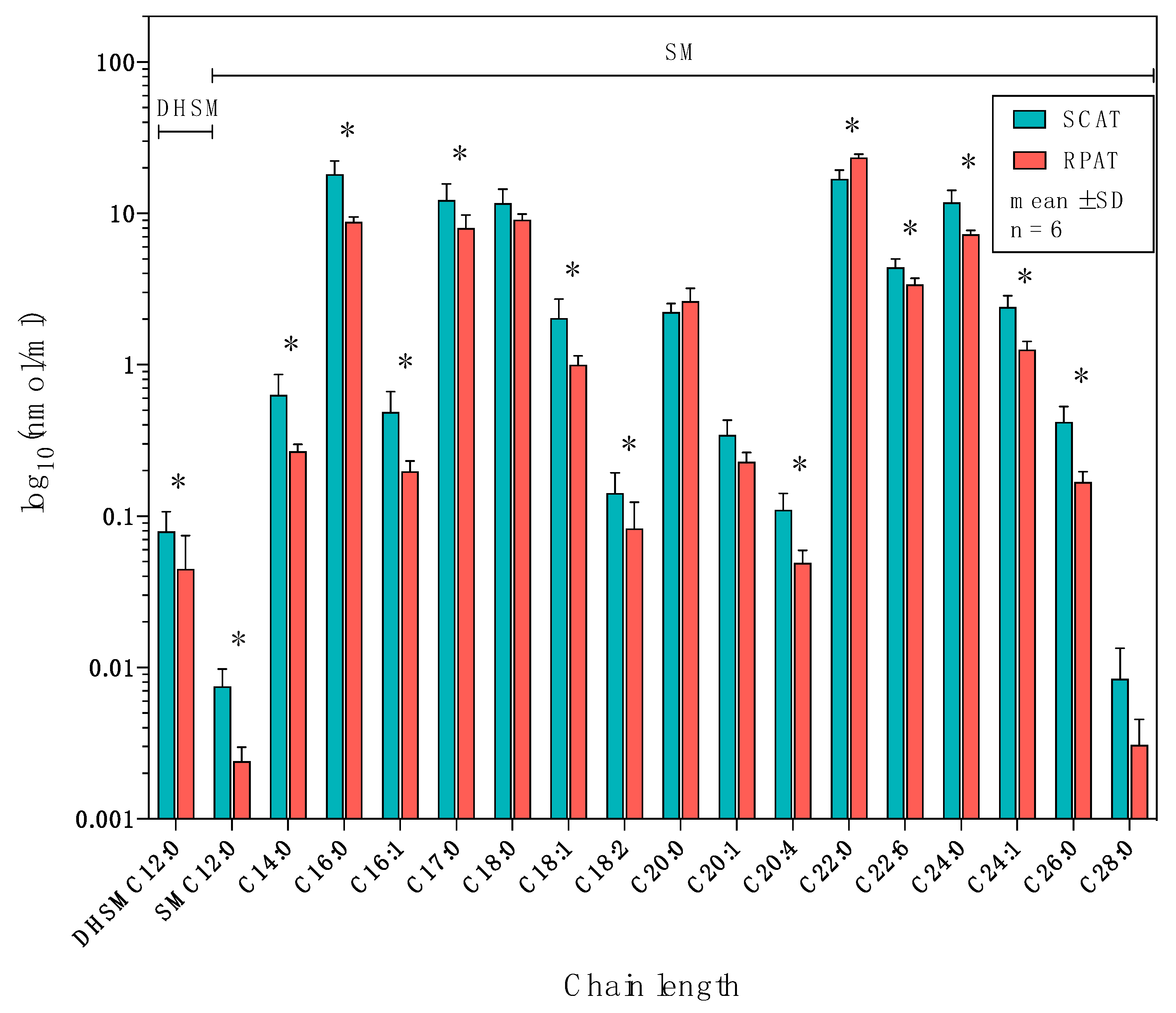
2.4. Distribution of Sphingolipids in the Sphingomyelinase Pathway
3. Discussion
3.1. Higher Concentration of Ceramides in RPAT
3.2. Insulin Resistance, Inflammation, and Oxidative Stress in RPAT and SCAT
4. Materials and Methods
4.1. Animals and Sampling
4.2. Sphingolipid Measurement
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- McFadden, J.W.; Rico, J.E. Invited review: Sphingolipid biology in the dairy cow: The emerging role of ceramide. J. Dairy Sci. 2019, 102, 7619–7639. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.E.; Bandaru, V.V.R.; Dorskind, J.M.; Haughey, N.J.; McFadden, J.W. Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation. J. Dairy Sci. 2015, 98, 7757–7770. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, A.; Clarke, B.A.; Gavrilova, O.; Ma, Y.; Zhu, H.; Ma, X.; Xu, L.; Tuymetova, G.; Larman, B.C.; Allende, M.L.; et al. De NovoSphingolipid Biosynthesis Is Required for Adipocyte Survival and Metabolic Homeostasis. J. Biol. Chem. 2017, 292, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Kolak, M.; Gertow, J.; Westerbacka, J.; Summers, S.A.; Liska, J.; Franco-Cereceda, A.; Orešič, M.; Yki-Järvinen, H.; Eriksson, P.; Fisher, R.M. Expression of ceramide-metabolising enzymes in subcutaneous and intra-abdominal human adipose tissue. Lipids Health Dis. 2012, 11, 115. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef]
- Merrill, J.A.H. Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nat. Cell Biol. 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Kabara, E.; Sordillo, L.M.; Holcombe, S.; Contreras, G.A. Adiponectin links adipose tissue function and monocyte inflammatory responses during bovine metabolic stress. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 49–58. [Google Scholar] [CrossRef]
- Kenéz, Á.; Ruda, L.; Dänicke, S.; Huber, K. Insulin signaling and insulin response in subcutaneous and retroperitoneal adipose tissue in Holstein cows during the periparturient period. J. Dairy Sci. 2019, 102, 11718–11729. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Drackley, J.K.; Khan, M.J.; Loor, J.J. Inflammation- and lipid metabolism-related gene network expression in visceral and subcutaneous adipose depots of Holstein cows. J. Dairy Sci. 2014, 97, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Locher, L.F.; Meyer, N.; Weber, E.-M.; Rehage, J.; Meyer, U.; Dänicke, S.; Huber, K. Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow. J. Dairy Sci. 2011, 94, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Kenéz, Á.; Locher, L.; Rizk, A.; Dänicke, S.; Rehage, J.; Huber, K. Lipolytic capacity of visceral adipose tissue in the dairy cow. In Energy and Protein Metabolism and Nutrition in Sustainable Animal Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 459–461. ISBN 978-90-8686-781-3. [Google Scholar]
- Ruda, L.; Raschka, C.; Huber, K.; Tienken, R.; Meyer, U.; Dänicke, S.; Rehage, J. Gain and loss of subcutaneous and abdominal fat depot mass from late pregnancy to 100 days in milk in German Holsteins. J. Dairy Res. 2019, 86, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Votruba, S.B.; Jensen, M.D. Regional Fat Deposition as a Factor in FFA Metabolism. Annu. Rev. Nutr. 2007, 27, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.E.; Mathews, A.T.; Lovett, J.; Haughey, N.J.; McFadden, J.W. Palmitic acid feeding increases ceramide supply in association with increased milk yield, circulating nonesterified fatty acids, and adipose tissue responsiveness to a glucose challenge. J. Dairy Sci. 2016, 99, 8817–8830. [Google Scholar] [CrossRef]
- Chazenbalk, G.; Bertolotto, C.; Heneidi, S.; Jumabay, M.; Trivax, B.; Aronowitz, J.; Yoshimura, K.; Simmons, C.F.; Dumesic, D.A.; Azziz, R. Novel Pathway of Adipogenesis through Cross-Talk between Adipose Tissue Macrophages, Adipose Stem Cells and Adipocytes: Evidence of Cell Plasticity. PLoS ONE 2011, 6, e17834. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered Adipose and Plasma Sphingolipid Metabolism in Obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Von Soosten, D.; Meyer, U.; Weber, E.M.; Rehage, J.; Flachowsky, G.; Dänicke, S. Effect of trans-10, cis-12 conjugated linoleic acid on performance, adipose depot weights, and liver weight in early-lactation dairy cows. J. Dairy Sci. 2011, 94, 2859–2870. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol. Physiol. 2010, 298, C961–C971. [Google Scholar] [CrossRef]
- De Koster, J.; Hostens, M.; Van Eetvelde, M.; Hermans, K.; Moerman, S.; Bogaert, H.; Depreester, E.; Van den Broeck, W.; Opsomer, G. Insulin response of the glucose and fatty acid metabolism in dry dairy cows across a range of body condition scores. J. Dairy Sci. 2015, 98, 4580–4592. [Google Scholar] [CrossRef] [PubMed]
- Malau-Aduli, A.E.O.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. A comparison of the fatty acid composition of triacylglycerols in adipose tissue from Limousin and Jersey cattle. Aust. J. Agric. Res. 1997, 48, 715. [Google Scholar] [CrossRef]
- Merrill, A.H.; Wang, E.; Mullins, R.E. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: Effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry 1988, 27, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Cowart, L.A.; Hannun, Y.A. Selective Substrate Supply in the Regulation of Yeastde NovoSphingolipid Synthesis. J. Biol. Chem. 2007, 282, 12330–12340. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Hannun, Y.A. The Acid Sphingomyelinase/Ceramide Pathway: Biomedical Significance and Mechanisms of Regulation. Curr. Mol. Med. 2010, 10, 454–466. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Ternes, P.; Holthuis, J.C.M. The Multigenic Sphingomyelin Synthase Family. J. Biol. Chem. 2006, 281, 29421–29425. [Google Scholar] [CrossRef]
- Yano, M.; Watanabe, K.; Yamamoto, T.; Ikeda, K.; Senokuchi, T.; Lu, M.; Kadomatsu, T.; Tsukano, H.; Ikawa, M.; Okabe, M.; et al. Mitochondrial Dysfunction and Increased Reactive Oxygen Species Impair Insulin Secretion in Sphingomyelin Synthase 1-null Mice. J. Biol. Chem. 2011, 286, 3992–4002. [Google Scholar] [CrossRef]
- Rico, J.E.; Giesy, S.L.; Haughey, N.J.; Boisclair, Y.R.; McFadden, J.W. Intravenous Triacylglycerol Infusion Promotes Ceramide Accumulation and Hepatic Steatosis in Dairy Cows. J. Nutr. 2018, 148, 1529–1535. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Rico, J.E.; Myers, W.A.; Laub, D.J.; Davis, A.N.; Zeng, Q.; McFadden, J.W. Hot topic: Ceramide inhibits insulin sensitivity in primary bovine adipocytes. J. Dairy Sci. 2018, 101, 3428–3432. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Gencer, S.; Peterson, Y.K.; Ward, K.E.; Mukhopadhyay, A.; Oaks, J.; Bielawski, J.; Szulc, Z.M.; Thomas, R.J.; Selvam, S.P.; et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 2013, 5, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.M.; Scheid, M.P.; Duronio, V. Ceramide Inhibits Protein Kinase B/Akt by Promoting Dephosphorylation of Serine 473. J. Biol. Chem. 2000, 275, 13330–13335. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.; Golde, D.W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell 1994, 77, 325–328. [Google Scholar] [CrossRef]
- Brodlie, M.; Mckean, M.C.; Johnson, G.E.; Gray, J.; Fisher, A.J.; Corris, P.A.; Lordan, J.L.; Ward, C. Ceramide Is Increased in the Lower Airway Epithelium of People with Advanced Cystic Fibrosis Lung Disease. Am. J. Respir. Crit. Care Med. 2010, 182, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Andrieu-Abadie, N.; Gouazé, V.; Salvayre, R.; Levade, T. Ceramide in apoptosis signaling: Relationship with oxidative stress. Free. Radic. Biol. Med. 2001, 31, 717–728. [Google Scholar] [CrossRef]
- Goldkorn, T.; Balaban, N.; Shannon, M.; Chea, V.; Matsukuma, K.; Gilchrist, D.; Wang, H.; Chan, C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J. Cell Sci. 1998, 111 Pt 21, 3209–3220. [Google Scholar]
- Heinrich, M.; Neumeyer, J.; Jakob, M.; Hallas, C.; Tchikov, V.; Winoto-Morbach, S.; Wickel, M.; Schneider-Brachert, W.; Trauzold, A.; Hethke, A.; et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004, 11, 550–563. [Google Scholar] [CrossRef]
- Sakakura, C.; Sweeney, E.A.; Shirahama, T.; Hagiwara, A.; Yamaguchi, T.; Takahashi, T.; Hakomori, S.-I.; Igarashi, Y. Selectivity of Sphingosine-Induced Apoptosis. Biochem. Biophys. Res. Commun. 1998, 246, 827–830. [Google Scholar] [CrossRef]
- Osawa, Y.; Uchinami, H.; Bielawski, J.; Schwabe, R.F.; Hannun, Y.A.; Brenner, D.A. Roles for C16-ceramide and Sphingosine 1-Phosphate in Regulating Hepatocyte Apoptosis in Response to Tumor Necrosis Factor-α. J. Biol. Chem. 2005, 280, 27879–27887. [Google Scholar] [CrossRef]
- Seumois, G.; Fillet, M.; Gillet, L.; Faccinetto, C.; Desmet, C.; François, C.; Dewals, B.; Oury, C.; Vanderplasschen, A.; Lekeux, P.; et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. Leukoc. Biol. 2007, 81, 1477–1486. [Google Scholar] [CrossRef]
- Liu, R.; Pulliam, D.A.; Liu, Y.; Salmon, A.B. Dynamic differences in oxidative stress and the regulation of metabolism with age in visceral versus subcutaneous adipose. Redox Biol. 2015, 6, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Niesler, C.U.; Siddle, K.; Prins, J.B. Human Preadipocytes Display a Depot-Specific Susceptibility to Apoptosis. Diabetes 1998, 47, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H., Jr.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, Y.H.; Christiane Bäßler, S.; Koch, C.; Scheu, T.; Meyer, U.; Dänicke, S.; Huber, K.; Kenéz, Á. Sphingolipid Profiling Reveals Different Extent of Ceramide Accumulation in Bovine Retroperitoneal and Subcutaneous Adipose Tissues. Metabolites 2020, 10, 473. https://doi.org/10.3390/metabo10110473
Leung YH, Christiane Bäßler S, Koch C, Scheu T, Meyer U, Dänicke S, Huber K, Kenéz Á. Sphingolipid Profiling Reveals Different Extent of Ceramide Accumulation in Bovine Retroperitoneal and Subcutaneous Adipose Tissues. Metabolites. 2020; 10(11):473. https://doi.org/10.3390/metabo10110473
Chicago/Turabian StyleLeung, Yue Hei, Sonja Christiane Bäßler, Christian Koch, Theresa Scheu, Ulrich Meyer, Sven Dänicke, Korinna Huber, and Ákos Kenéz. 2020. "Sphingolipid Profiling Reveals Different Extent of Ceramide Accumulation in Bovine Retroperitoneal and Subcutaneous Adipose Tissues" Metabolites 10, no. 11: 473. https://doi.org/10.3390/metabo10110473
APA StyleLeung, Y. H., Christiane Bäßler, S., Koch, C., Scheu, T., Meyer, U., Dänicke, S., Huber, K., & Kenéz, Á. (2020). Sphingolipid Profiling Reveals Different Extent of Ceramide Accumulation in Bovine Retroperitoneal and Subcutaneous Adipose Tissues. Metabolites, 10(11), 473. https://doi.org/10.3390/metabo10110473