The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction and Optimization of Fractionation by VLC for the Isolation of Alkaloids from Narcissus triandrus L. c.v. ‘Hawera’
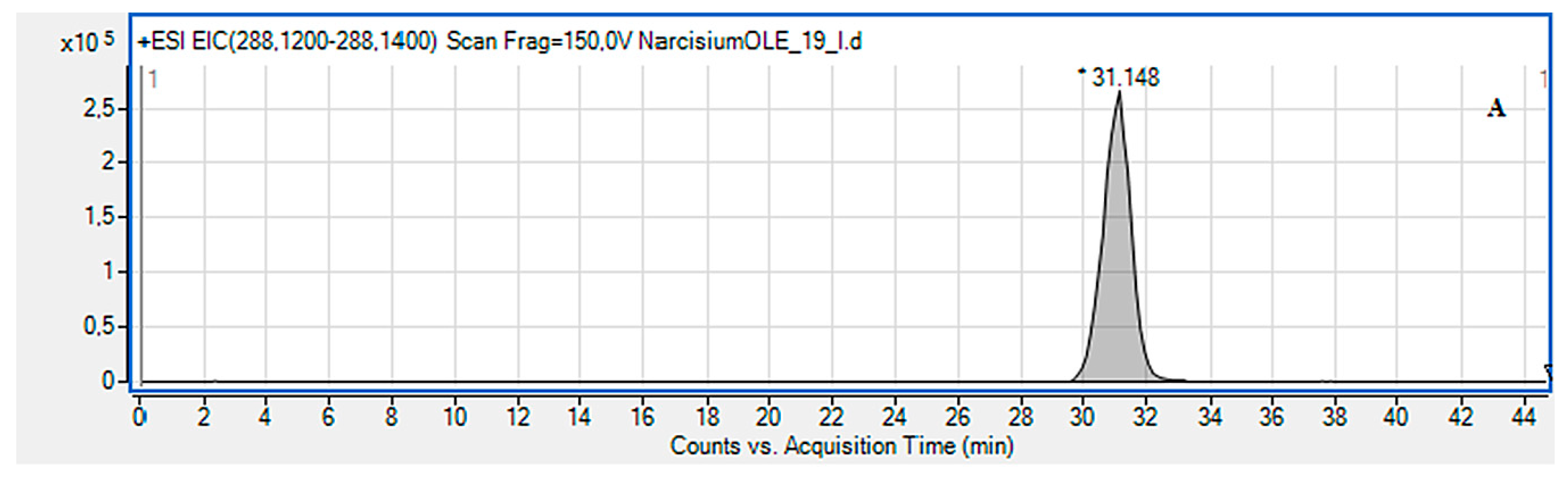
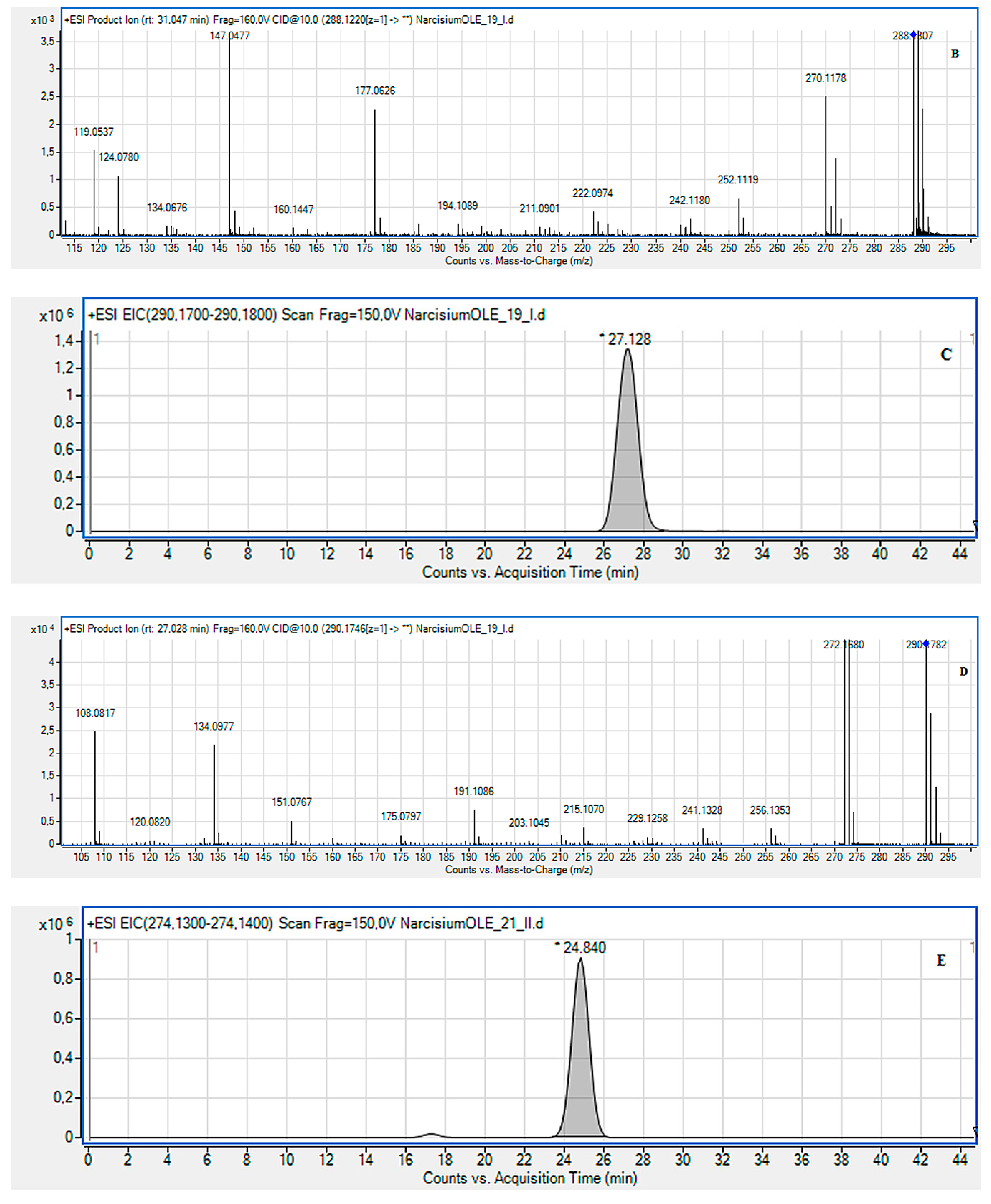
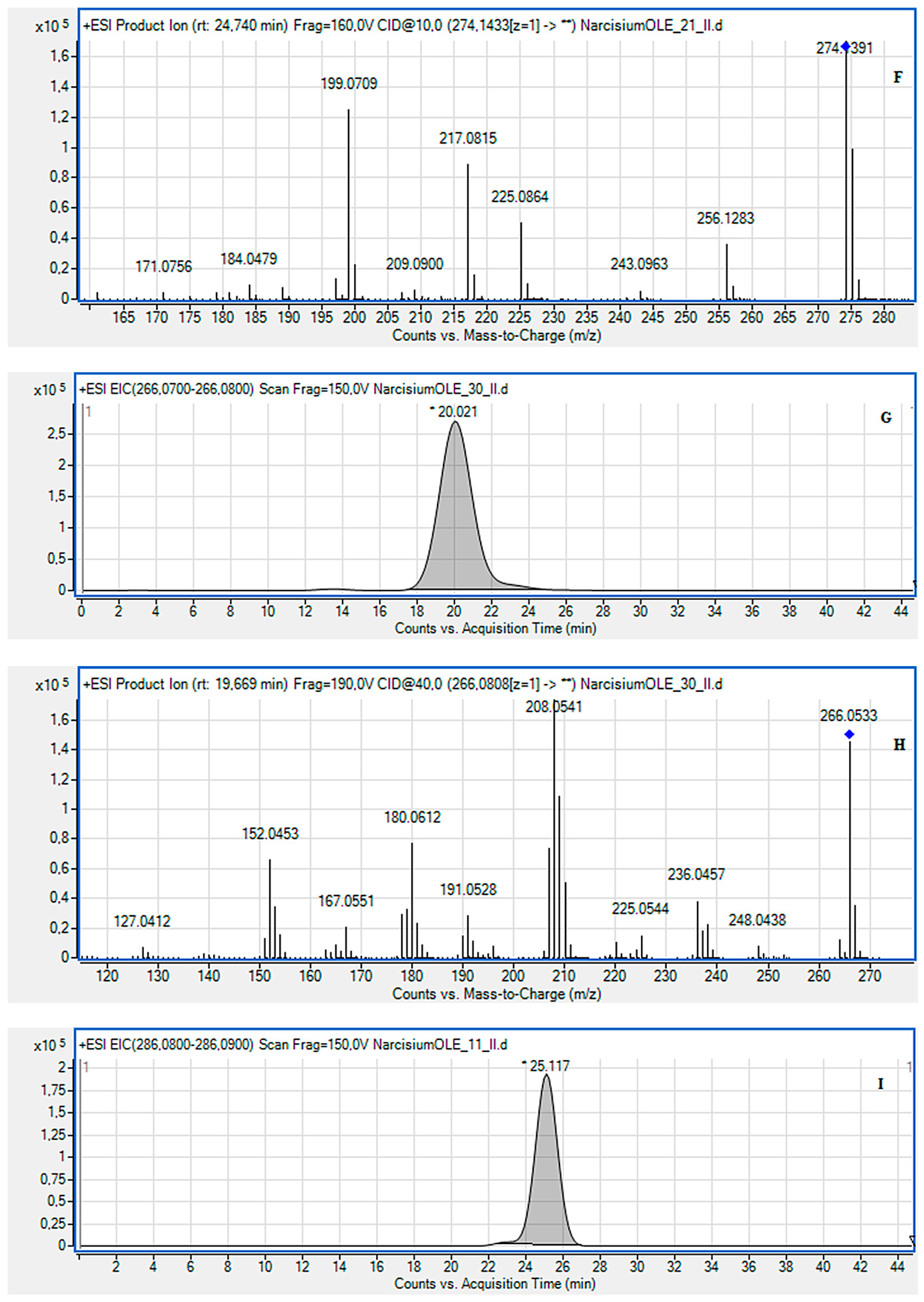
2.2. LC-MS Identification of the Isolated Compounds
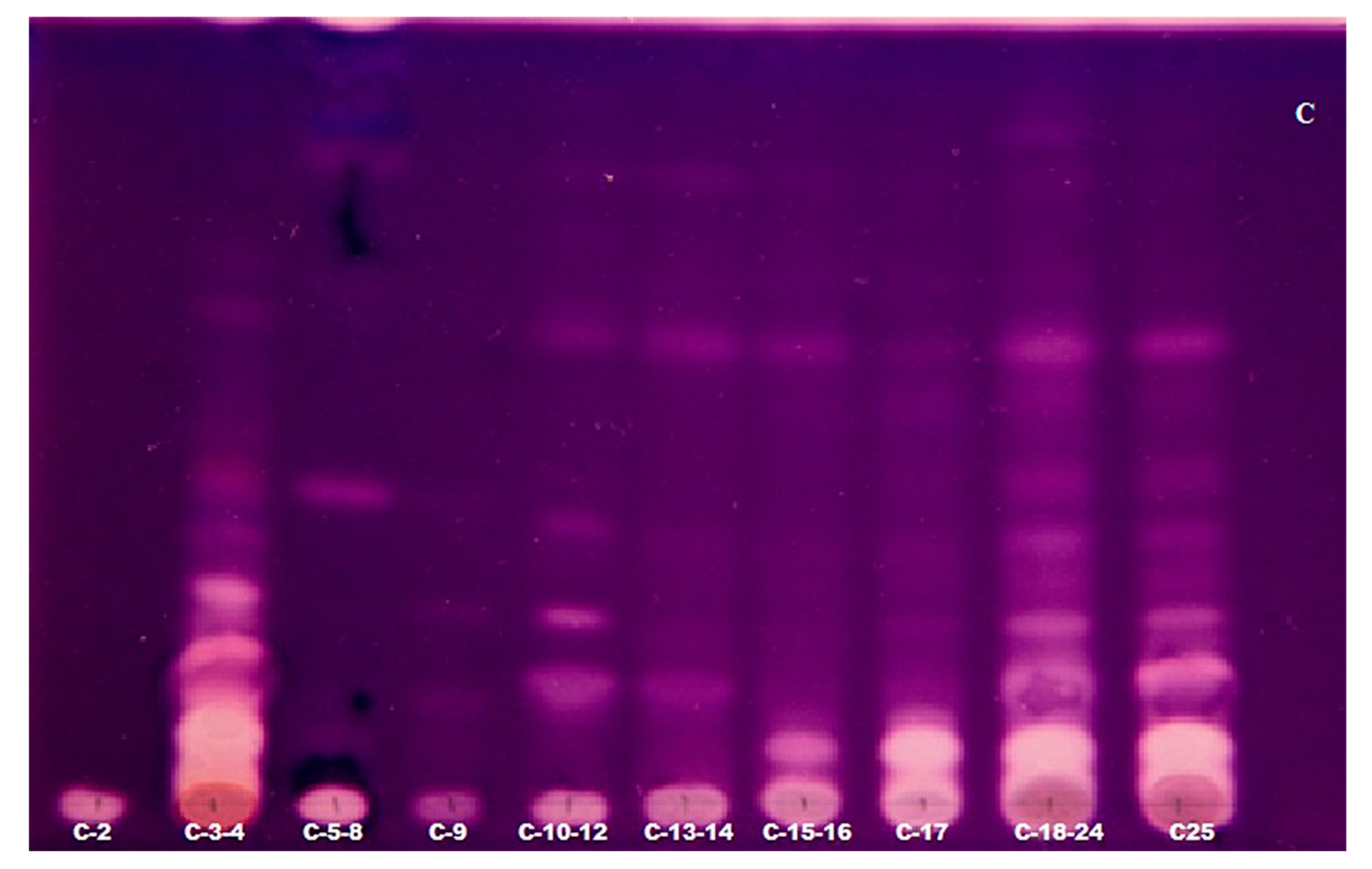
2.3. TLC-Bioautography for the Detection of Potent AChE Inhibitors
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation and Alkaloid Extraction
3.3. Method Optimization of VLC
3.4. LC-MS Identification of the Isolated Compounds
3.5. TLC with Bioautography of Anticholinesterase Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; ShI, X.; Zang, J.; Zang, X.; Martin, R.C.G. Hepatic protection and anticancer activity of curcuma: A potential chemopreventive strategy against hepatocellular carcinoma. Int. J. Oncol. 2014, 44, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.D.; Tamilselvan, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Grabarska, A.; Łuszczki, J.; Czernicka, L.; Nowosadzka, E.; Gumbarewicz, E.; Jarząb, A.; Audo, G.; Upadhyay, S.; Głowniak, K.; et al. Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoid sseparated by centrifugal partition chromatography. Phytother. Res. 2018, 5, 933–942. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Widelska, G.; Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Olech, M.; Nowak, R.; Wojtunik-Kulesza, K.; Jozwiak, G.; Waksmundzka-Hajnos, M. Influence of Production Parameters on the Content of Polyphnolic Compounds in Extruded Porridge Enriched with Chokeberry Fruit (Aronia melanocarpa (Michx.) Elliott). Open Chem. 2019, 17, 166–167. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef]
- Chaves, S.K.M.; Feitosa, C.M.; da S. Araujo, L. Alkaloids Pharmacological Activities Prospects for the Development of Phytopharmaceuticals for Neurodegenerative Diseases. Curr. Pharm. Biotechnol. 2016, 17, 629–695. [Google Scholar] [CrossRef]
- Berkov, S.; Martinez-Frances, V.; Bastida, J.; Codina, C.; Rios, S. Evolution of alkaloid biosynthesis in the genus Narcissus. Phytochemistry 2014, 99, 95–106. [Google Scholar] [CrossRef]
- Lopez, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Vergura, S.; Santoro, E.; Masi, M.; Evidente, A.; Scafato, P.; Superchi, S.; Mazzeo, G.; Longhi, G.; Abbate, S. Absolute configuration assignment of anticancer Amaryllidaceae alkaloid jonquailine. Fitoterapia 2018, 129, 78–84. [Google Scholar] [CrossRef]
- Zabłocka, A. Choroba Alzheimera jako przykład schorzenia neurodegeneracyjnego. Postepy Hig. Med. Dosw. 2006, 60, 209–216. [Google Scholar]
- Ghisalberti, E. Detection and Isolation of Bioactive Natural Products. In Bioactive Natural Products: Detection, Isolation, and Structural Determination, 2nd ed.; Colegate, S.M., Molyneux, R.J., Eds.; Taylor & Francis: Philadelphia, PA, USA, 2008; pp. 11–76. [Google Scholar]
- Maurya, A.; Kalani, K.; Verma, S.C.; Singh, R.; Srivastava, A. Vacuum Liquid Chromatography: Simple, Efficient and Versatile Separation Technique for Natural Products. Org. Med. Chem. IJ. 2018, 7, 1–3. [Google Scholar] [CrossRef]
- Targett, N.M.; Kilcoyne, J.P.; Green, B. Vacuum Liquid Chromatography: An Alternative to Common Chromatographic Methods. J. Org. Chem. 1979, 44, 4962–4964. [Google Scholar] [CrossRef]
- Upadhyay, H.C.; Saini, D.C.; Srivastava, S.K. Phytochemical Analysis of Ammannia multiflora. Res. J. Phytochem. 2011, 5, 170–176. [Google Scholar] [CrossRef]
- Bucar, F.; Wube, A.; Schmid, M. Natural products isolation–how to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Noviany, N.; Hadi, S. The Isolation of α-viniferin, a Timer Stilbene, from Shorea ovalis Blume. Adv. Nat. Appl. Sci. 2009, 3, 107–112. [Google Scholar] [CrossRef]
- Zarate, R.; Sukrasno; Yeoman, M.M. Application of two rapid techniques of column chromatography to separate the pungent principles of ginger, Zingiber officinale Roscoe. J. Chromatogr. A 1992, 609, 407–413. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of diterpenoid alkaloid mixtures using vacuum liquid chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- Pigni, N.B.; Berkov, S.; Elamrani, A.; Benaissa, M.; Viladomat, F.; Codina, C.; Bastida, J. Two New Alkaloids from Narcissus serotinus L. Molecules 2010, 15, 7083–7089. [Google Scholar] [CrossRef]
- Tallini, L.R.; Torras-Claveria, L.; de Souza Borges, W.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. N-oxide alkaloids from Crinum amabile (Amaryllidaceae). Molecules 2018, 23, 1277. [Google Scholar] [CrossRef]
- Mroczek, T.; Mazurek, J. Pressurized liquid extraction and anticholinesterase activity-based this-layer-chromatography with bioautography of Amaryllidaceae alklaloids. Anal. Chim. Acta 2009, 633, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T. Qualitative and quantitative two-dimensional thin-layerchromatography/high performance liquid chromatography/diode-array/electrospray-ionization-time-of-flight mass spectrometry of cholinesterase inhibitors. J. Pharm. Biomed. Anal. 2016, 129, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T. Highly Efficient, Selective and Sensitive Molecular Screening of Acetylcholinesterase Inhibitors of Natural Origin by Solid-Phase Extraction-Liquid Chromatography/Electrospray Ionisation-Octopole-Orthogonal Acceleration Time-of-Flight-Mass Spectrometry and Novel Thin-Layer Chromatography-Based. Bioautography J. Chromatogr. A 2009, 1216, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Marston, A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A 2011, 1218, 2676–2683. [Google Scholar] [CrossRef]
- Guillou, C.; Beunard, J.L.; Gras, E.; Thal, C. An Efficient Total Synthesis of (±) galanthamine, Angew. Chem. Int. Ed. 2001, 40, 4745–4746. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop–the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Russo, P.; Frustaci, A.; Fini, M.; Cesario, A. From Traditional European Medicine to Discovery of New Drug Candidates for the Treatment of Dementia and Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2013, 20, 976–983. [Google Scholar] [CrossRef]
- Coyle, J.; Kershaw, P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: Effect on the course of Alzheimer’s disease. Biol. Psychiat. 2001, 49, 289–299. [Google Scholar] [CrossRef]
- Sticher, O. Natural products isolation. Nat. Prod. Rep. 2008, 7, 517–554. [Google Scholar] [CrossRef]
- Bores, G.M.; Huger, F.P.; Petko, W.; Mutlib, A.E.; Camacho, F.; Rush, D.K.; Selk, D.E.; Wolf, V.; Kosley, R.W.; Davis, L.; et al. Pharmacological evaluation of novel Alzheimer’s disease therapeutics: Acetylcholinesterase inhibitors related to galanthamine. J. Pharmacol. Exp. Ther. 1996, 277, 728–738. [Google Scholar]


| The Number of Experiments | 1-A | 2-B | 3-C |
|---|---|---|---|
| Type of column | glass | polypropylene cartridge | polypropylene cartridge |
| Sorbent filling ratio (Al2O3(150 MeSh): silica gel (60 F254)) | 1:1 Al2O3(25 g): silica gel (57 g) | 1:3 Al2O3(17 g): silica gel (25 g) | 3:1 Al2O3(57 g): silica gel (8 g) |
| Fractions obtained | A-19 A-20-22 A-23-24 A-25 A-26 A-27-33 A-34 | B-3 B-4-7 B-8-9 B-10 B-11 B-12 B-13-14 B-15-17 B-18-20 B-21 B-22-25 B-26 B-27-29 B-30 B-31 | C-2 C-3-4 C-5-8 C-9 C-10-12 C-13-14 C-15-16 C-17 |
| % of Isolated Compounds to the Total Amount of All Alkaloid Compounds Obtained from Individual Fractions [%] | Fraction Number | ||||||
|---|---|---|---|---|---|---|---|
| A-19 | A-20-22 | A-23-24 | A-25 | A-26 | A-27-33 | A-34 | |
| Sanguinine | 1.2 | ||||||
| Lycoramine | 54.3 | 9.9 | 27.5 | 16.4 | 5.6 | ||
| Lycorine | 7.2 | 52.9 | 41.2 | 23.6 | 17.8 | 10.0 | 10.0 |
| Ungeremine | 9.9 | 16.0 | 16.8 | 18.6 | 22.6 | 14.6 | |
| 4,N-didehydro-nor-augustamine | 17.7 | 15.5 | 7.2 | 15.9 | 18.1 | 11.9 | |
| Tetrahydro-nor-augustamine | 4.7 | 10.7 | 8.8 | 12.5 | 21.4 | 16.6 | |
| Mesembrinole | 6.2 | 7.1 | 24.6 | ||||
| Galanthamine | 1.4 | 3.3 | |||||
| % of Isolated Compounds to the Total Amount of All Alkaloid Compounds Obtained from Individual Fractions [%] | Fraction Number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-10 | B-11 | B-12 | B-13-14 | B-15-17 | B-18-20 | B-21 | B-22-25 | B-26 | B-27-29 | B-30 | B-31 | |
| Sanguinine | 3.4 | 9.2 | 9.1 | 5.8 | 7.5 | 1.4 | 2.9 | |||||
| Lycoramine | 39.0 | 38.1 | 36.9 | |||||||||
| Lycorine | 11.6 | 26.6 | 29.2 | 22.6 | 17.1 | 21.2 | 22.2 | 22.8 | 26.1 | 9.1 | ||
| Ungeremine | 7.7 | 4.5 | 5.8 | 9.2 | 7.6 | 7.2 | 6.3 | 11.5 | 9.2 | 24.9 | 26.1 | |
| 4,N-didehydro-nor-augustamine | 10.8 | 4.7 | 6.6 | 6.0 | 3.6 | 5.9 | 4.0 | 8.1 | ||||
| Tetrahydro-nor-augustamine | 5.8 | 3.4 | 4.3 | 4.7 | 0.8 | 0.8 | ||||||
| Mesembrinole | 27.4 | 15.6 | 12.1 | 9.0 | 4.6 | 9.7 | ||||||
| Haemanthamine | 3.5 | |||||||||||
| Lycorine-N-oxide | 0.7 | |||||||||||
| Galanthamine-N-oxide | 2.1 | 1.6 | ||||||||||
| Galanthamine | 1.9 | 1.7 | ||||||||||
| Tazettine | 3.4 | 8.3 | 9.9 | 5.7 | ||||||||
| % of Isolated Compounds to the Total Amount of All Alkaloid Compounds Obtained from Individual Fractions [%] | Fraction Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C-2 | C-3-4 | C-5-8 | C-9 | C-10-12 | C-13-14 | C-15-16 | C-17 | C-18-24 | C-25 | |
| Sanguinine | 1.7 | 8.7 | ||||||||
| Lycoramine | 7.5 | 26.5 | 53.5 | |||||||
| Lycorine | 23.8 | 23.3 | 2.4 | 20.2 | 25.4 | 22.1 | 15.6 | 30.1 | 7.8 | |
| Ungeremine | 4.2 | 8.2 | 6.7 | 4.1 | 4.1 | 10.3 | 11.2 | 7.8 | 2.0 | 10.0 |
| 4,N-didehydro-nor-augustamine | 4.2 | 9.7 | 12.9 | 4.3 | ||||||
| Tetrahydro-nor-augustamine | 5.5 | 2.2 | 3.3 | 2.8 | 1.3 | 9.3 | 3.7 | |||
| Mesembrinole | 6.7 | 13.4 | 36.2 | 10.3 | 4.3 | |||||
| Galanthamine | 5.9 | |||||||||
| Lycoramine-N-oxide | 2.1 | 2.4 | ||||||||
| Haemanthamine | 12.2 | 6.9 | 3.4 | 2.6 | ||||||
| Lycorine-N-oxide | 2.8 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczek, T.; Dymek, A.; Widelski, J.; Wojtanowski, K.K. The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites 2020, 10, 395. https://doi.org/10.3390/metabo10100395
Mroczek T, Dymek A, Widelski J, Wojtanowski KK. The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites. 2020; 10(10):395. https://doi.org/10.3390/metabo10100395
Chicago/Turabian StyleMroczek, Tomasz, Aleksandra Dymek, Jarosław Widelski, and Krzysztof Kamil Wojtanowski. 2020. "The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography" Metabolites 10, no. 10: 395. https://doi.org/10.3390/metabo10100395
APA StyleMroczek, T., Dymek, A., Widelski, J., & Wojtanowski, K. K. (2020). The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites, 10(10), 395. https://doi.org/10.3390/metabo10100395







