Advancements in Encapsulation Technologies: The Potential of Polyphenols as an Antidiabetic Therapy
Abstract
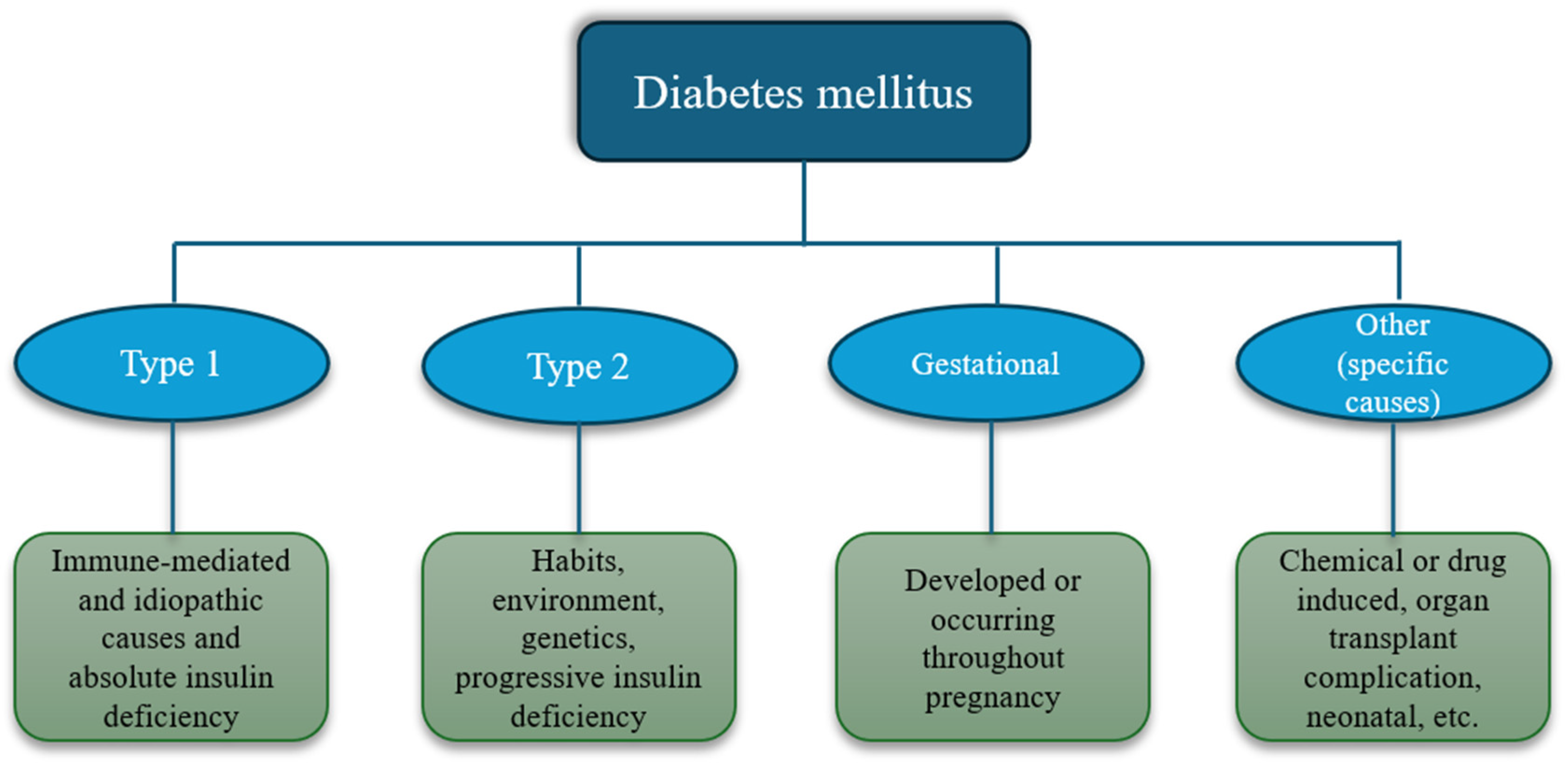
1. Introduction
2. Polyphenols
3. Microencapsulation
3.1. Physical Methods
3.2. Physico-Chemical Methods
| Source | Encapsulation Method | Wall Material | Conditions | Results | Reference |
|---|---|---|---|---|---|
| Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds | Spray Drying | Maltodextrin (5%) | Temperature: 100° C; flow rate: 7.5 mL/min, and pressure: 6 bar. | The microparticles showed spherical and heterogeneous structures and good encapsulation efficiency. | [67] |
| Blackberry Pomace (Rubus fruticosus) | Spray Drying | Maltodextrin DE 10, in a 1:1 (w/w) ratio | Inlet drying air temperature: 170 °C; atomization pressure: 4 bar; drying air flow: 3.5 m3/h, and flow rate: 0.5 L/h. | Microparticles have a rounded outer structure and are agglomerated into different sizes. | [64] |
| Chipilin (Crotalaria longirostrata) methanolic extracts | Spray Drying | Maltodextrin, Arabic gum, Cajanus gum, cocoa shell pectin, Cajanus protein, and soy protein. | Inlet air temperature: 120 °C; feed flow: 3 mL min−1; drop pressure: 1.35 bar | Microcapsules with mostly irregular amorphous structures, smooth surfaces, and depressions. Size between 3 and 8 μm | [65] |
| Sambucus nigra L. (elderberry) | Spray Drying | Modified chitosan, sodium alginate, and Arabic gum. | Flow rate: 4 mL/min (15%); inlet temperature: 115 °C; air pressure: 5–6 bar, and aspiration rate: 100% (36 m3/h) | Very small particles (between 5 and 19 μm). | [66] |
| Extract from Lippia citriodora leaves | Spray Drying | Maltodextrin and inulin | Inlet air temperature 135–195 °C; airflow: 0.30 m3/min; feeding flow: 2 mL/min, atomization air flow: 13 L/min | Inulin increased powder and polar compounds recovery, whereas maltodextrin achieved a higher encapsulation efficiency. | [63] |
| Ciriguela (Spondias purpurea L.) | Freeze-drying | Maltodextrin 10 DE and arabic gum | 48 h in a freeze dryer at −80 °C and 0.28 mbar chamber pressure. | Microcapsules with irregular shape, extensive wrinkles, and a serrated surface. | [84] |
| Blackberry (Rubus fruticosus) | Freeze-drying | Chitosan, xanthan, β-cyclodextrin, and hydrogel | Mixture: 0.003 mol of polymer and the same proportion of extract, diluted in 50 mL of water. The solution was frozen at −80 °C for 24 h, with subsequent lyophilization. | Only chitosan and xanthan showed the characteristic shape. | [68] |
| Blueberry (Vaccinium myrtillus) Juice | Freeze-drying | HP-β-cyclodextrin and β-cyclodextrin | β-CD in 15% (w/w) ratio to hot (75 °C) blueberry juice. The precipitated product was freeze-dried at −50 °C | Formation of amorphous material and a 78.1% product yield. | [69] |
| Pomegranate (Punica granatum L.) | Freeze-drying | Maltodextrin (20 DE) | The extract and maltodextrin mixture (1:2 (w/w)) was lyophilized at −30 °C and vacuum pressure: 0.04 mbar. | Homogeneous coating on particle surface. | [85] |
| Black chokeberry (Aronia melanocarpa) | Indirect extrusion | Sodium alginate, low-molecular-weight chitosan, carrageenan, Low-methoxyl pectin | Alginate was mixed in equal proportions (1:1 g/g) with other encapsulants. Encapsulator; vibrating nozzle: 150 m; pressure: 200 mbar; frequency: 400 Hz; electrode: 1000 V; solidification temperature: 30 °C and complexation time: 10 min. | Hydrogel beads differ in shape and structure. The most regular capsules were obtained with the mixture of alginate + carrageenan. | [70] |
| Papaya fruit (Carica papaya L.) | Extrusion | Pectin-alginate | The papaya extract was encapsulated through the in situ and two-step methodologies. Alginate:pectin ratio was 55:45. | Bioactive compounds are dispersed in the encapsulation matrix, improving their thermal stability. | [86] |
| Proanthocyanidin cinnamon extract | Complex coacervation | Gelatin and five different polysaccharides (gum Arabic, pectin, cashew tree gum, carboxymethylcellulose, and κ-carrageenan | The proanthocyanidin-rich cinnamon extract was dispersed in distilled water. The gelatin dispersion was added, and then the polysaccharide solution. The decanted material was frozen at −20 °C and dried in freeze-dryer. | Particles presented resistance when submitted to different stress conditions, except pH lower than 2 and temperatures higher than 50 °C. | [77] |
| Polyphenols from oat bran | Complex coacervation | Whey protein concentrates 10% Maltodextrin 10% | The wall materials were mixed in ratios 10:0, 8:2, 6:4, 4:6, and 2:8 by gentle magnetic stirring for 1 h. BAS extract was then added to the wall material at 10% (1:10 ratio) and the microcapsules solution was formed using a Magnetic Stirrer for 15 min. | The encapsulation efficiency was 95.28%. The release percentage of polyphenols coated in a capsule ranged between 70 and 83% after 2 h of digestion. | [87] |
| (−)-Epigallocatechin gallate (≥94%) | Liposomes | Phospholipon | Phospholipon and Epigallocatechin gallate were dissolved in ethanol. Citric acid (0.1%) was added while stirring, and the mixture was heated to 60 °C. The microparticles were prepared using an encapsulator. | Encapsulation efficiency (>97%) and sustained release; in 14 days, no more than 15% of EGCG was released. The sizes of the liposomes were estimated at 1–2 μm. | [88] |
| Grape-seed extract | Liposomes | Soy lecithin | Grape-seed extract was incorporated into liposomes (1.1% w/w soy lecithin) using high-pressure homogenization (22,500 psi). | Entrapment efficiency for uncoated liposomes was 88.2 ± 4.7%. The release rate after 24 h from uncoated liposomes was 0.55*h. | [72] |
| Green tea extract (C. sinensis) | Ionic gelation | Amidated low methoxyl pectin, calcium chloride, hydrogenated palm oil | Association of a double emulsion (water/oil/water) with ionic gelation. The final emulsion was sprayed through a double-fluid atomizer on a CaCl2 crosslinking solution acidified with citric acid (pH 3). | 72.6 ± 0.4% encapsulation efficiency for ionic gelation microparticles. | [81] |
| Anthocyanins from Hibiscus sabdariffa L. calyces | Ionic gelation | Rapeseed oil, pectin, calcium chloride | Ionic gelation using two techniques: drip-extrusion and atomization, both using a double emulsion (Hibiscus extract/rapeseed oil/pectin) and a cross-linked solution (CaCl2). | The median diameter (D50) of the particles ranged from 78 to 1100 μm, and encapsulation efficiency ranged from 67.9 to 93.9%. | [83] |
| Securigera securidaca (L) seed extract | Co-crystallization | Saccharose | Sucrose and S. securidaca extract were mixed on a heater at 132 °C. The co-crystallized product was dried in an oven at 40 °C for 15 h, then ground and sieved. | The production efficiency and moisture content of the extract-containing co-crystallized powder were 84% and 0.14%, respectively. | [89] |
| Pomegranate Peel Extract | Co-Crystallization | Food-grade crystal sucrose | Sucrose solution and extract were mixed at 700 rpm. The mixture is placed in a water bath and stirred until it reaches 45 °C. The powder is kept in a desiccator for 24 h. | The co-crystallized powder had low moisture content (0.59%), low hygroscopicity (0.011%), high apparent density (0.803 g/cm3) and solubility (61 s). | [90] |
4. Nanoencapsulation
5. Current Evidence Regarding the Efficacy of Encapsulated Polyphenols
5.1. In Vitro
5.2. In Vivo
| Compound | Polymer/Particle Size | Dosage | In Vivo Model | Effect * | Ref. |
|---|---|---|---|---|---|
| Chrysin | PLGA/176.0 ± 2.1 nm | One administration of 20 mg/kg | STZ-induced diabetes in male albino rats (180–200 g) | ↓ Blood glucose ↓ TG, LDL ↑ HDL | [133] |
| Curcumin | Chitosan/n.s. | 150 mg/kg once a day, for 28 days | STZ-induced type 1 diabetes in C57Bl/6 mice | ↓ Blood glucose ↑ Insulin secretion ↓ Fibrosis in the kidney | [134] |
| Ferulic acid | Chitosan/211.3 ± 5.1 nm | 10 mg/kg once a day, for 14 days | STZ-induced diabetes in Wistar albino rats (110–150 g) | ↓ Blood glucose ↑ Plasma insulin levels ↓ TC, TG -Recovered islets of Langerhans in the pancreas | [130] |
| Hesperidin | MgAl-double layered hydroxide/330–380 nm | 50 mg/kg once a day, for 30 days | Nicotinamide + STZ-induced diabetes in male albino rats (200–300 g) | ↓ Plasma glucose, HbA1c ↑ Insulin, HOMA-B -Restored the pancreatic Islets of Langerhans | [135] |
| Liquiritin | Phospholipid complex/91.8 ± 1.9 nm | 200 mg/kg once a day, for 28 days | STZ-diabetes induced in male ICR mice (18–22 g) | ↓ Blood glucose -Improved the glomerular and renal cortical structure of the kidney | [136] |
| Mangiferin | Labrafil M 2130 CS/138.4 ± 3.4 nm | One administration of 40 mg/kg | High-fat diet + STZ-diabetes induced in male Wistar rats (250 g) | ↓ Blood glucose ↓ TC, TG ↑ HDL ↓ AST, ALT | [137] |
| Mangiferin | NSC-alginate/124 nm | 10 mg/kg once a day, for 28 days | STZ-induced diabetes in Wistar rats (100–150 g) | ↓ Blood glucose ↓ TC, TG, LDL ↑ HDL | [138] |
| Myricetin | Chitosan/184.4 ± 4.1 nm | 50 mg/kg once a day, for 28 days | STZ-induced diabetes in male Wistar rats (~250 g) | ↓ Blood glucose ↓ TG, TC ↑ BW | [139] |
| Naringenin | Phospholipid LECIVA-S70/564.4 nm | Single dose of 25 mg/kg or 50 mg/kg, for 28 days | STZ-induced diabetes in male Sprague Dawley rats (180–220 g) | ↓ Plasma glucose level ↓ TC, TG, BUN ↓ ALT, AST | [140] |
| Naringenin | PLGA/129 nm | One dose of 10 mg/kg, and a second dose after 10 days, period of 7–49 days | STZ-induced diabetes in male Wistar rats | ↓ Blood glucose ↑ Insulin level ↓ HbA1c -Restored pancreas and kidney cells | [141] |
| Quercetin | Eudragit L-100/144.7 ± 1.7 nm | 200 mg/kg once a day, during 21 days | STZ-induced diabetes in albino female Wistar rats (150–200 g) | ↓ Blood glucose ↓ TG, TC, LDL ↓ ALP, ALT, AST ↓ cellular damage in the pancreas | [142] |
| Quercetin | PLGA/179.9 ± 11.2 nm | 150 mg/kg every 5th day, during 15 days | STZ-stimulated male Sprague-Dawley rats (~250 g) | ↓ Blood glucose | [143] |
| Quercetin | Poloxamer-180-stearic acid/157.1 to 528.2 nm | 5 or 10 mg/kg, for 21 days | STZ-induced diabetic retinopathy in male adult zebra fish (<8 months) | ↓ Plasma glucose | [144] |
| Resveratrol | Chitosan/38.0 nm | 100 mg/kg, for 28 days | STZ-indued gestational diabetes mellitus in Wistar albino rats (180–200 g) | ↓ Blood glucose ↑ Insulin level ↓ TC, TG, LDL ↑ HDL | [145] |
| Plant Specie | Components of the Extract | Encapsulating Material/Particle Size | In Vivo Model, Dosage | Effect * | Ref. |
|---|---|---|---|---|---|
| Cinnamomoum osmophloeum Kanehira | Cinnamaldehyde, benzoic acid, caffeic acid, caffeoylquinic acid, cinnamic acid, coumaric acid, rutin, kaempferol, eugenol, quercetin, and derivatives | Nanoemulsion (soybean oil, lecithin and Tween 80)/ 36.6 nm | Nicotinamide + STZ-induced diabetes in male Wistar rats (7 weeks old), 60 mg/kg (cinammaldehyde equivalents) | ↓ Blood glucose, HOMA-IR ↓ TC, TG, AST, ALT, BUN | [149] |
| Coccinia grandis | Phenolics and flavonoids | Gelatin/ 468 ± 14 nm | High-fat diet + STZ-induced diabetes in male Wistar rats (135–165 g), single dose of 330 mg/kg | ↓ Plasma glucose | [150] |
| Coffea arabica | Caffeine, chlorogenic acid | Maltodextrin + whey protein/ 1–2 µm | Fructose-induced obesity in male Wistar rats (85–120 g), 100 mg/kg per day (during 28 days) | ↓ Glucose, HOMA-IR ↓ TC, TG, AST, ALT ↓ Liver-TG, liver-TC | [146] |
| Murraya koenigii | Phenolics and flavonoids | Gelatin/ 520 ± 33 nm | High-fat diet + STZ-induced diabetes in male Wistar rats (135–165 g), single dose of 65 mg/kg | ↓ Plasma glucose | [150] |
| Posidonia oceanica | Hydroxybenzoic acid, protocatechuic acid, ferulic acid, gallic acid, coumaric acid, sinapic acid, vanillic acid, catechin, epicatechin, luteolin, naringenin, apigenin, among others. | Bovine gelatine/ 274.7 ± 30.5 | STZ-induced diabetes in male Wistar albino rats (150–170 g), 100 mg/kg (for 28 days) | ↓ Glucose, HOMA-IR ↑ GLUT4 | [151] |
| Senna auriculata | Phenolics and flavonoids | Gelatin/ 563 ± 4 nm | High-fat diet + STZ-induced diabetes in male Wistar rats (135–165 g), Single dose of 45 mg/kg | ↓ Plasma glucose | [150] |
| Vaccinium meridionale | Delphinidin 3-hexoside, cyanidin-3-galactoside, cyanidin-3-glucoside, cyanidin 3-arabinoside | Pro-nanosome Nio-N/219.7 ± 3.1 nm | High-fat diet-induced obesity in C57BL/6 mice, 160 µg/mL (during 28 days) | ↓ Glucose ↓ TC, leptin | [147] |
6. ADMET Analysis of Polyphenols with Antidiabetic Properties
7. Future Directions and Perspectives for Clinical Translation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ADME | Absorption, distribution, metabolism, and excretion |
| ALP | Alkaline phosphate |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BBB | Blood–brain barrier |
| BUN | Blood urea nitrogen |
| BW | Body weight |
| CAT | Catalase |
| DM | Diabetes mellitus |
| FPG | Fasting plasma glucose |
| GLUT4 | Insulin-regulated glucose transporter |
| GPX | Glutathione peroxidase |
| Hb1A1c | Glycosylated hemoglobin |
| HDL | High density lipoproteins |
| HLM | The human liver microsomal |
| HOMA-B | Homeostasis model assessment of β-cell function |
| HOMA-IR | Homeostasis model assessment-insulin resistance |
| IC50 | Inhibitory Concentration 50 |
| LUV | Unilamellar vesicles |
| LDL | Low density lipoproteins |
| LMPH | Longzhua mushroom polysaccharide hydrogel |
| MLV | Multilamellar vesicles |
| NLCs | Nanostructured Lipid Carriers |
| NSC | N-succinylated chitosan |
| OGTT | Oral glucose tolerance test |
| PGA | polyglycolides |
| P-gp | P-glycoprotein |
| PLA | Polylactides |
| PLA-PEG | poly(lactide)-poly(ethylene glycol) |
| PLGA | DL-polylactide/glycolide copolymer |
| PLGA-PEG | poly(lactide-co-glycolide)-poly(ethylene glycol) |
| SOD | Superoxide dismutase |
| SLNs | Solid Lipid Nanoparticles |
| STZ | streptozotocin |
| TBRAS | Thiobarbituric acid reactive substances |
| TC | Total cholesterol |
| TG | Triglycerides |
| T2DM | Type 2 Diabetes mellitus |
| 2-hPG | 2 hPlasma glucose |
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Paduraru, L.; Nutas, R.M.; Ujoc, A.M.; Yahya, G.; Metwally, K.; Cavalu, S. Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities. Int. J. Mol. Sci. 2023, 24, 12676. [Google Scholar] [CrossRef]
- Sharma, P.; Hajam, Y.A.; Kumar, R.; Rai, S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomed Plus 2022, 2, 100188. [Google Scholar] [CrossRef]
- Vlacho, B.; Rossell-Rusiñol, J.; Granado-Casas, M.; Mauricio, D.; Julve, J. Overview on chronic complications of diabetes mellitus. In Chronic Complications of Diabetes Mellitus; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–10. [Google Scholar]
- International Diabetes Federation. Diabetes Atlas. Available online: https://diabetesatlas.org/atlas-reports (accessed on 7 June 2025).
- World Health Organization. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 1 August 2024).
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Arigela, C.S.; Gan, S.H.; Salam, S.K.N.; Krishnan, K.T.; Rahman, N.A.; Jeffree, M.S. A review on oxidative stress, diabetic complications, and the roles of honey polyphenols. Oxidative Med. Cell. Longev. 2020, 2020, 8878172. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxidative Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Arkoub-Djermoune, L.; Boulekbache-Makhlouf, L.; Zeghichi-Hamri, S.; Bellili, S.; Boukhalfa, F.; Madani, K. Influence of the Thermal Processing on the Physico-Chemical Properties and the Antioxidant Activity of A Solanaceae Vegetable: Eggplant. J. Food Qual. 2016, 39, 181–191. [Google Scholar] [CrossRef]
- Aryal, D.; Joshi, S.; Thapa, N.K.; Chaudhary, P.; Basaula, S.; Joshi, U.; Bhandari, D.; Rogers, H.M.; Bhattarai, S.; Sharma, K.R.; et al. Dietary phenolic compounds as promising therapeutic agents for diabetes and its complications: A comprehensive review. Food Sci. Nutr. 2024, 12, 3025–3045. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Arribas, A.; Moreno, M.; Moreno, G.A.; Bermejo, E.; Zapardiel, A.; Chicharro, M. Characterization of White Wines by Electrochemical Indexes Obtained Using Carbon Nanotube-modified Electrodes. Electroanalysis 2018, 30, 1461–1471. [Google Scholar] [CrossRef]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Núñez, O. Detection and Quantitation of Frauds in the Authentication of Cranberry-Based Extracts by UHPLC-HRMS (Orbitrap) Polyphenolic Profiling and Multivariate Calibration Methods. J. Agric. Food Chem. 2018, 66, 9353–9365. [Google Scholar] [CrossRef]
- Brinsi, C.; Jedidi, S.; Sammari, H.; Selmi, H.; Sebai, H. Antidiarrheal, anti-inflammatory and antioxidant effects of Anethum graveolens L. fruit extract on castor oil-induced diarrhea in rats. Neurogastroenterol. Motil. 2024, 36, e14892. [Google Scholar] [CrossRef] [PubMed]
- Arslaner, A.; Türkoğlu, Z. A potential antiviral and food-derived healty ingredient: Resveratrol. Food Health 2021, 7, 54–63. [Google Scholar] [CrossRef]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernandez-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism; InTech Open: Rijeka, Croatia, 2019. [Google Scholar]
- Xuan Hoan, N.; Anh, L.; Quan, D.; Cuong, D.; Thai Ha, H.; Minh, N.; Hieu, D.; Thuat, N.; Pham, T.; Tuyen, D. Functional-Antioxidant Food; InTech Open: Rijeka, Croatia, 2021. [Google Scholar]
- Pinna, N.; Ben Abbou, S.; Ianni, F.; Angeles Flores, G.; Pietercelie, A.; Perretti, G.; Blasi, F.; Angelini, P.; Cossignani, L. Phenolic compounds from pumpkin pulp: Extraction optimization and biological properties. Food Chem. X 2024, 23, 101628. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.; Moreau, R.; Majumder, K. Antioxidant and Anti-Inflammatory Capacities of Three Dry Bean Varieties after Cooking and In Vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2024, 72, 18445–18454. [Google Scholar] [CrossRef]
- Marzagalli, M.; Battaglia, S.; Raimondi, M.; Fontana, F.; Cozzi, M.; Ranieri, F.R.; Sacchi, R.; Curti, V.; Limonta, P. Anti-Inflammatory and Antioxidant Properties of a New Mixture of Vitamin C, Collagen Peptides, Resveratrol, and Astaxanthin in Tenocytes: Molecular Basis for Future Applications in Tendinopathies. Mediat. Inflamm. 2024, 2024, 5273198. [Google Scholar] [CrossRef]
- Çetin, R.; Bahadir, S.; Basar, İ.; Aslanoglu, B.; Atlas, B.; Kaya, S.; Güzel, B.C.; Turan, Y. Neuroprotective effects of the combined treatment of resveratrol and urapidil in experimental cerebral ischemia-reperfusion injury in rats. Acta Cir. Bras. 2024, 39, e395329. [Google Scholar] [CrossRef]
- Özturk, N.; Ceylan, H.; Demir, Y. The hepatoprotective potential of tannic acid against doxorubicin-induced hepatotoxicity: Insights into its antioxidative, anti-inflammatory, and antiapoptotic mechanisms. J. Biochem. Mol. Toxicol. 2024, 38, e23798. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Xu, Q.; Xiang, Q.; Chen, Y.; Lv, L.; Zheng, B.; Wang, Q.; Wang, S.; Li, L. Epigallocatechin-3-Gallate Improves Intestinal Gut Microbiota Homeostasis and Ameliorates Clostridioides difficile Infection. Nutrients 2022, 14, 3756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, J.; Wang, Y.; Yi, X. High-tannin food enhances spatial memory and scatter-hoarding in rodents via the microbiota-gut-brain axis. Microbiome 2024, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Vitelli-Storelli, F.; Becerra-Tomas, N.; Vázquez-Ruiz, Z.; Díaz-López, A.; Corella, D.; Castañer, O.; Romaguera, D.; Vioque, J.; et al. Associations between Dietary Polyphenols and Type 2 Diabetes in a Cross-Sectional Analysis of the PREDIMED-Plus Trial: Role of Body Mass Index and Sex. Antioxidants 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wang, X. Ameliorating effect of Chinese jujube polyphenol on blood glucose oxidative stress in type 2 diabetic rats. J. Diabetes Complicat. 2024, 38, 108804. [Google Scholar] [CrossRef] [PubMed]
- Choockong, C.; Itharat, A.; Pipatrattanaseree, W.; Ninlaor, T.; Piwngam, K.; Intharit, N.; Sukkhum, S.; Davies, N.M. The most commonly used spices in Thai traditional medicine: In vitro evaluation of anti-hyperglycemic, antioxidant, polyphenol content, and nitric oxide production inhibitory activities. Res. Pharm. Sci. 2024, 19, 13–28. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, S.Y.; Kim, J.Y. Comparative evaluation of the antihyperglycemic effects of three extracts of sea mustard (Undaria pinnatifida): In vitro and in vivo studies. Food Res. Int. 2024, 190, 114623. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, H.; Cui, W.; Ouyang, J.; Zhou, F.; Wen, S.; Fang, W.; Zhang, S.; Huang, J.; Liu, Z. Theaflavins mitigate diabetic symptoms in GK rats by modulating the INSR/PI3K-Akt/GSK-3 pathway and intestinal microbiota. Int. J. Biol. Macromol. 2024, 277, 134331. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Ugarriza, L.; Gómez, C.; Sureda, A.; Tur, J.A. Long-Term Impact of Nutritional Intervention with Increased Polyphenol Intake and Physical Activity Promotion on Oxidative and Inflammatory Profiles in Patients with Metabolic Syndrome. Nutrients 2024, 16, 2121. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.G.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Gerloff, T. Impact of genetic polymorphisms in transmembrane carrier-systems on drug and xenobiotic distribution. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 69–77. [Google Scholar] [CrossRef]
- Lampe, J.W. Interindividual differences in response to plant-based diets: Implications for cancer risk. Am. J. Clin. Nutr. 2009, 89, 1553S–1557S. [Google Scholar] [CrossRef]
- Hidalgo-Liberona, N.; González-Domínguez, R.; Vegas, E.; Riso, P.; Del Bo’, C.; Bernardi, S.; Peron, G.; Guglielmetti, S.; Gargari, G.; Kroon, P.A. Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability. J. Agric. Food Chem. 2020, 68, 12476–12484. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Flavanol plasma bioavailability is affected by metabolic syndrome in rats. Food Chem. 2017, 231, 287–294. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of plant phenols with food macronutrients: Characterisation and nutritional–physiological consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. Gut microbiota-polyphenol interactions in chicken: A review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Yang, X.; Wang, Z.; Yao, X.; Guo, Y. Enhanced bioavailability and anti-hyperglycemic activity of young apple polyphenols by complexation with whey protein isolates. J. Food Sci. 2022, 87, 1257–1267. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Singh, R.; Westfall, S.; Herman, F.; Faith, J.; Ho, L. The role of the gut microbiota in the metabolism of polyphenols as characterized by gnotobiotic mice. J. Alzheimer’s Dis. 2018, 63, 409–421. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.; Cai, X.; Wang, C.; Cao, Y.; Xiao, J. Rosmarinic acid restores colonic mucus secretion in colitis mice by regulating gut microbiota-derived metabolites and the activation of inflammasomes. J. Agric. Food Chem. 2023, 71, 4571–4585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gu, X.; Zhuang, T.; Xu, Y.; Yang, L.; Zhou, M. Gut microbiota: A pivotal hub for polyphenols as antidepressants. J. Agric. Food Chem. 2020, 68, 6007–6020. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Shivashankara, K.; Acharya, S. Bioavailability of dietary polyphenols and the cardiovascular diseases. Open Nutraceuticals J. 2010, 3, 227–241. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol mechanisms against gastric cancer and their interactions with gut microbiota: A review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Lila, M.A.; Hoskin, R.T.; Grace, M.H.; Xiong, J.; Strauch, R.; Ferruzzi, M.; Iorizzo, M.; Kay, C. Boosting the bioaccessibility of dietary bioactives by delivery as protein–polyphenol aggregate particles. J. Agric. Food Chem. 2022, 70, 13017–13026. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of anthocyanins-critical review of techniques and wall materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A review of applications in the food and pharmaceutical industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; de la Luz Cádiz-Gurrea, M.; Fernández-Ochoa, Á.; Arráez-Román, D.; Segura-Carretero, A. Spray-drying microencapsulation of bioactive compounds from lemon verbena green extract. Foods 2020, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.d.; Rodrigues, L.M.; da Costa, S.C.; Bergamasco, R.d.C.; Madrona, G.S. Microencapsulation of bioactive compounds from blackberry pomace (Rubus fruticosus) by spray drying technique. Int. J. Food Eng. 2017, 13, 20170047. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.R.; Abud-Archila, M. Spray drying encapsulation of a native plant extract rich in phenolic compounds with combinations of maltodextrin and non-conventional wall materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Spray Drying Encapsulation of Elderberry Extract and Evaluating the Release and Stability of Phenolic Compounds in Encapsulated Powders. Food Bioprocess Technol. 2019, 12, 1381–1394. [Google Scholar] [CrossRef]
- Ferreira, L.M.D.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.D.; Remígio, M.S.D.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyzowska, A.; Adamiec, J. Effect of Microencapsulation by Spray-Drying and Freeze-Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Int. Food Res. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- de Souza, V.B.; Thomazini, M.; Echalar Barrientos, M.A.; Nalin, C.M.; Ferro-Furtado, R.; Genovese, M.I.; Favaro-Trindade, C.S. Functional properties and encapsulation of a proanthocyanidin-rich cinnamon extract (Cinnamomum zeylanicum) by complex coacervation using gelatin and different polysaccharides. Food Hydrocoll. 2018, 77, 297–306. [Google Scholar] [CrossRef]
- Soliman, T.N.; Mohammed, D.M.; El-Messery, T.M.; Elaaser, M.; Zaky, A.A.; Eun, J.B.; Shim, J.H.; El-Said, M.M. Microencapsulation of Plant Phenolic Extracts Using Complex Coacervation Incorporated in Ultrafiltered Cheese Against AlCl3-Induced Neuroinflammation in Rats. Front. Nutr. 2022, 9, 929977. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mahoonak, A.S.; Akbari, M. Physicochemical properties and antioxidant stability of microencapsulated marjoram extract prepared by co-crystallization method. J. Food Process Eng. 2019, 42, e12949. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Pandey, V.K.; Tripathi, A.; Mukarram, S.A.; Harsányi, E.; Kovács, B. Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization. Molecules 2023, 28, 4764. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Alvim, I.D.; Cortez, M.A.S. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. J. Food Technol. 2019, 56, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Berling, C.L.; Germer, S.P.M.; Alvim, I.D.; Hubinger, M.D. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef]
- da Silva Júnior, M.E.; Araújo, M.V.R.L.; Martins, A.C.S.; dos Santos Lima, M.; da Silva, F.L.H.; Converti, A.; Maciel, M.I.S. Microencapsulation by spray-drying and freeze-drying of extract of phenolic compounds obtained from ciriguela peel. Sci. Rep. 2023, 13, 15222. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Thakur, A. Microencapsulation of wild pomegranate flavedo phenolics by lyophilization: Effect of maltodextrin concentration, structural morphology, functional properties, elemental composition and ingredient for development of functional beverage. LWT 2020, 133, 110077. [Google Scholar] [CrossRef]
- Vallejo-Castillo, V.; Rodríguez-Stouvenel, A.; Martínez, R.; Bernal, C. Development of alginate-pectin microcapsules by the extrusion for encapsulation and controlled release of polyphenols from papaya (Carica papaya L.). J. Food Biochem. 2020, 44, e13331. [Google Scholar] [CrossRef]
- Bannikova, A.; Zyainitdinov, D.; Evteev, A.; Drevko, Y.; Evdokimov, I. Microencapsulation of polyphenols and xylooligosaccharides from oat bran in whey protein-maltodextrin complex coacervates: In-vitro evaluation and controlled release. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100236. [Google Scholar] [CrossRef]
- Istenič, K.; Cerc Korošec, R.; Poklar Ulrih, N. Encapsulation of (-)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016, 96, 4623–4632. [Google Scholar] [CrossRef]
- Nik, A.B.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019, 3, 243–250. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Goula, A.M. Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization. Appl. Sci. 2023, 13, 8680. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Techniques for Nanoencapsulation of Food Ingredients; Springer: Berlin/Heidelberg, Germany, 2014; Volume 8. [Google Scholar]
- Contreras-Angulo, L.A.; Gutiérrez-Grijalva, E.P.; Cabanillas-Bojórquez, L.A.; Jiménez-Ortega, L.A.; Heredia, J.B. Chapter 7—Nanoformulations applied to the delivery of alkaloids. In Phytochemical Nanodelivery Systems as Potential Biopharmaceuticals; Heredia, J.B., Gutiérrez-Grijalva, E.P., Licea-Claverie, A., Gutierrez-Uribe, J.A., Patra, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 257–281. [Google Scholar]
- Garcia-Carrasco, M.; Parra-Aguilar, I.F.; Gutiérrez-Grijalva, E.P.; Licea-Claverie, A.; Basilio Heredia, J. Chapter 18—Nano-formulations in drug delivery. In Food, Medical, and Environmental Applications of Nanomaterials; Pal, K., Sarkar, A., Sarkar, P., Bandara, N., Jegatheesan, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 473–491. [Google Scholar]
- Pedrozo, R.C.; Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz. J. Pharm. 2020, 56, e17692. [Google Scholar] [CrossRef]
- Rambaran, T.F. Nanopolyphenols: A review of their encapsulation and anti-diabetic effects. SN Appl. Sci. 2020, 2, 1335. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Junior, A.C.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and crosslinked chitosan nanoparticles: Synthesis, characterization and their role as Pickering emulsifiers. Carbohydr. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Jiang, T.; Jin, K.; Liu, X.; Pang, Z. 8—Nanoparticles for tumor targeting. In Biopolymer-Based Composites; Jana, S., Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 221–267. [Google Scholar]
- Costa-Almeida, R.; Soares, R.; Costa, R. Polyphenol-Based Nanoparticles as Multifaceted Diabetes Modulators. In Functional Bionanomaterials: From Biomolecules to Nanoparticles; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 251–270. [Google Scholar]
- Rai, V.K.; Gupta, G.D.; Pottoo, F.H.; Barkat, M.A. Potential of Nano-Structured Drug Delivery System for Phytomedicine Delivery. In Nanophytomedicine: Concept to Clinic; Beg, S., Barkat, M.A., Ahmad, F.J., Eds.; Springer: Singapore, 2020; pp. 89–111. [Google Scholar]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-β-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]
- Palacio, J.; Monsalve, Y.; Ramírez-Rodríguez, F.; López, B. Study of encapsulation of polyphenols on succinyl-chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101610. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Extraction of catechins from decaffeinated green tea for development of nanoemulsion using palm oil and sunflower oil based lipid carrier systems. J. Food Eng. 2015, 147, 14–23. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of photoprotection potential of catechin loaded nanoemulsion gel against UVA induced oxidative stress. J. Photochem. Photobiol. B Biol. 2016, 160, 318–329. [Google Scholar] [CrossRef]
- Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine Serum Albumin Nanoparticles Containing Quercetin: Characterization and Antioxidant Activity. J. Nanosci. Nanotechnol. 2016, 16, 1346–1353. [Google Scholar] [CrossRef]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Romeo, A.; Musumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic Acid-Loaded Polymeric Nanoparticles for Potential Ocular Delivery. Pharmaceutics 2021, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Anal. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Neelakandan, M.; Manoharan, S.; Muralinaidu, R.; Thara, J.M. Tumor preventive and antioxidant efficacy of chlorogenic acid–loaded chitosan nanoparticles in experimental skin carcinogenesis. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Ma, Y.; Goff, H.D.; Zhong, F. Regulation of nano-encapsulated tea polyphenol release from gelatin films with different Bloom values. Food Hydrocoll. 2020, 108, 106045. [Google Scholar] [CrossRef]
- Gaber Ahmed, G.H.; Fernández-González, A.; Díaz García, M.E. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Jafari, S.M.; Assadpour, E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocoll. 2020, 101, 105572. [Google Scholar] [CrossRef]
- Oskoueian, E.; Karimi, E.; Noura, R.; Ebrahimi, M.; Shafaei, N.; Karimi, E. Nanoliposomes encapsulation of enriched phenolic fraction from pistachio hulls and its antioxidant, anti-inflammatory, and anti-melanogenic activities. J. Microencapsul. 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Kumar, M.N.V.R.; Reddy, G.B. Efficacy of Biodegradable Curcumin Nanoparticles in Delaying Cataract in Diabetic Rat Model. PLoS ONE 2013, 8, e78217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; An, F.-F.; Liu, J.; Jin, S.; Zhang, J.-C.; Wang, P.C.; Zhang, X.; Lee, C.-S.; Liang, X.-J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef]
- Chockalingam, S.; Thada, R.; Dhandapani, R.K.; Panchamoorthy, R. Biogenesis, characterization, and the effect of vicenin-gold nanoparticles on glucose utilization in 3T3-L1 adipocytes: A bioinformatic approach to illuminate its interaction with PTP 1B and AMPK. Biotechnol. Prog. 2015, 31, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar] [CrossRef]
- Salah, M.; Mansour, M.; Zogona, D.; Xu, X. Nanoencapsulation of anthocyanins-loaded β-lactoglobulin nanoparticles: Characterization, stability, and bioavailability in vitro. Food Res. Int. 2020, 137, 109635. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, J.; Mei, Z.; Luo, Z.; Ding, L.; Jiang, X.; Bai, W. Synthesis, structural characterization, and evaluation of cyanidin-3-O-glucoside-loaded chitosan nanoparticles. Food Chem. 2020, 330, 127239. [Google Scholar] [CrossRef]
- Liang, T.; Guan, R.; Shen, H.; Xia, Q.; Liu, M. Optimization of Conditions for Cyanidin-3-OGlucoside (C3G) Nanoliposome Production by Response Surface Methodology and Cellular Uptake Studies in Caco-2 Cells. Molecules 2017, 22, 457. [Google Scholar] [CrossRef]
- Verônica Cardoso de Souza, B.; de Morais Sousa, M.; Augusto Gasparotto Sattler, J.; Cristina Sousa Gramoza Vilarinho Santana, A.; Bruno Fonseca de Carvalho, R.; de Sousa Lima Neto, J.; de Matos Borges, F.; Angelica Neri Numa, I.; Braga Ribeiro, A.; César Cunha Nunes, L. Nanoencapsulation and bioaccessibility of polyphenols of aqueous extracts from Bauhinia forficata link. Food Chem. 2022, 5, 100144. [Google Scholar] [CrossRef]
- Kerbab, K.; Sansone, F.; Zaiter, L.; Esposito, T.; Celano, R.; Franceschelli, S.; Pecoraro, M.; Benayache, F.; Rastrelli, L.; Picerno, P.; et al. Halimium halimifolium: From the Chemical and Functional Characterization to a Nutraceutical Ingredient Design. Planta Med. 2019, 85, 1024–1033. [Google Scholar] [CrossRef]
- Zorzenon, M.R.T.; Formigoni, M.; da Silva, S.B.; Hodas, F.; Piovan, S.; Ciotta, S.R.; Jansen, C.A.; Dacome, A.S.; Pilau, E.J.; Mareze-Costa, C.E.; et al. Spray drying encapsulation of stevia extract with maltodextrin and evaluation of the physicochemical and functional properties of produced powders. J. Food Sci. 2020, 85, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.D.; Attanayake, A.P.; Karunaratne, D.N.; Arawwawala, L.; Pamunuwa, G.K. Bael (Aegle marmelos L. Correa) fruit extracts encapsulated alginate nanoparticles as a potential dietary supplement with improved bioactivities. J. Food Sci. 2023, 88, 4942–4961. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Tamrakar, A.K.; Mahajan, S.; Prasad, G. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef]
- Pandey, S.; Dvorakova, C.M. Future Perspective of Diabetic Animal Models. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef]
- Yao, Y.; Luong, T.N.; Lepik, M.; Aftab, N.; Fong, V.H.; Vieira, A. Synergism of Antioxidant Phytochemicals: Comparisons Among Purified Polyphenols and Dietary-Plant Extracts; International Society for Horticultural Science: Orlando, FL, USA, 2012; pp. 121–127. [Google Scholar]
- Revathi, G.; Elavarasi, S.; Saravanan, K.; Ashokkumar, M.; Egbuna, C. Greater efficiency of polyherbal drug encapsulated biosynthesized chitosan nano-biopolymer on diabetes and its complications. Int. J. Biol. Macromol. 2023, 240, 124445. [Google Scholar] [CrossRef]
- El-Hussien, D.; El-Zaafarany, G.M.; Nasr, M.; Sammour, O. Chrysin nanocapsules with dual anti-glycemic and anti-hyperlipidemic effects: Chemometric optimization, physicochemical characterization and pharmacodynamic assessment. Int. J. Pharm. 2021, 592, 120044. [Google Scholar] [CrossRef]
- Sudirman, S.; Lai, C.-S.; Yan, Y.-L.; Yeh, H.-I.; Kong, Z.-L. Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model. Sci. Rep. 2019, 9, 15233. [Google Scholar] [CrossRef]
- El-Shahawy, A.A.G.; Abdel-Moneim, A.; Ebeid, A.S.M.; Eldin, Z.E.; Zanaty, M.I. A novel layered double hydroxide-hesperidin nanoparticles exert antidiabetic, antioxidant and anti-inflammatory effects in rats with diabetes. Mol. Biol. Rep. 2021, 48, 5217–5232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, C.; Weng, W.; Bao, R.; Adu-Frimpong, M.; Toreniyazov, E.; Ji, H.; Xu, X.-M.; Yu, J. Enhancement of oral bioavailability and hypoglycemic activity of liquiritin-loaded precursor liposome. Int. J. Pharm. 2021, 592, 120036. [Google Scholar] [CrossRef]
- Foudah, A.I.; Ayman Salkini, M.; Alqarni, M.H.; Alam, A. Preparation and evaluation of antidiabetic activity of mangiferin-loaded solid lipid nanoparticles. Saudi J. Biol. Sci. 2024, 31, 103946. [Google Scholar] [CrossRef]
- Wang, Y.; Karmakar, T.; Ghosh, N.; Basak, S.; Gopal Sahoo, N. Targeting mangiferin loaded N-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis—A case study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022, 370, 131376. [Google Scholar] [CrossRef]
- Upadhyay, M.; Hosur, R.V.; Jha, A.; Bharti, K.; Mali, P.S.; Jha, A.K.; Mishra, B.; Kumar, A. Myricetin encapsulated chitosan nanoformulation for management of type 2 diabetes: Preparation, optimization, characterization and in vivo activity. Biomater. Adv. 2023, 153, 213542. [Google Scholar] [CrossRef]
- Joshi, R.; Laddha, A.P.; Kulkarni, Y.A.; Wairkar, S. Improved performance of naringenin herbosomes over naringenin in streptozotocin-induced diabetic rats: In vitro and in vivo evaluation. Asian Pac. J. Trop. Biomed. 2021, 11, 385–393. [Google Scholar] [CrossRef]
- Maity, S.; Chakraborti, A.S. Formulation, physico-chemical characterization and antidiabetic potential of naringenin-loaded poly D, L lactide-co-glycolide (N-PLGA) nanoparticles. Eur. Polym. J. 2020, 134, 109818. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Pathak, K.; Das, R.J.; Saikia, R.; Sarma, H.; Gogoi, N.; Gogoi, U.; Das, A.; Alasiri, A.S.; Abdel-Wahab, B.A.; et al. Design and optimization of quercetin-loaded polymeric Eudragit L-100 nanoparticles for anti-diabetic activity with improved oral delivery: In-vitro and in-vivo evaluation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2411–2428. [Google Scholar] [CrossRef]
- Chitkara, D.; Nikalaje, S.K.; Mittal, A.; Chand, M.; Kumar, N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv. Transl. Res. 2012, 2, 112–123. [Google Scholar] [CrossRef]
- Wang, S.; Du, S.; Wang, W.; Zhang, F. Therapeutic investigation of quercetin nanomedicine in a zebrafish model of diabetic retinopathy. Biomed. Pharmacother. 2020, 130, 110573. [Google Scholar] [CrossRef]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.-F. Biological investigations on therapeutic effect of chitosan encapsulated nano resveratrol against gestational diabetes mellitus rats induced by streptozotocin. J. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Benyelles, M.; Merzouk, H.; Merzouk, A.Z.; Imessaoudene, A.; Medjdoub, A.; Mebarki, A. Valorization of Encapsulated Coffee Parchment Extracts as Metabolic Control for High Fructose Diet-Induced Obesity, Using Wistar Rat as Animal Model. Waste Biomass Valori 2024, 15, 265–281. [Google Scholar] [CrossRef]
- Colorado, D.; Fernandez, M.; Orozco, J.; Lopera, Y.; Muñoz, D.L.; Acín, S.; Balcazar, N. Metabolic activity of anthocyanin extracts loaded into non-ionic niosomes in diet-induced obese mice. Pharm. Res. 2020, 37, 152. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Int. Food Res. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chen, B.-H. A comparative study on improving streptozotocin-induced type 2 diabetes in rats by hydrosol, extract and nanoemulsion prepared from cinnamon leaves. Antioxidants 2023, 12, 29. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Attanayake, A.P.; Kalansuriya, P. Gelatine nanoparticles encapsulating three edible plant extracts as potential nanonutraceutical agents against type 2 diabetes mellitus. J. Microencapsul. 2024, 41, 94–111. [Google Scholar] [CrossRef]
- Ammar, N.M.; Hassan, H.A.; Mohammed, M.A.; Serag, A.; Abd El-Alim, S.H.; Elmotasem, H.; El Raey, M.; El Gendy, A.N.; Sobeh, M.; Abdel-Hamid, A.-H.Z. Metabolomic profiling to reveal the therapeutic potency of Posidonia oceanica nanoparticles in diabetic rats. RSC Adv. 2021, 11, 8398–8410. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Protox3.0. Available online: https://tox-new.charite.de/protox3/index.php?site=home (accessed on 15 October 2025).
- Korobkova, E.A. Effect of Natural Polyphenols on CYP Metabolism: Implications for Diseases. Chem. Res. Toxicol. 2015, 28, 1359–1390. [Google Scholar] [CrossRef] [PubMed]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxidative Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Sprouse, A.A.; van Breemen, R.B. Pharmacokinetic Interactions between Drugs and Botanical Dietary Supplements. Drug Metab. Dispos. 2016, 44, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Jiang, W.; Zhang, C.; Yin, R.; Ouyang, Z.; Wei, Y. Effect of food components on cytochrome P450 expression and activity. Hum. Nutr. Metab. 2025, 40, 200304. [Google Scholar] [CrossRef]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, A.Y.; Kim, H.C.; Ryu, D.; Jo, S.A.; Jung, Y.-S. Effects of Natural Polyphenols on Oxidative Stress-Mediated Blood-Brain Barrier Dysfunction. Antioxidants 2022, 11, 197. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood–Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Singh, K.; Tarapcsák, S.; Gyöngy, Z.; Ritter, Z.; Batta, G.; Bosire, R.; Remenyik, J.; Goda, K. Effects of Polyphenols on P-Glycoprotein (ABCB1) Activity. Pharmaceutics 2021, 13, 2062. [Google Scholar] [CrossRef]
- Nabekura, T.; Kawasaki, T.; Furuta, M.; Kaneko, T.; Uwai, Y. Effects of Natural Polyphenols on the Expression of Drug Efflux Transporter P-Glycoprotein in Human Intestinal Cells. ACS Omega 2018, 3, 1621–1626. [Google Scholar] [CrossRef]
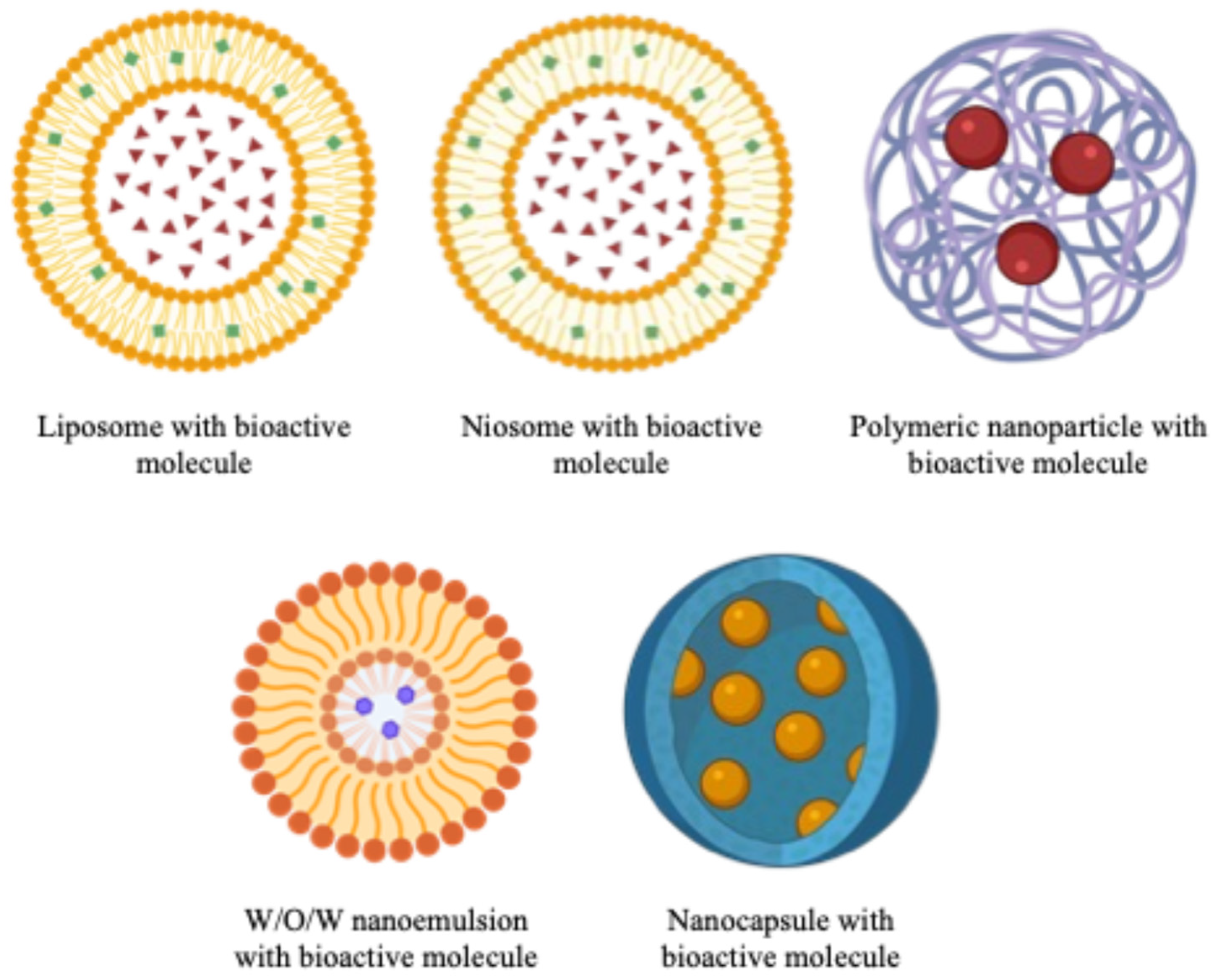
| Polyphenols Loaded | Nanosystem | Encapsulating Material | Technique Nanoencapsulation | Effect * | Ref. |
|---|---|---|---|---|---|
| Epigallocatechin-3-gallate | Nanoparticle | Bovine β-lactoglobulin | Co-assembled with preheated | ↑ Inhibition of proliferation of human malignant melanoma cells and esophageal carcinoma cells | [101] |
| Epigallocatechin-3-gallate | Nanoparticles | Succinyl-chitosan (modified chitosan), pentasodium tripolyphosphate | Ionic crosslinking | 65% nanoencapsulation efficiency | [102] |
| Propyl gallate | Nanoparticles | Succinyl-chitosan (modified chitosan), pentasodium tripolyphosphate | Ionic crosslinking | 27% nanoencapsulation efficiency | [102] |
| Gallic acid | Nanoparticles | Succinyl-chitosan (modified chitosan), pentasodium tripolyphosphate | Ionic crosslinking | 88% nanoencapsulation efficiency | [102] |
| Catechin | Nanoemulsion | Palm oil and sunflower oil | Nanoemulsification | Physically and chemically stable emulsions, with no significant variation in droplet diameter, conductivity, refractive index and pH | [103] |
| Catechin | Nanoemulsion | Ethyl oleate, the surfactant spans 80, and the cosurfactant trancutol CG | Nanoemulsification | ↑ skin permeability ↑ %relative bioavailability ↑ SOD, GPX and CAT ↓ TBRAS | [104] |
| Rutin | Nanoparticle | Bovine serum albumins | Nanospray drying | 32% nanoencapsulation efficiency ↑ antioxidant effect for ABTS radical | [94] |
| Quercetin | Nanoparticle | Bovine serum albumins and glutaraldehyde as a crosslinking agent | Desolvation | 85% nanoencapsulation efficiency ↑ antioxidant effect for ABTS radical | [105] |
| Quercetin | Nanoniosome | Surfactants (span 60 and 80, tween 60 and 80), polymers (polyethylene glycol, propylene glycol, glycerol, and cholesterol). | Thin-layer hydration combined with sonication | Tween 60/Span 60 showed better nanoencapsulation efficiency | [106] |
| Trans-Ferulic acid | Nanoparticle | Nanoparticle A: poly (lactic acid) Nanoparticle B: poly (lactic acid)/poly (lactic-co-glycolic acid) | Nanoprecipitation | 75% nanoencapsulation efficiency Controlled liberation in in vitro analysis No toxic effects at concentrations of 2.5 mg/ml | [107] |
| Chlorogenic acid | Nanoparticle | Chitosan, pentasodium tripolyphosphate | Ionic gelation | 59% nanoencapsulation efficiency 69% release after 100 h | [108,109] |
| Phloretin | Nanoparticle | Chitosan, sodium tripolyphosphate | Ionotropic gelation | ↑ mitochondrial-mediated apoptotic ↓ oxidative stress | [110] |
| Tea Polyphenol | Nanoparticle | Chitosan, sulfobutylether-β-cyclodextrin | Inclusion complexes | ↑ antioxidant activity Chemically stable nanocapsule | [111] |
| Phenolics of grape pomace | Nanocapsules | Chitosan, soy protein | Nanoemulsification | 95 and 75% nanoencapsulation efficiency ↑ antioxidant capacity | [112] |
| Phenolics of apple pomace | Nanocapsules | Chitosan, soy protein | Nanoemulsification | 95 and 75% nanoencapsulation efficiency ↑ antioxidant capacity | [112] |
| Olive leaf phenolics | Nanoparticle | Whey protein concentrate and tween 20 as surfactant | Electrospray | 232.3–659.8 nm nanoparticle size 0.074–0.65 Polydispersity index | [113] |
| Phenolics of pistachio hulls | Nanoliposome | Lecithin | Sonication | ↑ antioxidant capacity ↑ anti-inflammatory activity Anti-melanogenic activity | [114] |
| Oleuropin | Nanoemulsion | Soybean oil, span 80 (surfactant), whey protein concentrate, and pectin | Double emulsification | 91% nanoencapsulation efficiency 40.4% liberation | [115] |
| Curcumin | Nanoparticle | Polyvinyl alcohol, Poly(lactide-co-glycolic) acid | Modified emulsion- diffusion-evaporation method | ↑ bioavailability ↑ delay diabetic cataract in rats | [116] |
| Curcumin | Nanoparticle | Poly (maleic anhydride-alt-1-octadecene), poly (ethylene glycol)-amine and 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide | Sonication | ↑ dispersibility ↑ stability ↑ therapeutic efficiency | [117] |
| Vicenin-2 | Nanoparticle | Chloroauric acid | Ultrasonication | Cell viability 78.21% ↑ glucose uptake Strong interaction with PTP1B and AMPK | [118] |
| Sylbin | Nanoparticle | Chitosan, poly(lactide-co-glycolic) acid, pluronic F-127 | Solvent diffusion and polyelectrolyte deposition | 92.11% nanoencapsulation efficiency ↓ blood glucose ↓ glycosylated hemoglobin | [119] |
| Anthocyanin from raspberry | Nanoparticle | β-Lactoglobulin, N-(3-Dimethylaminopropyl)-N-ethyl carbodiimide hydrochloride (cross-linking) | Desolvation | 77% nanoencapsulation efficiency ↑ antioxidant activity ↑ stability, bioavailability | [120] |
| Cyanidin 3-O-Glucoside | Nanoparticle | Nanoparticle1: Chitosan, and PGA Nanoparticle 2: Chitosan oligosaccharide and polyglutamic acid Nanoparticle 3: Carboxymethyl chitosan, CaCl2 | Ionic crosslinking | 53.88% nanoencapsulation efficiency 75% release at pH 5.3 | [121] |
| Cyanidin 3-O-Glucoside | Nanoliposome | Phosphatidylcholine and cholesterol | Reverse-phase evaporation | Optimal conditions: concentration 0.17 mg/mL, temperature 41.41 °C, and relation 2.87 | [122] |
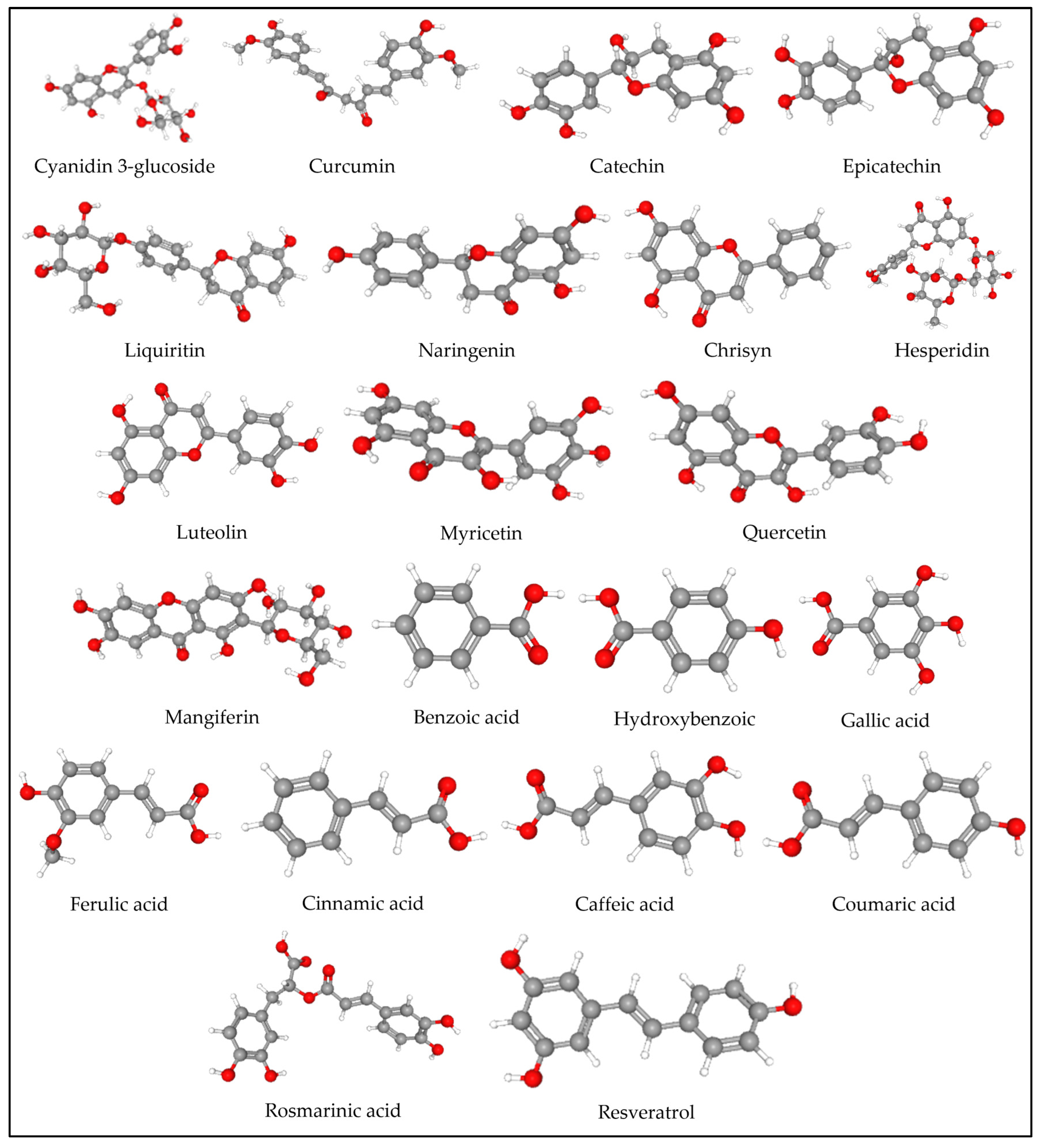
| Molecule | Class of Compound | PubChem CID | Chemical Formula | Molecular Weight | H Bond Donors | H Bond Acceptor | Log p | Lipinski Rule of 5 |
|---|---|---|---|---|---|---|---|---|
| Cyanidin 3-glucoside | Anthocyanin | 197081 | C21H21ClO11 | 484.8 | 8 | 11 | −1.5 | No |
| Curcumin | Curcuminoids | 969516 | C21H20O6 | 368.4 | 2 | 6 | 3.2 | Yes |
| (+)-Catechin | Flavanol | 9064 | C15H14O6 | 290.27 | 5 | 6 | 1.4 | Yes |
| (−)-Epicatechin | Flavanol | 72276 | C15H14O6 | 290.27 | 5 | 6 | 1.8 | Yes |
| Liquiritin | Flavanone | 503737 | C21H22O9 | 418.4 | 5 | 9 | 0.4 | Yes |
| Naringenin | Flavanone | 439246 | C15H12O5 | 272.25 | 3 | 5 | 2.2 | Yes |
| Chrysin | Flavone | 5281607 | C15H10O4 | 254.24 | 2 | 4 | 2.5 | Yes |
| Hesperidin | Flavone | 10621 | C28H34O15 | 610.6 | 8 | 15 | −1.1 | No |
| Luteolin | Flavone | 5280445 | C15H10O6 | 286.24 | 4 | 6 | 2.0 | Yes |
| Myricetin | Flavonol | 5281672 | C15H10O8 | 318.23 | 6 | 8 | 1.6 | No |
| Quercetin | Flavonol | 5280343 | C15H10O7 | 302.23 | 5 | 7 | 1.5 | Yes |
| Mangiferin | Glucosylxanthone | 5281647 | C19H18O11 | 422.3 | 8 | 11 | −0.4 | No |
| Benzoic acid | Hydroxybenzoic acid | 243 | C7H6O2 | 122.12 | 1 | 2 | 1.87 | Yes |
| Hydroxybenzoic acid | Hydroxybenzoic acid | 135 | C7H6O3 | 138.12 | 2 | 3 | 1.58 | yes |
| Gallic acid | Hydroxybenzoic acid | 370 | C7H6O5 | 170.12 | 4 | 5 | 0.7 | Yes |
| Ferulic acid | Hydroxycinnamic acid | 445858 | C10H10O4 | 194.18 | 2 | 4 | 1.5 | Yes |
| Cinnamic acid | Hydroxycinnamic acid | 444539 | C9H8O2 | 148.16 | 1 | 2 | 2.1 | Yes |
| Caffeic acid | Hydroxycinnamic acid | 689043 | C9H8O4 | 180.16 | 3 | 4 | 1.2 | Yes |
| Coumaric acid | Hydroxycinnamic acid | 637542 | C9H8O3 | 164.16 | 2 | 3 | 1.5 | Yes |
| Rosmarinic acid | Hydroxycinnamic acid | 5281792 | C18H16O8 | 360.3 | 5 | 8 | 2.4 | Yes |
| Resveratrol | Stilbene | 445154 | C14H12O3 | 228.24 | 3 | 3 | 3.1 | Yes |
| Molecule | Predicted LD50 (mg/kg) | Predicted Toxicity Class | Hepatotoxicity | Neuro Toxicity | Nephrotoxicity | Respiratory Toxicity | Cardiotoxicity | Carcinogenicity | Inmuno Toxicity | Mutagenicity | Cytotoxicity | Clinical Toxicity | Nutricional Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyanidin 3-glucoside | 5000 | 5 | Inactive | Inactive | Active | Active | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Active |
| Curcumin | 2000 | 4 | Inactive | Inactive | Active | Inactive | Active | Inactive | Active | Inactive | Inactive | Active | Inactive |
| (+)-Catechin | 10,000 | 6 | Inactive | Inactive | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Active | Active |
| (−)-Epicatechin | 10,000 | 6 | Inactive | Inactive | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Active | Active |
| Liquiritin | 2300 | 5 | Inactive | Inactive | Active | Active | Inactive | Inactive | Active | Inactive | Inactive | Active | Active |
| Naringenin | 2000 | 4 | Inactive | Inactive | Active | Active | Inactive | Inactive | Inactive | Inactive | Active | Active | Active |
| Chrysin | 3919 | 5 | Inactive | Inactive | Active | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Hesperidin | 12,000 | 6 | Inactive | Inactive | Active | Active | Inactive | Inactive | Active | Inactive | Inactive | Active | Active |
| Luteolin | 3919 | 5 | Inactive | Inactive | Active | Active | Inactive | Active | Inactive | Active | Inactive | Inactive | Active |
| Myricetin | 159 | 3 | Inactive | Inactive | Active | Active | Inactive | Active | Inactive | Active | Inactive | Inactive | Active |
| Quercetin | 159 | 3 | Inactive | Inactive | Active | Active | Inactive | Active | Inactive | Active | Inactive | Inactive | Active |
| Mangiferin | 2 | 1 | Inactive | Inactive | Active | Active | Inactive | Inactive | Active | Active | Inactive | Active | Active |
| Benzoic acid | 290 | 3 | Active | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Hydroxybenzoic acid | 2200 | 5 | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Gallic acid | 2000 | 4 | Inactive | Inactive | Active | Active | Inactive | Active | Inactive | Inactive | Inactive | Active | Inactive |
| Ferulic acid | 1772 | 4 | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Active | Inactive | Inactive | Active | Inactive |
| Cinnamic acid | 2500 | 5 | Active | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Caffeic acid | 2980 | 5 | Inactive | Inactive | Active | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Active | Inactive |
| Coumaric acid | 2850 | 5 | Inactive | Inactive | Active | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive |
| Rosmarinic acid | 5000 | 5 | Inactive | Inactive | Active | Inactive | Inactive | Inactive | Active | Inactive | Inactive | Active | Inactive |
| Resveratrol | 1560 | 4 | Inactive | Inactive | Active | Inactive | Active | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Predicted toxicity class |  | ||||||||||||
| Molecule | Inhibitor | ||||||
|---|---|---|---|---|---|---|---|
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | CYP2B6 | CYP2C8 | |
| Cyanidin 3-glucoside | No | No | No | No | No | No | Yes |
| Curcumin | No | No | Yes | No | No | Yes | Yes |
| (+)-Catechin | No | No | No | No | No | No | Yes |
| (−)-Epicatechin | No | No | No | No | No | Yes | Yes |
| Liquiritin | No | No | No | No | No | No | No |
| Naringenin | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Chrysin | Yes | No | No | Yes | Yes | Yes | Yes |
| Hesperidin | No | No | No | No | No | No | No |
| Luteolin | Yes | No | No | Yes | Yes | Yes | Yes |
| Myricetin | Yes | No | Yes | No | Yes | No | Yes |
| Quercetin | Yes | No | No | No | Yes | No | Yes |
| Mangiferin | No | No | No | No | No | No | Yes |
| Benzoic acid | No | No | No | No | No | No | No |
| Hydroxybenzoic acid | No | No | No | No | No | No | No |
| Gallic acid | No | No | No | No | No | No | No |
| Ferulic acid | No | No | No | No | No | No | Yes |
| Cinnamic acid | No | No | No | No | No | No | Yes |
| Caffeic acid | No | No | No | No | No | No | Yes |
| Coumaric acid | No | No | No | No | No | No | Yes |
| Rosmarinic acid | No | No | No | No | No | No | No |
| Resveratrol | Yes | No | No | No | Yes | No | Yes |
| Molecule | HLM Stability * | BBB * | P-gp Inhibitor * | GI Absorption * |
|---|---|---|---|---|
| Cyanidin 3-glucoside | No | No | No | Low |
| Curcumin | Yes | No | No | High |
| (+)-Catechin | No | No | No | High |
| (−)-Epicatechin | No | No | No | High |
| Liquiritin | No | No | No | Low |
| Naringenin | Yes | No | Yes | High |
| Chrysin | Yes | No | No | High |
| Hesperidin | No | No | No | Low |
| Luteolin | Yes | No | No | High |
| Myricetin | Yes | No | No | Low |
| Quercetin | Yes | No | No | High |
| Mangiferin | No | No | No | Low |
| Benzoic acid | No | No | No | High |
| Hydroxybenzoic acid | No | No | No | High |
| Gallic acid | No | No | No | High |
| Ferulic acid | No | No | No | High |
| Cinnamic acid | No | No | No | High |
| Caffeic acid | No | No | No | High |
| Coumaric acid | No | No | No | High |
| Rosmarinic acid | No | No | No | Low |
| Resveratrol | No | No | No | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanillas-Ponce de León, R.; Cardenas-Torres, F.I.; Ontiveros, N.; Contreras-Angulo, L.A.; Elisande-Romero, C.A.; Leyva-López, N.; Bernal-Millán, M.d.J.; Heredia, J.B.; Gutiérrez-Grijalva, E.P. Advancements in Encapsulation Technologies: The Potential of Polyphenols as an Antidiabetic Therapy. Sci. Pharm. 2025, 93, 55. https://doi.org/10.3390/scipharm93040055
Cabanillas-Ponce de León R, Cardenas-Torres FI, Ontiveros N, Contreras-Angulo LA, Elisande-Romero CA, Leyva-López N, Bernal-Millán MdJ, Heredia JB, Gutiérrez-Grijalva EP. Advancements in Encapsulation Technologies: The Potential of Polyphenols as an Antidiabetic Therapy. Scientia Pharmaceutica. 2025; 93(4):55. https://doi.org/10.3390/scipharm93040055
Chicago/Turabian StyleCabanillas-Ponce de León, Rigoberto, Feliznando Isidro Cardenas-Torres, Noe Ontiveros, Laura Aracely Contreras-Angulo, Cristina Alicia Elisande-Romero, Nayely Leyva-López, Manuel de Jesús Bernal-Millán, Jose Basilio Heredia, and Erick Paul Gutiérrez-Grijalva. 2025. "Advancements in Encapsulation Technologies: The Potential of Polyphenols as an Antidiabetic Therapy" Scientia Pharmaceutica 93, no. 4: 55. https://doi.org/10.3390/scipharm93040055
APA StyleCabanillas-Ponce de León, R., Cardenas-Torres, F. I., Ontiveros, N., Contreras-Angulo, L. A., Elisande-Romero, C. A., Leyva-López, N., Bernal-Millán, M. d. J., Heredia, J. B., & Gutiérrez-Grijalva, E. P. (2025). Advancements in Encapsulation Technologies: The Potential of Polyphenols as an Antidiabetic Therapy. Scientia Pharmaceutica, 93(4), 55. https://doi.org/10.3390/scipharm93040055











