Modulation of Antimicrobial Peptide–Membrane Interactions by Lysyl-Phosphatidylglycerol in Staphylococcus aureus: An FTIR Spectroscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
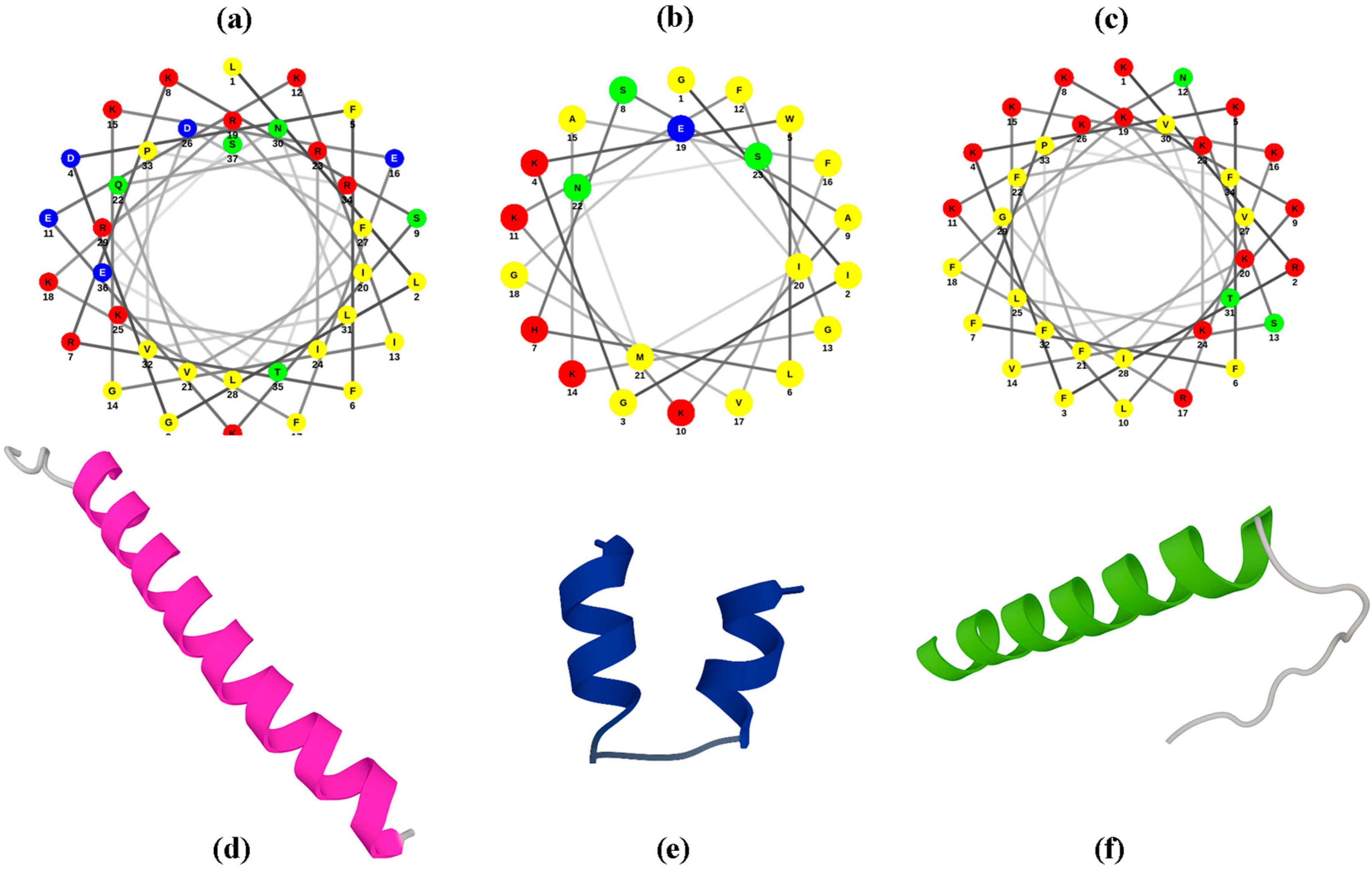
2.2. Prediction of the 3D Structure of Peptides Under Study
2.3. Phase Transition Measurements by Infrared Spectroscopy
3. Results
3.1. Prediction of the 3D Structure of the Peptides
3.2. Phase Transition Experiments by Infrared Spectroscopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| lysyl-PG | Lysyl-phosphatidylglycerol |
| AMP(s) | Antimicrobial peptide(s) |
| F5WMag | F5W Magainin |
| NA | NA-CATH:ATRA-1-ATRA-1 |
| FTIR | Fourier-transform infrared spectroscopy |
| S. aureus | Staphylococcus aureus |
| CL | Cardiolipin |
| STX | Staphyloxanthin |
| PG | Phosphatidylglycerol |
| DMPG | 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MprF | Multiple Peptide Resistance Factor |
| 3adLPG | Synthetic analog of lysyl-phosphatidylglycerol |
| νsCH2 | Symmetric stretching vibration of methylene groups |
| νC=O | Carbonyl stretching vibration |
| νasPO2− | Asymmetric stretching vibration of phosphate groups |
| Tm | Main transition temperature |
| Lβ–Lα | Gel phase (Lβ)–Liquid crystalline phase (Lα) |
| NMR | Nuclear magnetic resonance |
| EC50 | Half-maximal effective concentration |
| CD | Circular dichroism |
References
- Joyce, G.; Hammond, R.; White, D.C. Changes in membrane lipid composition in exponentially growing Staphylococcus aureus during the shift from 37 to 25 C. J. Bacteriol. 1970, 104, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Rivera, N.; Múnera-Jaramillo, J.; Jaramillo-Berrio, S.; Suesca, E.; Manrique-Moreno, M.; Leidy, C. Cardiolipin strongly inhibits the leakage activity of the short antimicrobial peptide atra-1 in comparison to ll-37, in model membranes mimicking the lipid composition of Staphylococcus aureus. Membranes 2023, 13, 304. [Google Scholar] [CrossRef]
- Rocha-Roa, C.; Orjuela, J.D.; Leidy, C.; Cossio, P.; Aponte-Santamaria, C. Cardiolipin prevents pore formation in phosphatidylglycerol bacterial membrane models. FEBS Lett. 2021, 595, 2701–2714. [Google Scholar] [CrossRef] [PubMed]
- Elmesseri, R.A.; Saleh, S.E.; Elsherif, H.M.; Yahia, I.S.; Aboshanab, K.M. Staphyloxanthin as a potential novel target for deciphering promising anti-Staphylococcus aureus agents. Antibiotics 2022, 11, 298. [Google Scholar] [CrossRef]
- Leidy, C.; Perez, M.I.; Méndez Reina, R.M.; Trier, S.; Herrfurth, C.; Lopez, G.-D.; Carazzone, C.; Feussner, I.; Bernal, A.; Forero-Shelton, M.; et al. Carotenoid Content and Composition in Exponential, Stationary and Biofilm States of Staphylococcus aureus and their Influence on Membrane Biophysical Properties. Biophys. J. 2020, 118, 321a. [Google Scholar] [CrossRef]
- Múnera-Jaramillo, J.; López, G.-D.; Suesca, E.; Carazzone, C.; Leidy, C.; Manrique-Moreno, M. The role of staphyloxanthin in the regulation of membrane biophysical properties in Staphylococcus aureus. Biochim. Biophys. Acta (BBA)-Biomembr. 2024, 1866, 184288. [Google Scholar] [CrossRef]
- Vásquez, A.; Leidy, C.; Manrique-Moreno, M. Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity. Antibiotics 2025, 14, 349. [Google Scholar] [CrossRef]
- Houtsmuller, U.; Van Deenen, L. On the accumulation of amino acid derivates of phosphatidylglycerol in bacteria. Biochim. Biophys. Acta (BBA)-Spec. Sect. Lipids Relat. Subj. 1964, 84, 96–98. [Google Scholar] [CrossRef][Green Version]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 2009, 1788, 2092–2100. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ramanathan, A.; Lopez, C.F. Cardiolipin-dependent properties of model mitochondrial membranes from molecular simulations. Biophys. J. 2019, 117, 429–444. [Google Scholar] [CrossRef]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: Implications for daptomycin resistance. Antimicrob. Agents Chemother. 2010, 54, 4476–4479. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.-J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Pept. Sci. 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Felsztyna, I.; Galassi, V.V.; Wilke, N. Selectivity of membrane-active peptides: The role of electrostatics and other membrane biophysical properties. Biophys. Rev. 2025, 17, 591–604. [Google Scholar] [CrossRef]
- Biri-Kovács, B.; Adorján, A.; Szabó, I.; Szeder, B.; Bősze, S.; Mező, G. Structure–Activity Relationship of HER2 Receptor Targeting Peptide and Its Derivatives in Targeted Tumor Therapy. Biomolecules 2020, 10, 183. [Google Scholar] [CrossRef]
- Maupetit, J.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An online resource for de novo peptide structure prediction. Nucleic Acids Res. 2009, 37, W498–W503. [Google Scholar] [CrossRef]
- Mol, A.R.; Castro, M.S.; Fontes, W. NetWheels: A web application to create high quality peptide helical wheel and net projections. bioRxiv 2018. bioRxiv: 416347. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.; Sudheendra, U.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.n.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef]
- Lee, C.-C.; Sun, Y.; Qian, S.; Huang, H.W. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys. J. 2011, 100, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Zhao, H.; Gan, T.-X.; Liu, X.-D.; Jin, Y.; Lee, W.-H.; Shen, J.-H.; Zhang, Y. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides 2008, 29, 1685–1691. [Google Scholar] [CrossRef]
- de Latour, F.A.; Amer, L.S.; Papanstasiou, E.A.; Bishop, B.M.; van Hoek, M.L. Antimicrobial activity of the Naja atra cathelicidin and related small peptides. Biochem. Biophys. Res. Commun. 2010, 396, 825–830. [Google Scholar] [CrossRef]
- Dean, S.N.; Bishop, B.M.; Van Hoek, M.L. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 2011, 11, 114. [Google Scholar] [CrossRef]
- Rehal, R.; Gaffney, P.R.; Hubbard, A.T.; Barker, R.D.; Harvey, R.D. The pH-dependence of lipid-mediated antimicrobial peptide resistance in a model staphylococcal plasma membrane: A two-for-one mechanism of epithelial defence circumvention. Eur. J. Pharm. Sci. 2019, 128, 43–53. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. Fourier transform infrared spectroscopy in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Membr. Lipids 2007, 400, 207–226. [Google Scholar]
- Islam, M.M.; Nawagamuwage, S.U.; Parshin, I.V.; Richard, M.C.; Burin, A.L.; Rubtsov, I.V. Probing the Hydrophobic Region of a Lipid Bilayer at Specific Depths Using Vibrational Spectroscopy. J. Am. Chem. Soc. 2023, 145, 26363–26373. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, D.; Ivica, J.; Separovic, F.; De Planque, M.R. Characterisation of cell membrane interaction mechanisms of antimicrobial peptides by electrical bilayer recording. Biophys. Chem. 2022, 281, 106721. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Blume, A.; Hübner, W. A Fourier transform infrared spectroscopic study of the interaction of alkaline earth cations with the negatively charged phospholipid 1, 2-dimyristoyl-sn-glycero-3-phosphoglycerol. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1466, 245–259. [Google Scholar] [CrossRef]
- Mendelsohn, R.; Moore, D.J. Vibrational spectroscopic studies of lipid domains in biomembranes and model systems. Chem. Phys. Lipids 1998, 96, 141–157. [Google Scholar] [CrossRef]
- Dhanikula, A.B.; Panchagnula, R. Fluorescence anisotropy, FT-IR spectroscopy and 31-P NMR studies on the interaction of paclitaxel with lipid bilayers. Lipids 2008, 43, 569–579. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A. Interactions at the lipid–water interface. Chem. Phys. Lipids 1998, 96, 99–123. [Google Scholar] [CrossRef]
- Nibali, V.C.; Branca, C.; Wanderlingh, U.; Verduci, R.; Bonaccorso, E.; Ciccolo, A.; D’Angelo, G. Insights on Hydrogen Bond Network of Water in Phospholipid Membranes: An Infrared Study at Varying Hydration. Membranes 2025, 15, 46. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.; Kondejewski, L.H.; Hodges, R.S.; McElhaney, R.N. Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1417, 211–223. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, Z.; Bian, Y.; Hu, G.; Wang, J.; Zhou, Y. Molecular dynamics simulations of human antimicrobial peptide LL-37 in model POPC and POPG lipid bilayers. Int. J. Mol. Sci. 2018, 19, 1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Al-Mualem, Z.A.; Baiz, C.R. Lipid landscapes: Vibrational spectroscopy for decoding membrane complexity. Annu. Rev. Phys. Chem. 2024, 75, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, H.; McElhaney, R. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Watanabe, N.; Suga, K.; Umakoshi, H. Functional hydration behavior: Interrelation between hydration and molecular properties at lipid membrane interfaces. J. Chem. 2019, 2019, 4867327. [Google Scholar] [CrossRef]
- Pérez, H.A.; Cejas, J.d.P.; Rosa, A.S.; Gimenez, R.E.; Disalvo, E.A.; Frias, M.A. Modulation of interfacial hydration by carbonyl groups in lipid membranes. Langmuir 2020, 36, 2644–2653. [Google Scholar] [CrossRef]
- Goñi, F.M.; Arrondo, J.L. A study of phospholipid phosphate groups in model membranes by Fourier transform infrared spectroscopy. Faraday Discuss. Chem. Soc. 1986, 81, 117–126. [Google Scholar] [CrossRef]
- Arrondo, J.L.R.; Goni, F.M. Infrared studies of protein-induced perturbation of lipids in lipoproteins and membranes. Chem. Phys. Lipids 1998, 96, 53–68. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. The structure and organization of phospholipid bilayers as revealed by infrared spectroscopy. Chem. Phys. Lipids 1998, 96, 9–21. [Google Scholar] [CrossRef]
- Popova, A.V.; Hincha, D.K. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: A Fourier transform infrared spectroscopy study. Biophys. J. 2003, 85, 1682–1690. [Google Scholar] [CrossRef]
- Güler, G.; Gärtner, R.M.; Ziegler, C.; Mäntele, W. Lipid-protein interactions in the regulated betaine symporter BetP probed by infrared spectroscopy. J. Biol. Chem. 2016, 291, 4295–4307. [Google Scholar] [CrossRef] [PubMed]
- Pašalić, L.; Pem, B.; Jakas, A.; Čikoš, A.; Groznica, N.; Mlinarić, T.; Accorsi, M.; Mangiarotti, A.; Dimova, R.; Bakarić, D. Peptide-induced hydration of lipid bilayers modulates packing pattern and conformations of hydrocarbon chains–A potential pathway for peptide translocation? bioRxiv 2025. bioRxiv: 2025.2002. 2004.636480.. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Binder, H.; Zschörnig, O. The effect of metal cations on the phase behavior and hydration characteristics of phospholipid membranes. Chem. Phys. Lipids 2002, 115, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Mishra, N.N.; Rubio, A.; Bayer, A.S. Causal role of single nucleotide polymorphisms within the mprF gene of Staphylococcus aureus in daptomycin resistance. Antimicrob. Agents Chemother. 2013, 57, 5658–5664. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Bonucci, A.; Caldaroni, E.; Balducci, E.; Pogni, R. A spectroscopic study of the aggregation state of the human antimicrobial peptide LL-37 in bacterial versus host cell model membranes. Biochemistry 2015, 54, 6760–6768. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides 2010, 31, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Nishida, M.; Matsuzaki, K. Action mechanism of PEGylated magainin 2 analogue peptide. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2578–2585. [Google Scholar] [CrossRef]
- Balleza, D. Peptide flexibility and the hydrophobic moment are determinants to evaluate the clinical potential of magainins. J. Membr. Biol. 2023, 256, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Henzler-Wildman, K.A.; Martinez, G.V.; Brown, M.F.; Ramamoorthy, A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry 2004, 43, 8459–8469. [Google Scholar] [CrossRef]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 2160–2172. [Google Scholar] [CrossRef]
- Majewska, M.; Zamlynny, V.; Pieta, I.S.; Nowakowski, R.; Pieta, P. Interaction of LL-37 human cathelicidin peptide with a model microbial-like lipid membrane. Bioelectrochemistry 2021, 141, 107842. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Sevcsik, E.; Pabst, G.; Richter, W.; Danner, S.; Amenitsch, H.; Lohner, K. Interaction of LL-37 with model membrane systems of different complexity: Influence of the lipid matrix. Biophys. J. 2008, 94, 4688–4699. [Google Scholar] [CrossRef] [PubMed]
- Henzler Wildman, K.A.; Lee, D.-K.; Ramamoorthy, A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Shahmiri, M.; Enciso, M.; Adda, C.G.; Smith, B.J.; Perugini, M.A.; Mechler, A. Membrane core-specific antimicrobial action of cathelicidin LL-37 peptide switches between pore and nanofibre formation. Sci. Rep. 2016, 6, 38184. [Google Scholar] [CrossRef]
- Kristian, S.A.; Datta, V.; Weidenmaier, C.; Kansal, R.; Fedtke, I.; Peschel, A.; Gallo, R.L.; Nizet, V. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 2005, 187, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.M.; Peschel, A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011, 80, 290–299. [Google Scholar] [CrossRef]
- Saravanan, R.; Li, X.; Lim, K.; Mohanram, H.; Peng, L.; Mishra, B.; Basu, A.; Lee, J.M.; Bhattacharjya, S.; Leong, S.S.J. Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnol. Bioeng. 2014, 111, 37–49. [Google Scholar] [CrossRef]
- Imura, Y.; Choda, N.; Matsuzaki, K. Magainin 2 in action: Distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys. J. 2008, 95, 5757–5765. [Google Scholar] [CrossRef]
- Wenk, M.R.; Seelig, J. Magainin 2 amide interaction with lipid membranes: Calorimetric detection of peptide binding and pore formation. Biochemistry 1998, 37, 3909–3916. [Google Scholar] [CrossRef]
- Rehal, R.P.; Marbach, H.; Hubbard, A.T.; Sacranie, A.A.; Sebastiani, F.; Fragneto, G.; Harvey, R.D. The influence of mild acidity on lysyl-phosphatidylglycerol biosynthesis and lipid membrane physico-chemical properties in methicillin-resistant Staphylococcus aureus. Chem. Phys. Lipids 2017, 206, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Wölk, C.; Giselbrecht, J.; Chan, K.A.; Harvey, R.D. A combined FTIR and DSC study on the bilayer-stabilising effect of electrostatic interactions in ion paired lipids. Colloids Surf. B Biointerfaces 2018, 169, 298–304. [Google Scholar] [CrossRef]
- Wölk, C.; Hause, G.; Gutowski, O.; Harvey, R.D.; Brezesinski, G. Enhanced chain packing achieved via putative headgroup ion-triplet formation in binary anionic lipid/cationic surfactant mixed monolayers. Chem. Phys. Lipids 2019, 225, 104827. [Google Scholar] [CrossRef]
- Rehal, R.; Barker, R.D.; Lu, Z.; Bui, T.T.; Demé, B.; Hause, G.; Wölk, C.; Harvey, R.D. Lipid domain formation and non-lamellar structures associated with varied lysylphosphatidylglycerol analogue content in a model Staphylococcal plasma membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183571. [Google Scholar] [CrossRef]
- Wölk, C.; Youssef, H.; Guttenberg, T.; Marbach, H.; Vizcay-Barrena, G.; Shen, C.; Brezesinski, G.; Harvey, R.D. Phase Diagram for a Lysyl-Phosphatidylglycerol Analogue in Biomimetic Mixed Monolayers with Phosphatidylglycerol: Insights into the Tunable Properties of Bacterial Membranes. ChemPhysChem 2020, 21, 702–706. [Google Scholar] [CrossRef]
- Sani, M.-A.; Whitwell, T.; Gehman, J.; Robins-Browne, R.; Pantarat, N.; Attard, T.; Reynolds, E.; O’Brien-Simpson, N.; Separovic, F. Maculatin 1.1 disrupts Staphylococcus aureus lipid membranes via a pore mechanism. Antimicrob. Agents Chemother. 2013, 57, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.-A.; Separovic, F. How membrane-active peptides get into lipid membranes. Acc. Chem. Res. 2016, 49, 1130–1138. [Google Scholar] [CrossRef] [PubMed]




| Peptide | Sequence | Charge | Hydrophobicity (%) | MIC (μM) | (μM) | Ref |
|---|---|---|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 | 37.8 | 5.99 | 0.25 | [21,29] |
| F5W Mag | GIGKWLHSAKKFGKAFVGEIMNS | +3 | 43.5 | 6.49 | - | [30] |
| NA | KRFKKFFKKLKNSVKKRFKKFFKKLKVIGVTFPF | +15 | 44.1 | - | 0.09 | [29] |
| Tm (°C) | |||||
|---|---|---|---|---|---|
| Peptide | Concentration (mol%) | DMPG:CL (80:20) | (72:18:10) | DMPG:CL:Lysyl-PG (64:16:20) | (56:14:30) |
| LL-37 | 0 | 28.76 ± 0.15 | 35.65 ± 0.14 | 38.57 ± 0.13 | 43.20 ± 0.21 |
| 1 | 30.32 ± 0.23 | 34.99 ± 0.24 | 38.06 ± 0.28 | 43.12 ± 0.17 | |
| 5 | 29.82 ± 0.31 | 36.40 ± 0.30 | 37.33 ± 0.37 | 41.71 ± 0.12 | |
| 10 | 26.02 ± 0.55 | 36.52 ± 0.20 | 41.12 ± 0.34 | 40.64 ± 0.12 | |
| F5W Mag | 0 | 28.76 ± 0.15 | 35.65 ± 0.14 | 38.57 ± 0.13 | 43.20 ± 0.21 |
| 1 | 29.53 ± 0.13 | 32.99 ± 0.12 | 40.01 ± 0.20 | 37.56 ± 0.11 | |
| 5 | 28.27 ± 0.11 | 33.36 ± 0.12 | 36.32 ± 0.11 | 34.12 ± 0.20 | |
| 10 | 27.77 ± 0.33 | 35.43 ± 0.10 | 38.73 ± 0.07 | 44.24 ± 0.17 | |
| NA | 0 | 28.76 ± 0.15 | 35.65 ± 0.14 | 38.57 ± 0.13 | 43.20 ± 0.21 |
| 1 | 28.84 ± 0.11 | 34.86 ± 0.09 | 39.47 ± 0.09 | 43.12 ± 0.17 | |
| 5 | 29.32 ± 0.09 | 35.27 ± 0.08 | 40.79 ± 0.11 | 41.71 ± 0.12 | |
| 10 | 28.48 ± 0.12 | 34.55 ± 0.07 | 41.20 ± 0.10 | 40.64 ± 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, A.; Echeverri-Gaviria, S.; Manrique-Moreno, M. Modulation of Antimicrobial Peptide–Membrane Interactions by Lysyl-Phosphatidylglycerol in Staphylococcus aureus: An FTIR Spectroscopy Study. Sci. Pharm. 2025, 93, 49. https://doi.org/10.3390/scipharm93040049
Vásquez A, Echeverri-Gaviria S, Manrique-Moreno M. Modulation of Antimicrobial Peptide–Membrane Interactions by Lysyl-Phosphatidylglycerol in Staphylococcus aureus: An FTIR Spectroscopy Study. Scientia Pharmaceutica. 2025; 93(4):49. https://doi.org/10.3390/scipharm93040049
Chicago/Turabian StyleVásquez, Andrea, Sofía Echeverri-Gaviria, and Marcela Manrique-Moreno. 2025. "Modulation of Antimicrobial Peptide–Membrane Interactions by Lysyl-Phosphatidylglycerol in Staphylococcus aureus: An FTIR Spectroscopy Study" Scientia Pharmaceutica 93, no. 4: 49. https://doi.org/10.3390/scipharm93040049
APA StyleVásquez, A., Echeverri-Gaviria, S., & Manrique-Moreno, M. (2025). Modulation of Antimicrobial Peptide–Membrane Interactions by Lysyl-Phosphatidylglycerol in Staphylococcus aureus: An FTIR Spectroscopy Study. Scientia Pharmaceutica, 93(4), 49. https://doi.org/10.3390/scipharm93040049








