Towards Best Practice in Hair Metabolomic Studies: Systematic Investigation on the Impact of Hair Length and Color
Abstract
:1. Introduction
2. Results and Discussion
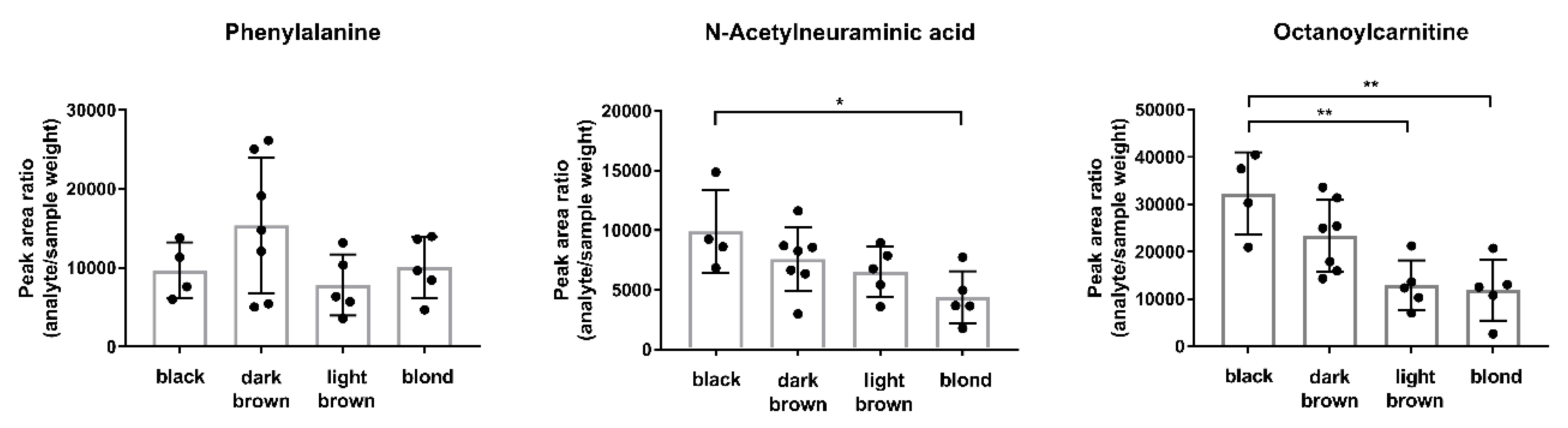
2.1. Hair Color Dependencies of Endogenous Compounds: Interindividual Differences
2.2. Segmental Hair Analysis of Cosmetically Untreated Hair Samples: Intra-Individual Differences
2.3. General Recommendations for Best Practice in Hair Metabolomics Studies and Future Perspectives
2.4. Limitations of the Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Hair Samples
3.3. Hair Sample Preparation
3.4. Liquid Chromatography High-Resolution Mass Spectrometry (HPLC-HRMS)
3.5. Data Processing and Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Benton, H.P.; Casazza, K.; Cooper, S.J.; Cui, X.; Du, X.; Engler, J.; Kabarowski, J.H.; Li, S.; Pathmasiri, W.; et al. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 2016, 51, 461–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delplancke, T.D.J.; Wu, Y.; Han, T.L.; Joncer, L.R.; Qi, H.; Tong, C.; Baker, P.N. Metabolomics of pregnancy complications: Emerging application of maternal hair. BioMed Res. Int. 2018, 2018, 2815439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, W.J.; Choi, J.Y.; Park, B.; Seo, J.H.; Seo, Y.H.; Lee, S.; Jeong, C.H.; Lee, S. Hair metabolomics in animal studies and clinical settings. Molecules 2019, 24, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulek, K.; Han, T.L.; Villas-Boas, S.G.; Wishart, D.S.; Soh, S.E.; Kwek, K.; Gluckman, P.D.; Chong, Y.S.; Kenny, L.C.; Baker, P.N. Hair metabolomics: Identification of fetal compromise provides proof of concept for biomarker discovery. Theranostics 2014, 4, 953–959. [Google Scholar] [CrossRef]
- Eisenbeiss, L.; Binz, T.M.; Baumgartner, M.R.; Kraemer, T.; Steuer, A.E. Cheating on forensic hair testing? Detection of potential biomarkers for cosmetically altered hair samples using untargeted hair metabolomics. Analyst 2020. [Google Scholar] [CrossRef] [PubMed]
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49. [Google Scholar] [CrossRef]
- Miolo, G.; Stocchero, G.; Vogliardi, S.; Menilli, L.; Scrivano, S.; Montisci, M.; Favretto, D. A study on photostability of ethyl glucuronide in hair irradiated under artificial sunlight. J. Anal. Toxicol. 2020, 44, 58–64. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Dudzik, D.; Barbas-Bernardos, C.; Garcia, A.; Barbas, C. Quality assurance procedures for mass spectrometry untargeted metabolomics. a review. J. Pharm. Biomed. Anal. 2018, 147, 149–173. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbeiss, L.; Steuer, A.E.; Binz, T.M.; Baumgartner, M.R.; Kraemer, T. (Un)targeted hair metabolomics: First considerations and systematic evaluation on the impact of sample preparation. Anal. Bioanal. Chem. 2019, 411, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of eumelanin and pheomelanin and its pathophysiological implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noppe, G.; de Rijke, Y.B.; Dorst, K.; van den Akker, E.L.; van Rossum, E.F. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin. Endocrinol. (Oxf.) 2015, 83, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.F.; Baby, A.R.; Velasco, M.V. Effects of solar radiation on hair and photoprotection. J. Photochem. Photobiol. B 2015, 153, 240–246. [Google Scholar] [CrossRef]
- Grass, J.; Miller, R.; Carlitz, E.H.; Patrovsky, F.; Gao, W.; Kirschbaum, C.; Stalder, T. In vitro influence of light radiation on hair steroid concentrations. Psychoneuroendocrinology 2016, 73, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Delplancke, T.D.J.; de Seymour, J.V.; Tong, C.; Sulek, K.; Xia, Y.; Zhang, H.; Han, T.L.; Baker, P.N. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Sci. Rep. 2018, 8, 36. [Google Scholar] [CrossRef]
- Joo, K.M.; Kim, A.R.; Kim, S.N.; Kim, B.M.; Lee, H.K.; Bae, S.; Lee, J.H.; Lim, K.M. Metabolomic analysis of amino acids and lipids in human hair altered by dyeing, perming and bleaching. Exp. Dermatol. 2016, 25, 729–731. [Google Scholar] [CrossRef] [Green Version]
- Eisenbeiss, L.; Binz, T.M.; Baumgartner, M.R.; Steuer, A.E.; Kraemer, T. A possible new oxidation marker for hair adulteration: Detection of PTeCA (1H-pyrrole-2,3,4,5-tetracarboxylic acid) in bleached hair. Drug Test. Anal. 2020, 12, 230–238. [Google Scholar] [CrossRef]
- Salomone, A.; Tsanaclis, L.; Agius, R.; Kintz, P.; Baumgartner, M.R. European guidelines for workplace drug and alcohol testing in hair. Drug Test. Anal. 2016, 8, 996–1004. [Google Scholar] [CrossRef]
- Cooper, G.A.; Kronstrand, R.; Kintz, P.; Society of Hair Testing. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci. Int. 2012, 218, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Boxler, M.I.; Schneider, T.D.; Kraemer, T.; Steuer, A.E. Analytical considerations for (un)-targeted metabolomic studies with special focus on forensic applications. Drug Test. Anal. 2019, 11, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Steuer, A.E.; Arnold, K.; Schneider, T.D.; Poetzsch, M.; Kraemer, T. A new metabolomics-based strategy for identification of endogenous markers of urine adulteration attempts exemplified for potassium nitrite. Anal. Bioanal. Chem. 2017, 409, 6235–6244. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Compound Class | Formula | pKa | Analytics | Differences between Hair Colors | Differences between Hair Segments | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | B vs. C | A vs. C | A vs. D | B vs. D | C vs. D | ||||||
| Choline | 1,2-Aminoalcohols | C5H14NO | 13.97 | HSST ESI + | black to blond: ↓ * | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ ** | ↓ ** |
| Cysteic acid | Amino acids and derivatives | C3H7NO5S | −1.7 | HILIC ESI − | black to blond: ↓ ** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| black to light brown: ↓ * | |||||||||||
| Urocanic acid | Amino acids and derivatives | C6H6N2O2 | 3.85 | HILIC ESI + | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ *** | n.s. | n.s. |
| Citrulline | Amino acids and derivatives | C6H13N3O3 | 2.27 | HILIC ESI − | n.s. | ↓ **** | ↓ *** | ↓ **** | ↓ **** | ↓ *** | ↓ ** |
| Trimethyllysine | Amino acids and derivatives | C9H20N2O2 | 2.41 | HSST ESI + | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ * | ↓ ** |
| Pyroglutamic acid | Amino acids and derivatives | C5H7NO3 | 3.61 | HILIC ESI − | n.s. | ↓ **** | ↓ ** | ↓ **** | ↓ **** | ↓ *** | ↓ * |
| Cysteinesulfate | Amino acids and derivatives | C3H7NO5S2 | −1.9 | HILIC ESI − | n.s. | ↑ **** | ↑ *** | ↑ **** | ↑ **** | ↑ *** | n.s. |
| Ornithine | Amino acids and derivatives | C5H12N2O2 | 2.67 | HILIC ESI + | n.s. | ↓ **** | n.s. | ↓ * | ↓ * | n.s. | n.s. |
| Indole-3-lactic acid | Amino acids and derivatives | C11H11NO3 | 4.14 | HSST ESI + | n.s. | ↓ **** | ↓ ** | ↓ *** | ↓ *** | ↓ ** | ↓ *** |
| Taurine | Amino acids and derivatives | C2H7NO3S | −1.5 | HILIC ESI - | n.s. | ↓ *** | ↓ *** | ↓ **** | ↓ **** | ↓ ** | n.s. |
| Creatinine | Amino acids and derivatives | C4H7N3O | 9.21 | HSST ESI + | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ * | ↓ * |
| Serine | Amino acids and derivatives | C3H7NO3 | 2.03 | HILIC ESI − | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ * | n.s. |
| Arginine | Amino acids and derivatives | C6H14N4O2 | 2.41 | HSST ESI + | n.s. | ↓ **** | n.s. | ↓ **** | ↓ **** | n.s. | ↓ * |
| Lysine | Amino acids and derivatives | C6H14N2O2 | 2.74 | HILIC ESI + | n.s. | ↓ **** | n.s. | ↓ **** | ↓ **** | n.s. | n.s. |
| Phenylalanine | Amino acids and derivatives | C9H11NO2 | 2.47 | HSST ESI + | n.s. | ↓ **** | n.s. | ↓ **** | ↓ **** | n.s. | n.s. |
| Tyrosine | Amino acids and derivatives | C9H11NO3 | 2.0 | HILIC ESI + | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | n.s. | n.s. |
| Tryptophan | Amino acids and derivatives | C11H12N2O2 | 2.54 | HSST ESI + | n.s. | ↓ **** | n.s. | ↓ **** | ↓ **** | n.s. | ↓ * |
| Glutamic acid | Amino acids and derivatives | C5H9NO4 | 1.88 | HSST ESI + | n.s. | ↓ **** | ↓ ** | ↓ **** | ↓ **** | ↓ ** | ↓ ** |
| Aspartic acid | Amino acids and derivatives | C4H7NO4 | 1.7 | HSST ESI - | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ * | n.s. |
| Histidine | Amino acids and derivatives | C6H9N3O2 | 1.85 | HSST ESI + | n.s. | ↓ * | n.s. | ↓ * | ↓ *** | n.s. | n.s. |
| N-Acetylneuraminic acid | Carbohydrates and carbohydrate conjugates | C11H19NO9 | 3.0 | HILIC ESI + | black to blond: ↓ * | ↓ ** | ↓ * | ↓ **** | ↓ **** | ↓ ** | ↓ ** |
| Carnitine (C0) | Carnitines | C7H15NO3 | 4.2 | HSST ESI + | n.s. | ↓ **** | ↓ **** | ↓ **** | ↓ **** | ↓ **** | ↓ ** |
| Acetylcarnitine (C2) | Carnitines | C9H17NO4 | 4.09 | HILIC ESI + | n.s. | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ ** | ↓ * |
| Butyrylcarnitine (C4) | Carnitines | C11H21NO4 | 4.27 | HILIC ESI + | n.s. | ↓ **** | n.s. | ↓ **** | ↓ **** | ↓ * | n.s. |
| Hexanoylcarnitine (C6) | Carnitines | C13H25NO4 | 4.22 | HSST ESI + | black to blond: ↓ * | ↓ **** | ↓ * | ↓ **** | ↓ **** | ↓ * | n.s. |
| black to light brown: ↓ * | |||||||||||
| Octanoylcarnitine (C8) | Carnitines | C15H29NO4 | 4.22 | HSST ESI + | black to blond: ↓ ** | ↓ *** | ↓ ** | ↓ **** | ↓ **** | ↓ *** | n.s. |
| black to light brown: ↓ ** | |||||||||||
| Decanoylcarnitine (C10) | Carnitines | C17H33NO4 | 4.22 | HSST ESI + | black to light brown: ↓ * | ↓ **** | ↓ *** | ↓ **** | ↓ **** | ↓ **** | ↓ ** |
| Riboflavin | Cofactors | C17H20N4O6 | 5.97 | HSST ESI + | black to blond: ↓ ** | ↓ *** | n.s. | ↓ *** | ↓ *** | ↓ * | ↓ * |
| Nicotinamide | Cofactors | C6H6N2O | 13.39 | HSST ESI + | n.s. | ↓ ** | n.s. | ↓ ** | ↓ **** | n.s. | n.s. |
| Inosine | Nucleosides and nucleotides | C10H12N4O5 | 6.94 | HILIC ESI − | n.s. | ↓ ** | n.s. | ↓ *** | ↓ *** | ↓ ** | ↓ ** |
| Adenosine | Nucleosides and nucleotides | C10H13N5O4 | 12.45 | HSST ESI + | n.s. | ↓ ** | n.s. | ↓ **** | ↓ **** | n.s. | n.s. |
| Adenine | Nucleosides and nucleotides | C5H5N5 | 10.29 | HSST ESI + | n.s. | n.s. | ↑ * | n.s. | ↑ *** | ↑ *** | ↑ * |
| Theobromine | Purines and derivatives | C7H8N4O2 | 9.28 | HSST ESI + | n.s. | ↓ **** | ↓ ** | ↓ **** | ↓ **** | ↓ ** | ↓ *** |
| Theophylline | Purines and derivatives | C7H8N4O2 | 7.82 | HILIC ESI + | black to blond: ↓ * | ↓ *** | ↑ ** | ↓ *** | ↓ **** | ↓ ** | ↓ * |
| black to light brown: ↓ * | |||||||||||
| black to dark brown: ↓ * | |||||||||||
| Hypoxanthine | Purines and derivatives | C5H4N4O | 8.72 | HSST ESI + | n.s. | ↓ *** | ↓ ** | ↓ **** | ↓ **** | ↓ * | ↓ * |
| Uric acid | Purines and derivatives | C5H4N4O3 | 7.25 | HILIC ESI − | n.s. | ↓ ** | n.s. | ↓ *** | ↓ **** | ↓ ** | ↓ ** |
| Subject Number | Hair Color | Sampling Month | Hair Length (cm) | Number of Analyzed Segments |
|---|---|---|---|---|
| 1 | dark blond | 05/2019 | 15 | 5 |
| 11/2019 | 15 | 4 | ||
| 2 | dark blond | 05/2019 | 16 | 5 |
| 11/2019 | 16 | 4 | ||
| 3 | dark blond | 05/2019 | 14 | 5 |
| 11/2019 | 18 | 4 | ||
| 4 | dark brown | 11/2018 | 35 | 4 |
| 05/2019 | 32 | 4 | ||
| 5 | dark brown | 11/2018 | 37 | 4 |
| 05/2019 | 12 | 3 | ||
| 6 | dark brown | 11/2018 | 24 | 5 |
| 05/2019 | 33 | 5 | ||
| 7 | dark brown | 11/2018 | 12 | 4 |
| 05/2019 | 8 | 2 | ||
| 8 | dark brown | 10/2018 | 16 | 5 |
| 04/2019 | 14 | 3 | ||
| 9 | medium brown | 05/2019 | 23 | 6 |
| 11/2019 | 25 | 6 | ||
| 10 | medium brown | 10/2018 | 28 | 4 |
| 04/2019 | 25 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenbeiss, L.; Binz, T.M.; Baumgartner, M.R.; Kraemer, T.; Steuer, A.E. Towards Best Practice in Hair Metabolomic Studies: Systematic Investigation on the Impact of Hair Length and Color. Metabolites 2020, 10, 381. https://doi.org/10.3390/metabo10100381
Eisenbeiss L, Binz TM, Baumgartner MR, Kraemer T, Steuer AE. Towards Best Practice in Hair Metabolomic Studies: Systematic Investigation on the Impact of Hair Length and Color. Metabolites. 2020; 10(10):381. https://doi.org/10.3390/metabo10100381
Chicago/Turabian StyleEisenbeiss, Lisa, Tina M. Binz, Markus R. Baumgartner, Thomas Kraemer, and Andrea E. Steuer. 2020. "Towards Best Practice in Hair Metabolomic Studies: Systematic Investigation on the Impact of Hair Length and Color" Metabolites 10, no. 10: 381. https://doi.org/10.3390/metabo10100381







