Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus
Abstract
1. Introduction
2. Results
2.1. Glutathione Injection Test
2.2. Effect of Glutathione on Thermal Stress
2.2.1. Results of Serum Parameters
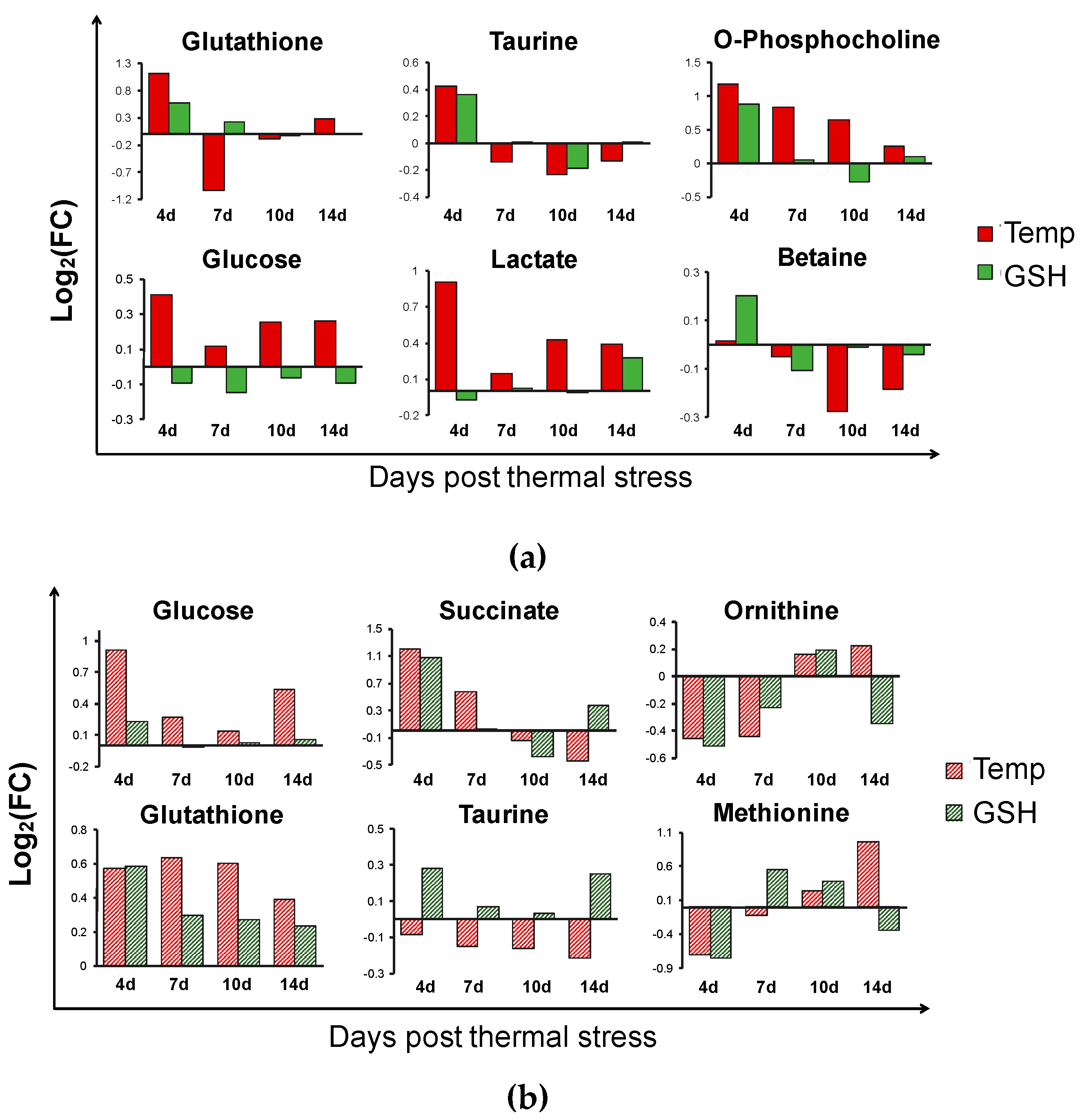
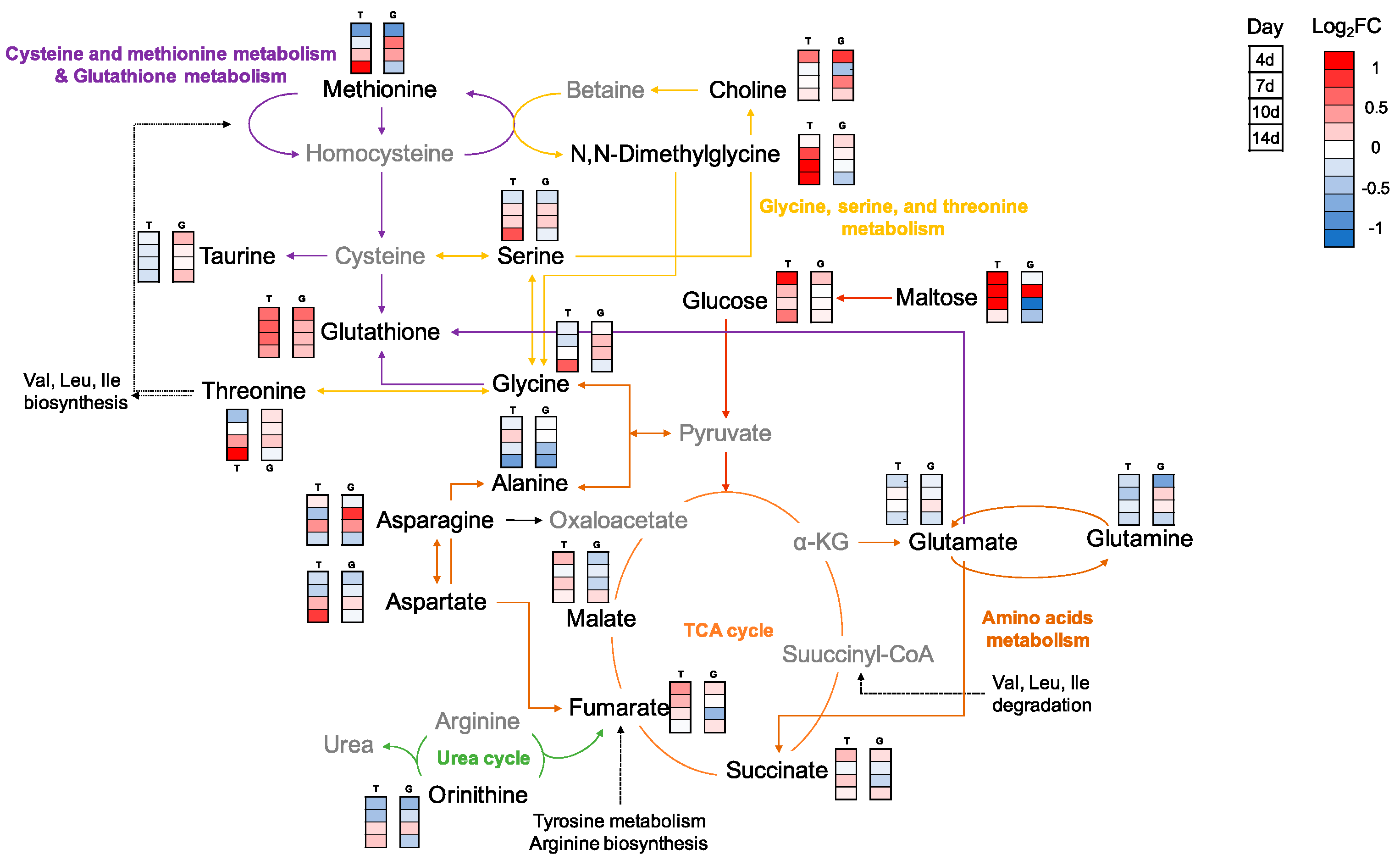
2.2.2. Metabolic Changes in Kidney and Liver under Thermal Stress
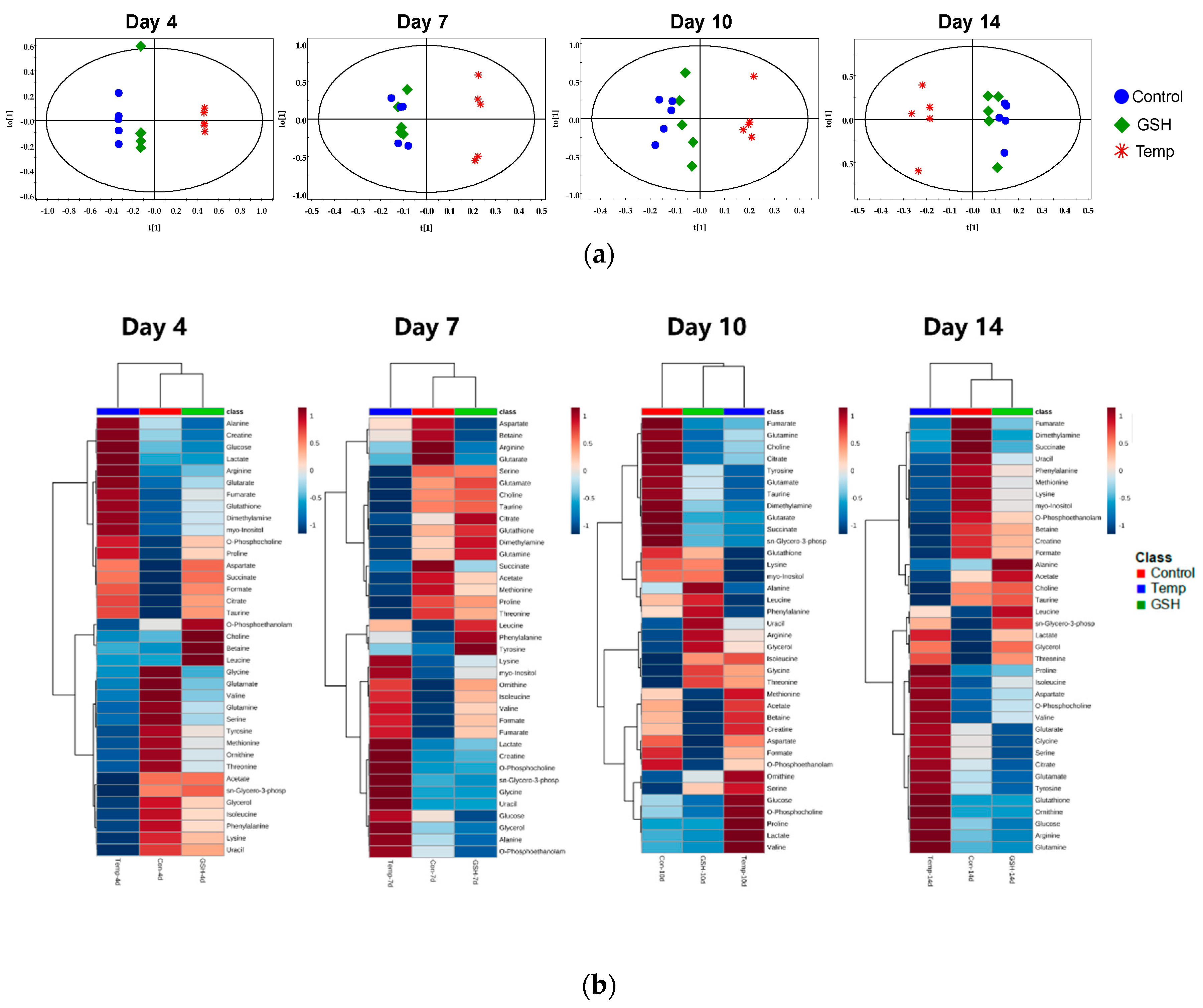
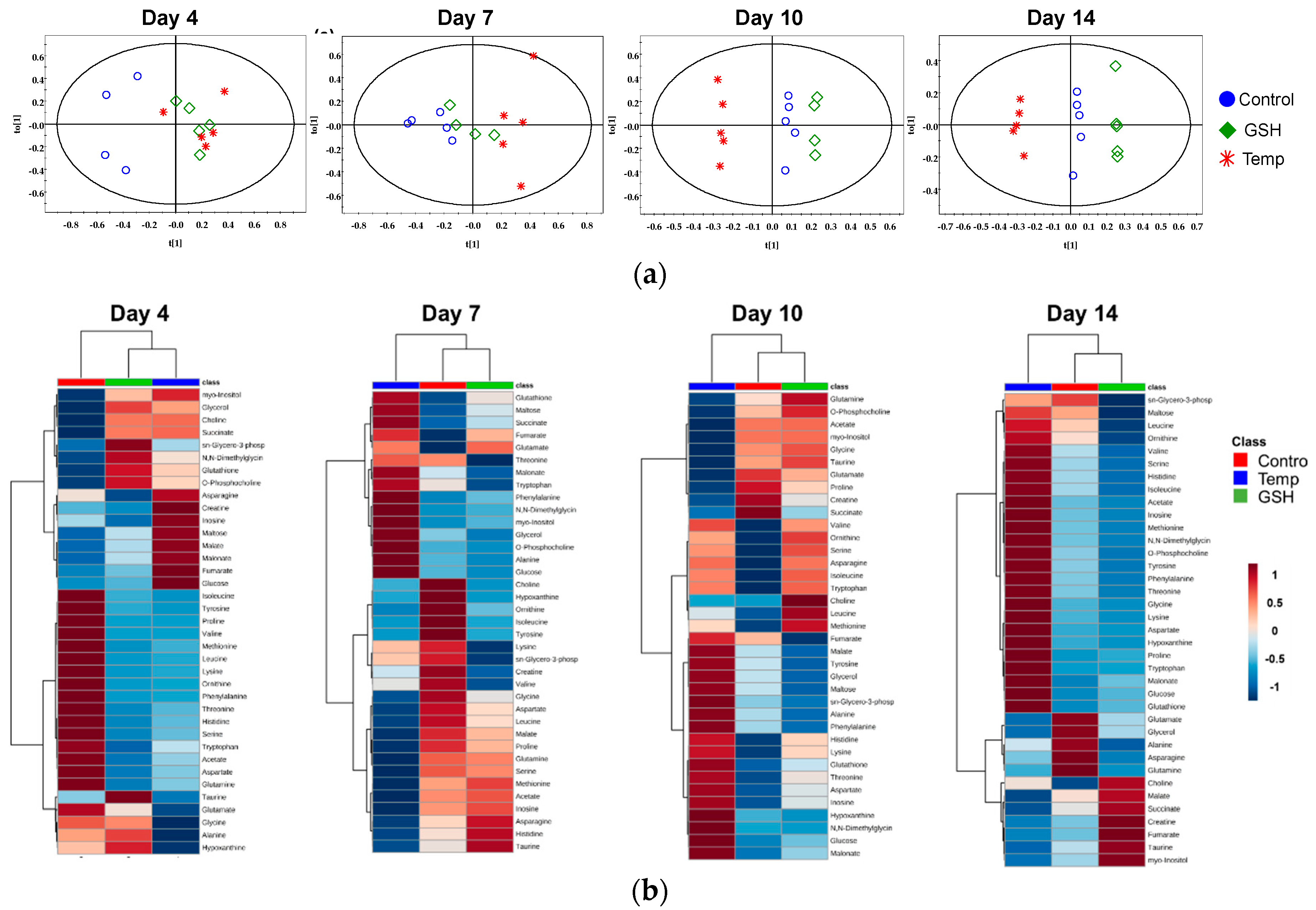
2.2.3. Results of Multivariate Data Analysis
3. Discussion
4. Materials and Methods
4.1. Glutathione Injection Test
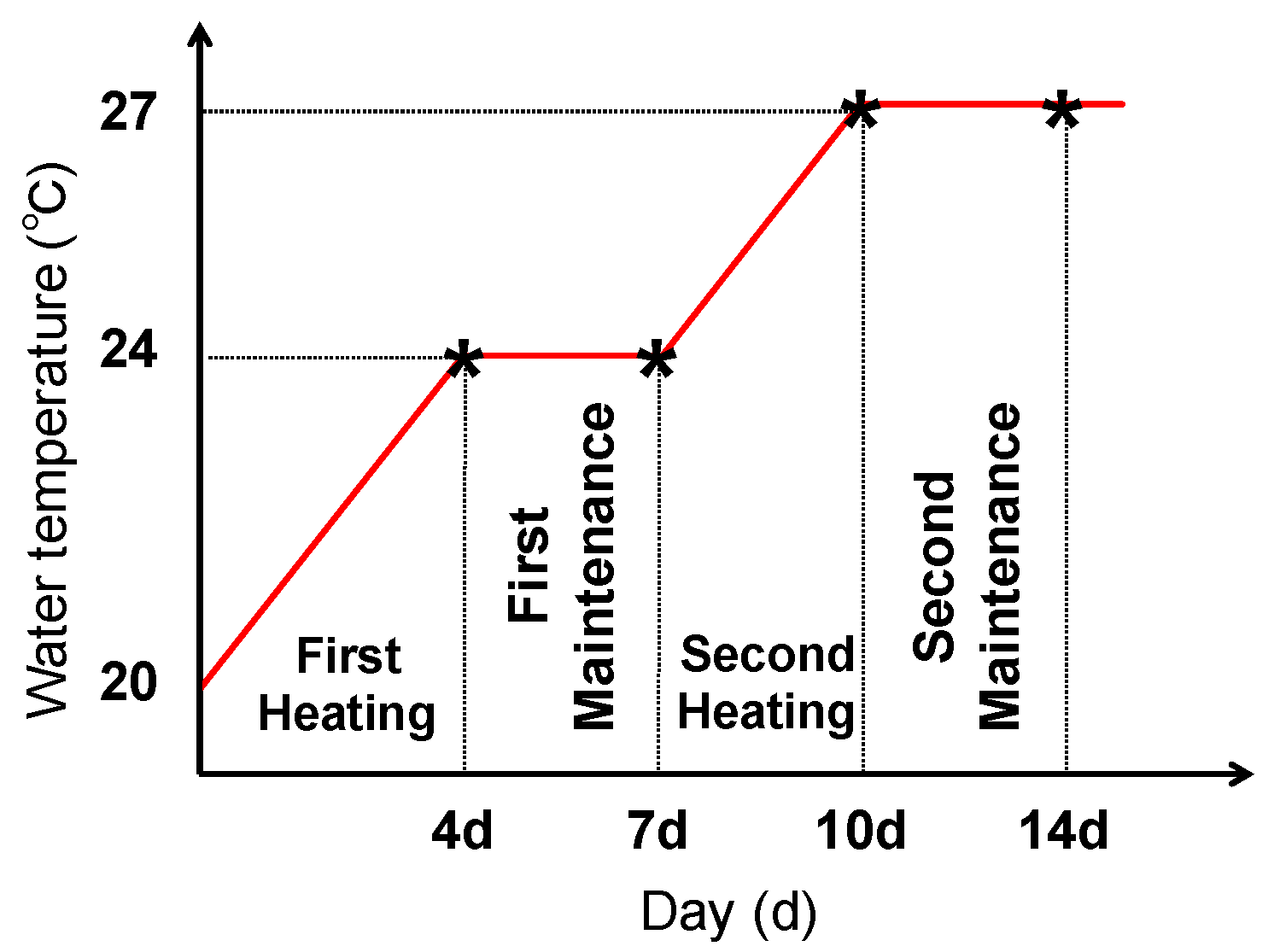
4.2. Thermal Stress Exposure
4.3. Analysis of Biochemical Parameters in Serum
4.4. 1H NMR Measurement
4.5. Statistical Analysis of 1H NMR Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Pankhurst, N.W.; Pankhurst, P.M. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Phys. A 2013, 164, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.A.; Buckley, B.A. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 2015, 218, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, V.; Bennett, W.A. Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish Physiol. Biochem. 2011, 37, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Madeira, D.; Mendonca, V.; Dias, M.; Roma, J.; Diniz, M.S. Effect of increasing temperature in the differential activity of oxidative stress biomarkers in various tissues of the Rock goby, Gobius paganellus. Mar. Environ. Res. 2014, 97, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Hu, W.T.; Guo, W.L.; Meng, A.Y.; Sun, Y.; Wang, S.F.; Xie, Z.Y.; Zhou, Y.C.; He, C.Z. A metabolomic investigation into the effects of temperature on Streptococcus agalactiae from Nile tilapia (Oreochromis niloticus) based on UPLC-MS/MS. Vet. Microbiol. 2017, 210, 174–182. [Google Scholar] [CrossRef]
- Oyarzun, R.; Munoz, J.L.P.; Pontigo, J.P.; Morera, F.J.; Vargas-Chacoff, L. Effects of acclimation to high environmental temperatures on intermediary metabolism and osmoregulation in the sub-Antarctic notothenioid Eleginops maclovinus. Mar. Biol. 2018, 165. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp. Biochem. Phys. C 2006, 143, 30–35. [Google Scholar] [CrossRef]
- Almroth, B.C.; Sturve, J.; Berglund, A.; Forlin, L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 2005, 73, 171–180. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.H.; Hong, S.H.; Kim, G.Y.; Cha, H.J.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.N.; Chae, W.S.; Park, E.K.; Yoo, C.Y.; Son, S.E.; Kim, S.; Lee, H.J. Effects of reduced glutathione on stress and inflammatory response in Korean native calves vaccinated with foot-and-mouth disease vaccine. J. Prev. Vet. Med. 2018, 42, 37–40. [Google Scholar] [CrossRef]
- Sukhovskaya, I.V.; Borvinskaya, E.V.; Smirnov, L.P.; Kochneva, A.A. Role of Glutathione in Functioning of the System of Antioxidant Protection in Fish (Review). Inland Water Biol. 2017, 10, 97–102. [Google Scholar] [CrossRef]
- Leggatt, R.A.; Brauner, C.J.; Schulte, P.M.; Iwama, G.K. Effects of acclimation and incubation temperature on the glutathione antioxidant system in killifish and RTH-149 cells. Comp. Biochem. Phys. A 2007, 146, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Leggatt, R.A.; Iwama, G.K. Exogenous glutathione can increase glutathione levels in tissues of rainbow trout (Oncorhynchus mykiss) through extracellular breakdown and intracellular synthesis. Comp. Biochem. Phys. C 2009, 150, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Otto, D.M.E.; Sen, C.K.; Hidiroglou, N.; Madere, R.; Moon, T.W. Role of exogenous glutathione in teleost fish and its effects on antioxidant defense responses in rainbow trout exposed to 3, 3’, 4, 4’-tetrachlorobiphenyl. Fish Physiol. Biochem. 1997, 16, 449–457. [Google Scholar] [CrossRef]
- Kullgren, A.; Jutfelt, F.; Fontanillas, R.; Sundell, K.; Samuelsson, L.; Wiklander, K.; Kling, P.; Koppe, W.; Larsson, D.G.J.; Bjornsson, B.T.; et al. The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic salmon (Salmo salar). Comp. Biochem. Phys. A 2013, 164, 44–53. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Luo, S.W.; Wang, A.L. Effects of high temperature on biochemical parameters, oxidative stress, DNA damage and apoptosis of pufferfish (Takifugu obscurus). Ecotoxicol. Environ. Saf. 2018, 150, 190–198. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics. Sci. Total Environ. 2018, 640–641, 1372–1381. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicators of fish stress? Pan-Am. J. Aquat. Sci. 2009, 4, 158–178. [Google Scholar]
- Viant, M.R.; Werner, I.; Rosenblum, E.S.; Gantner, A.S.; Tjeerdema, R.S.; Johnson, M.L. Correlation between heat-shock protein induction and reduced metabolic condition in juvenile steelhead trout (Oncorhynchus mykiss) chronically exposed to elevated temperature. Fish Physiol. Biochem. 2003, 29, 159–171. [Google Scholar] [CrossRef]
- Gandar, A.; Laffaille, P.; Canlet, C.; Tremblay-Franco, M.; Gautier, R.; Perrault, A.; Gress, L.; Mormede, P.; Tapie, N.; Budzinski, H.; et al. Adaptive response under multiple stress exposure in fish: From the molecular to individual level. Chemosphere 2017, 188, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, M.; Goodwin, L.O.; Orom, U.A.; Lombardi, S.; Tonelli, R.; Mengozzi, M.; Ghezzi, P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc. Natl. Acad. Sci. USA 2005, 102, 13998–14003. [Google Scholar] [CrossRef]
- Bing, L.; Sun, S.M.; Jian, Z.; Su, Y.L.; Zhang, W.X.; Ge, X.P. Transcriptome profiling and histology changes in juvenile blunt snout bream (Megalobrama amblycephala) liver tissue in response to acute thermal stress. Genomics 2019, 111, 242–250. [Google Scholar] [CrossRef]
- Kaur, M.; Atif, F.; Ali, M.; Rehman, H.; Raisuddin, S. Heat stress-induced alterations of antioxidants in the freshwater fish Channa punctata Bloch. J. Fish Biol. 2005, 67, 1653–1665. [Google Scholar] [CrossRef]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Elwan, H.A.M.; Xu, Q.Q.; Xie, C.; Dong, X.Y.; Zou, X.T. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 2019, 98, 2290–2298. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol. 2018, 77, 457–464. [Google Scholar] [CrossRef]
- Currie, S.; Ahmady, E.; Watters, M.A.; Perry, S.F.; Gilmour, K.M. Fish in hot water: Hypoxaemia does not trigger catecholamine mobilization during heat shock in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 281–287. [Google Scholar] [CrossRef]
- Lu, Y.L.; Wu, Z.H.; Song, Z.C.; Xiao, P.; Liu, Y.; Zhang, P.J.; You, F. Insight into the heat resistance of fish via blood: Effects of heat stress on metabolism, oxidative stress and antioxidant response of olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus. Fish Shellfish Immun. 2016, 58, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Steinhausen, M.F.; Sandblom, E.; Eliason, E.J.; Verhille, C.; Farrell, A.P. The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J. Exp. Biol. 2008, 211, 3915–3926. [Google Scholar] [CrossRef] [PubMed]
- Karakach, T.K.; Huenupi, E.C.; Soo, E.C.; Walter, J.A.; Afonso, L.O.B. H-1-NMR and mass spectrometric characterization of the metabolic response of juvenile Atlantic salmon (Salmo salar) to long-term handling stress. Metabolomics 2009, 5, 123–137. [Google Scholar] [CrossRef]
- Brandao, F.; Cappello, T.; Raimundo, J.; Santos, M.A.; Maisano, M.; Mauceri, A.; Pacheco, M.; Pereira, P. Unravelling the mechanisms of mercury hepatotoxicity in wild fish (Liza aurata) through a triad approach: Bioaccumulation, metabolomic profiles and oxidative stress. Metallomics 2015, 7, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immun. 2008, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ratriyanto, A.; Mosenthin, R.; Bauer, E.; Eklund, M. Metabolic, Osmoregulatory and Nutritional Functions of Betaine in Monogastric Animals. Asian Australas. J. Anim. Sci. 2009, 22, 1461–1476. [Google Scholar] [CrossRef]
- Kalujnaia, S.; Cramb, G. Regulation of Expression of the Myo-inositol Monophosphatase 1 Gene in Osmoregulatory Tissues of the European Eel Anguilla anguilla after Seawater Acclimation. Ann. N. Y. Acad. Sci. 2009, 1163, 433–436. [Google Scholar] [CrossRef]
- Kultz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Aragao, C.; Corte-Real, J.; Costas, B.; Dinis, M.T.; Conceicao, L.E.C. Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids 2008, 34, 143–148. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Cote, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Nakano, T.; Kameda, M.; Shoji, Y.; Hayashi, S.; Yamaguchi, T.; Sato, M. Effect of severe environmental thermal stress on redox state in salmon. Redox Biol. 2014, 2, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Bonga, S.E.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mai, K.S.; Trushenski, J.; Wu, G.Y. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Dani, V.D.; Lankadurai, B.P.; Nagato, E.G.; Simpson, A.J.; Simpson, M.J. Comparison of metabolomic responses of earthworms to sub-lethal imidacloprid exposure in contact and soil tests. Environ. Sci. Pollut. Res. 2019, 26, 18846–18855. [Google Scholar] [CrossRef] [PubMed]
- Enrique Padilla-Guerrero, I.; Barelli, L.; Angelica Gonzalez-Hernandez, G.; Carlos Torres-Guzman, J.; Bidochka, M.J. Flexible metabolism in Metarhizium anisopliae and Beauveria bassiana: Role of the glyoxylate cycle during insect pathogenesis. Microbiology 2011, 157, 199–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Somero, G.N.; Doyle, D. Temperature and rates of protein degradation in the fish Gillichthys mirabilis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 46, 463–474. [Google Scholar] [CrossRef]
- Ip, A.Y.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1. [Google Scholar] [CrossRef]
- Andersen, S.M.; Waagbø, R.; Espe, M. Functional amino acids in fish health and welfare. Front. Biosci. 2016, 8, 143–169. [Google Scholar]
- Lin, C.Y.; Wu, H.F.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
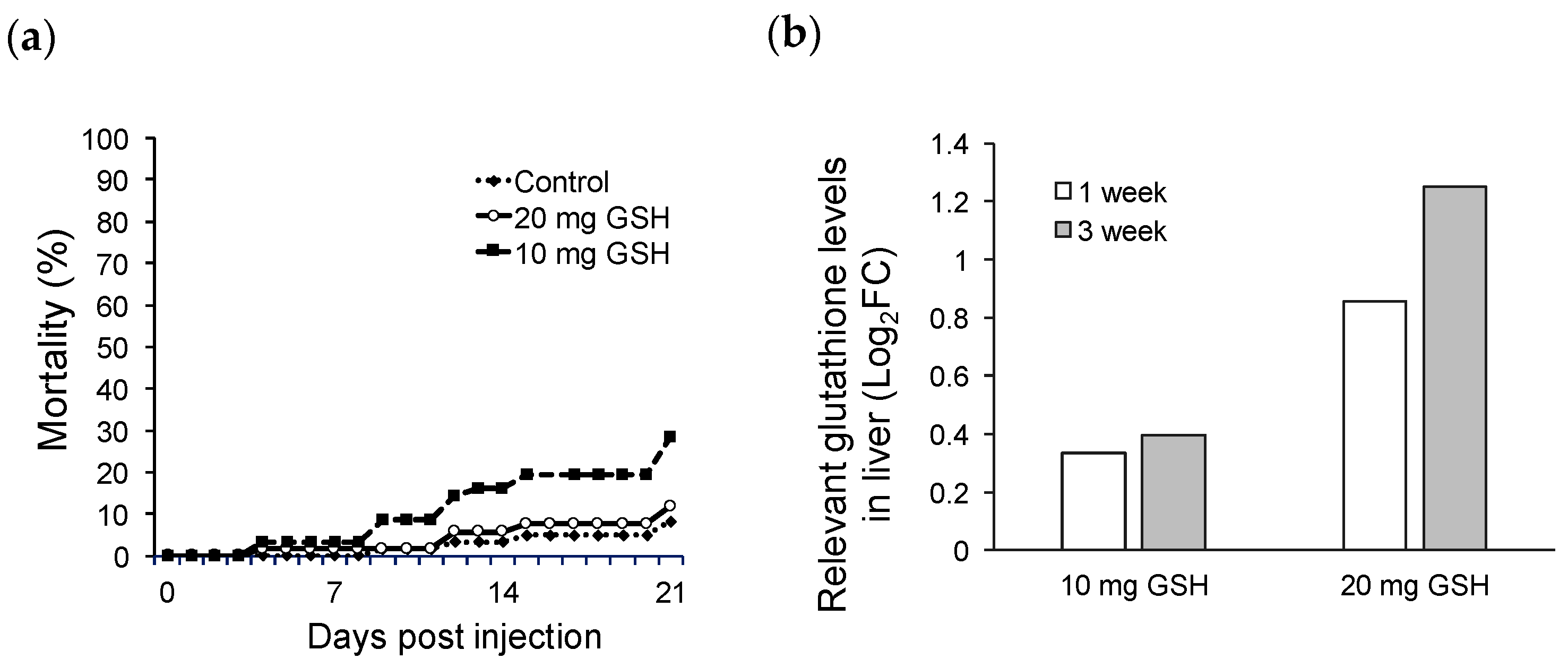
| Week | Control (%) | 20 mg GSH (%) | 10 mg GSH (%) |
|---|---|---|---|
| 0 | 0/5 (0) | ||
| 1 | 1/5 (20) | 1/5 (20) | 2/5 (40) |
| 3 | 3/5 (60) | 0/5 (0) | 2/5 (40) |
| No. | Pathway Name | Hits | Total | p-Value |
|---|---|---|---|---|
| Amino acid metabolism | ||||
| 1 | Alanine, aspartate and glutamate metabolism | 8 | 28 | 6.41 × 10−7 |
| 2 | Arginine biosynthesis | 6 | 14 | 1.23 × 10−6 |
| 3 | Glycine, serine and threonine metabolism | 7 | 33 | 3.03 × 10−5 |
| 4 | Valine, leucine and isoleucine biosynthesis | 8 | 4 | 4.37 × 10−5 |
| 5 | Arginine and proline metabolism | 48 | 5 | 0.004448 |
| 6 | Phenylalanine, tyrosine and tryptophan biosynthesis | 4 | 2 | 0.0049789 |
| 7 | Histidine metabolism | 16 | 3 | 0.010452 |
| 8 | Phenylalanine metabolism | 10 | 2 | 0.043482 |
| Metabolism of other amino acids | ||||
| 9 | Glutathione metabolism | 28 | 4 | 0.0082529 |
| 10 | D-Glutamine and D-glutamate metabolism | 6 | 2 | 0.011982 |
| 11 | beta-Alanine metabolism | 21 | 3 | 0.022376 |
| Carbohydrate metabolism | ||||
| 12 | Glyoxylate and dicarboxylate metabolism | 32 | 8 | 1.98 × 10−6 |
| 13 | Citrate cycle (TCA cycle) | 20 | 4 | 0.0023246 |
| 14 | Pyruvate metabolism | 22 | 4 | 0.0033598 |
| 15 | Galactose metabolism | 27 | 3 | 0.043482 |
| Lipid metabolism | ||||
| 16 | Glycerophospholipid metabolism | 36 | 4 | 0.019962 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, A.; Ma, S.; Lee, W.; Lee, S.; Yoon, D.; Kim, D.-H.; Kim, S. Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus. Metabolites 2020, 10, 3. https://doi.org/10.3390/metabo10010003
Kim S, Kim A, Ma S, Lee W, Lee S, Yoon D, Kim D-H, Kim S. Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus. Metabolites. 2020; 10(1):3. https://doi.org/10.3390/metabo10010003
Chicago/Turabian StyleKim, Seonghye, Ahran Kim, Seohee Ma, Wonho Lee, Sujin Lee, Dahye Yoon, Do-Hyung Kim, and Suhkmann Kim. 2020. "Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus" Metabolites 10, no. 1: 3. https://doi.org/10.3390/metabo10010003
APA StyleKim, S., Kim, A., Ma, S., Lee, W., Lee, S., Yoon, D., Kim, D.-H., & Kim, S. (2020). Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus. Metabolites, 10(1), 3. https://doi.org/10.3390/metabo10010003






