Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Isolation of Compounds
2.3. Solid-Phase Extraction (SPE) for PAs/PANOs Enrichment
2.4. UHPLC-ESI-Q-TOF–MS and MS/MS Analysis
2.5. Data Processing
2.6. Molecular Networking and Chemical Dereplication
2.7. Total Phenolic Content (TPC) and Flavonoid Content (TFC) Assays
2.8. Biological Activity Assays
2.8.1. Antioxidant Assays
2.8.2. Enzyme Inhibition Assays
3. Results
3.1. Analysis of AC Extract
3.2. Molecular Network
3.3. LC–MS Analysis of PAs/PANOs Fraction
3.4. TPC and TFC
3.5. Biological Activity
3.5.1. Antioxidant Activity Assays
3.5.2. Enzyme Inhibitory Activity Assays
4. Discussion
4.1. PCs Analysis
4.1.1. Hydroxycinnamic Acid Derivatives
Coumaroyl Derivatives
Caffeoyl Derivatives
- Monomers
- Dimers
- Trimers
- Tetramers
4.1.2. Flavonoids and Flavonoid Glycosides
Kaempferol Derivatives
Quercetin Derivatives
Other Flavonoid Derivatives
4.2. Molecular Network
4.3. PA Analysis
4.4. Evaluation of TPC and TFC
4.5. Evaluation of Biological Activity
4.5.1. Antioxidant Activity
4.5.2. Enzyme Inhibitory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alkanna corcyrensis |
| PCs | Phenolic compounds |
| PAs | Pyrrolizidine alkaloids |
| PANOs | Pyrrolizidine alkaloid-N-oxides |
| SPE | Solid-phase extraction |
| ACN | Acetonitrile |
| FA | Formic acid |
| HILIC | Hydrophilic Interaction Liquid Chromatography |
| PLAT | Platynecine type alkaloid |
| RET | Retronecine type alkaloid |
| RETNO | Respective N-oxides type alkaloid |
| TRA | Trachelanthamidine type alkaloid |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| ABTS | 2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid |
| TE | Trolox Equivalents |
| RE | Rutin Equivalents |
| GAE | Gallic Acid Equivalents |
| UHPLC-ESI-Q-TOF–MS/MS | Ultra-High-Performance Liquid Chromatography-Electrospray Ionization-Quadrupole Time-of-Flight Tandem Mass Spectrometry |
| CUPRAC | Cupric Ion Reducing Antioxidant Capacity |
| FRAP | Ferric Reducing Antioxidant Power |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| FBMN | Feature-based Molecular Network |
| GNPS | Global Natural Products Social Molecular Networking |
References
- World Flora Online WFO Plant List. World Flora Online. Taxon: WFO 7000000079. Available online: https://wfoplantlist.org/taxon/wfo-4000001217-2023-12 (accessed on 5 January 2025).
- Strid, A. Alkanna Tausch. In Mountain Flora of Greece, 2nd ed.; Strid, A., Tan, K., Eds.; Edinburgh University Press: Edinburgh, UK, 1991; pp. 39–41. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013; p. 372. [Google Scholar]
- Hayek, A. Prodromus Florae Peninsulae Balcanicae. 2. Band: Dicotyledoneae Sympetalae. In Repertorium Specierum Novarum Regni Vegetabilis Beihefte; Verlag des Repertoriums: Berlin, Germany, 1928; Volume 30, p. 71. [Google Scholar]
- Abdel-Gelil, O.E.; Atwa, N.A.; Moustafa, A.R.A.; Mansour, S.R. Alkanna Species: A Promising Herbal Medicine and Its Uses. J. Food Sci. Nutr. Res. 2018, 2, 309–315. [Google Scholar] [CrossRef]
- Nikolova, M.; Aneva, I.; Zhelev, P.; Semerdjieva, I.; Zheljazkov, V.D.; Vladimirov, V.; Stoyanov, S.; Berkov, S.; Yankova-Tsvetkova, E. Metabolic Profiles, Genetic Diversity, and Genome Size of Bulgarian Population of Alkanna tinctoria. Plants 2022, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Papageorgiou, V.P. Radical Scavenging Activity of Alkanna tinctoria Root Extracts and Their Main Constituents, Hydroxynaphthoquinones. Phytother. Res. 2005, 19, 141–147. [Google Scholar] [CrossRef]
- Khan, U.A.; Rahman, H.; Qasim, M.; Hussain, A.; Azizllah, A.; Murad, W.; Khan, Z.; Anees, M.; Adnan, M. Alkanna tinctoria Leaves Extracts: A Prospective Remedy against Multidrug Resistant Human Pathogenic Bacteria. BMC Complement. Altern. Med. 2015, 15, 127. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Karapanagiotis, I.; Vasiliou, A.; Kokkini, S.; Papageorgiou, V.P. Analysis of Alkannin Derivatives from Alkanna Species by High-Performance Liquid Chromatography/Photodiode Array/Mass Spectrometry. Biomed. Chromatogr. 2006, 20, 1359–1374. [Google Scholar] [CrossRef]
- Tappeiner, J.; Vasiliou, A.; Ganzera, M.; Fessas, D.; Stuppner, H.; Papageorgiou, V.P.; Assimopoulou, A.N. Quantitative Determination of Alkannins and Shikonins in Endemic Mediterranean Alkanna Species. Biomed. Chromatogr. 2014, 28, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Ezzaitouni, M.; Chileh-Chelh, T.; Rincón-Cervera, M.Á.; Gómez-Mercado, F.; Benteima, H.; López-Ruiz, R.; Guil-Guerrero, J.L. Biocompounds and Bioactivities of Selected Greek Boraginaceae Seeds. Appl. Sci. 2024, 14, 6026. [Google Scholar] [CrossRef]
- Ganos, C.; Zengin, G.; Chinou, I.; Aligiannis, N.; Graikou, K. Phytochemical Profiling and Biological Assessment of the Aerial Parts from Three Mediterranean Alkanna Species (A. orientalis, A. tinctoria, A. kotschyana) in the Boraginaceae Family. Plants 2024, 13, 278. [Google Scholar] [CrossRef]
- ElSohly, H.; El-Feraly, F.; Joshi, A.; Walker, L. Antiviral Flavonoids from Alkanna orientalis. Planta Medica 1997, 63, 384. [Google Scholar] [CrossRef]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and Analyzing Pyrrolizidine Alkaloids in Medicinal Plants: A Review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae: Structures, Distribution, and Biological Properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Petrova, G.; Yankova-Tsvetkova, E.; Doncheva, T.; Kostova, N.; Nikolova, R.; Zheljazkov, V.D. Genetic Diversity, Reproductive Capacity and Alkaloids Content in Three Endemic Alkanna Species. PLoS ONE 2020, 15, e0233516. [Google Scholar] [CrossRef] [PubMed]
- Tsiokanos, E.; Tsafantakis, N.; Obé, H.; Beuerle, T.; Leti, M.; Fokialakis, N.; Grondin, A. Profiling of Pyrrolizidine Alkaloids Using a Retronecine-Based Untargeted Metabolomics Approach Coupled to the Quantitation of the Retronecine-Core in Medicinal Plants Using UHPLC-QTOF. J. Pharm. Biomed. Anal. 2023, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Lis-Cieplak, A.; Trześniowska, K.; Stolarczyk, K.; Stolarczyk, E.U. Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules 2024, 29, 3269. [Google Scholar] [CrossRef] [PubMed]
- Colegate, S.M.; Edgar, J.A.; Knill, A.M.; Lee, S.T. Solid-Phase Extraction and HPLC-MS Profiling of Pyrrolizidine Alkaloids and their N-Oxides: A Case Study of Echium Plantagineum. Phytochem. Anal. 2005, 16, 108–119. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, PAs Eur Pharm. 20826E, 11th ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2022; ISBN 978-3-7692-8026-5. [Google Scholar]
- Perez De Souza, L.; Alseekh, S.; Naake, T.; Fernie, A. Mass Spectrometry-Based Untargeted Plant Metabolomics. Curr. Protoc. Plant Biol. 2019, 4, e20100. [Google Scholar] [CrossRef]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass Spectrometry-Based Metabolomics in Microbiome Investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of Untargeted Metabolomics Approach and Molecular Networking Analysis to Identify Unique Natural Components in Wild Morchella Sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Tufa, T.; Damianakos, H.; Zengin, G.; Graikou, K.; Chinou, I. Antioxidant and Enzyme Inhibitory Activities of Disodium Rabdosiin Isolated from Alkanna sfikasiana Tan, Vold and Strid. S. Afr. J. Bot. 2019, 120, 157–162. [Google Scholar] [CrossRef]
- Panou, E.; Zengin, G.; Milic, N.; Ganos, C.; Graikou, K.; Chinou, I. A Comparative UPLC/HRMS Molecular Networking-Enhanced Study on the Phenolic Profiles and Bioactivities of Three Medicinally Significant Species of Onosma (Boraginaceae). Plants 2024, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Varvouni, E.-F.; Zengin, G.; Graikou, K.; Ganos, C.; Mroczek, T.; Chinou, I. Chemical Profile and Biological Properties of the Endemic Turkish Species Phyllocara aucheri. S. Afr. J. Bot. 2021, 137, 340–344. [Google Scholar] [CrossRef]
- Yan, K.-J.; Chu, Y.; Huang, J.-H.; Jiang, M.-M.; Li, W.; Wang, Y.-F.; Huang, H.-Y.; Qin, Y.-H.; Ma, X.-H.; Zhou, S.-P.; et al. Qualitative and Quantitative Analyses of Compound Danshen Extract Based on 1 H NMR Method and Its Application for Quality Control. J. Pharm. Biomed. Anal. 2016, 131, 183–187. [Google Scholar] [CrossRef]
- Risikobewertung, B.F. Aktualisierte Risikobewertung zu Gehalten an 1,2-ungesättigten Pyrrolizidinalkaloiden (PA) in Lebensmitteln: Stellungnahme Nr. 026/2020 des BfR vom 17. Juni 2020. BfR-Stellungnahmen 2020, 2020, 26. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Data Conversion with ProteoWizard msConvert. In Proteomics; Comai, L., Katz, J.E., Mallick, P., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1550, pp. 339–368. ISBN 978-1-4939-6745-2. [Google Scholar]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching Molecular Structure Databases with Tandem Mass Spectra Using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.-F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic Classification of Unknown Metabolites Using High-Resolution Fragmentation Mass Spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminformatics 2016, 8, 61. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Terzić, M.; Abul, N.; Gulcin, I.; Koyuncu, I.; Basarali, M.K.; Đorđević, T.; Cziáky, Z.; Jekő, J.; Cespedes- Acuna, C.L. A Multidimensional Study for Design Functional Foods: Chemical Profiling, Antioxidant Potential, Enzyme Inhibition, and Cytotoxic Effects of Alkanna tubulosa Extracts. Food Biosci. 2024, 60, 104280. [Google Scholar] [CrossRef]
- Kurt-Celep, I.; Zheleva-Dimitrova, D.; Sinan, K.I.; Uba, A.I.; Nilofar; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Cakilcioglu, U.; Dall’Acqua, S.; Zengin, G. Uncovering Chemical Profiles, Biological Potentials, and Protection Effect against ECM Destruction in H2O2 -treated HDF Cells of the Extracts of Stachys tundjeliensis. Arch. Pharm. 2024, 357, 2300528. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Lee, Y.G.; Lee, S.-H.; Kim, W.-S.; Park, K.-H.; Moon, J.-H. An Ether and Three Ester Derivatives of Phenylpropanoid from Pear (Pyrus pyrifolia Nakai Cv. Chuhwangbae) Fruit and Their Radical-Scavenging Activity. Food Sci. Biotechnol. 2014, 23, 253–259. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández De Simón, B.; Aranda, I.; Sanz, M.; Sánchez-Gómez, D.; Pinto, E. Non-targeted Metabolomic Profile of Fagus sylvatica L. Leaves Using Liquid Chromatography with Mass Spectrometry and Gas Chromatography with Mass Spectrometry. Phytochem. Anal. 2015, 26, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-Based Profiling of African Baobab Fruit (Adansonia digitata L.) Using a Multiplex Approach of MS and NMR Techniques in Relation to Its Biological Activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, H.; Wang, Z.-N.; Luo, H.-Z.; Jia, A.-Q. Phytochemical Constituents of Onosma bracteatum Wall. Phytochem. Lett. 2021, 45, 1–5. [Google Scholar] [CrossRef]
- Nguyen, T.-K.-O.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid Profiling Reveals a Class of Hydroxycinnamoyl Glucaric Acid Conjugates in Isatis tinctoria Leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Zheng, Q.-A.; Gadagi, R.S.; Rimmer, S.R. Phytoalexins and Polar Metabolites from the Oilseeds Canola and Rapeseed: Differential Metabolic Responses to the Biotroph Albugo candida and to Abiotic Stress. Phytochemistry 2008, 69, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Beladjila, K.A.; Cotugno, R.; Berrehal, D.; Kabouche, Z.; De Tommasi, N.; Braca, A.; De Leo, M. Cytotoxic Triterpenes from Salvia buchananii Roots. Nat. Prod. Res. 2018, 32, 2025–2030. [Google Scholar] [CrossRef]
- Gravina, C.; Formato, M.; Piccolella, S.; Fiorentino, M.; Stinca, A.; Pacifico, S.; Esposito, A. Lavandula austroapennina (Lamiaceae): Getting Insights into Bioactive Polyphenols of a Rare Italian Endemic Vascular Plant. Int. J. Mol. Sci. 2023, 24, 8038. [Google Scholar] [CrossRef]
- Krzyżanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel Phenolic Constituents of Pulmonaria officinalis L. LC-MS/MS Comparison of Spring and Autumn Metabolite Profiles. Molecules 2018, 23, 2277. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.-Y.; Lee, H.J.; Ma, Y.-K.; Kwon, J.; Park, S.H.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Park, K.-H.; et al. Hydroxycinnamoylmalic Acids and Their Methyl Esters from Pear (Pyrus pyrifolia Nakai) Fruit Peel. J. Agric. Food Chem. 2011, 59, 10124–10128. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Baracz, T.; Skowrońska, W.; Piwowarski, J.P.; Majdan, M.; Malarz, J.; Stojakowska, A.; Zidorn, C.; Granica, S. The Contribution of Phenolics to the Anti-Inflammatory Potential of the Extract from Bolivian Coriander (Porophyllum ruderale subsp. ruderale). Food Chem. 2022, 371, 131116. [Google Scholar] [CrossRef]
- Hussein, H.M.; Abdel Kawy, M.A.; Eltanany, B.M.; Pont, L.; Benavente, F.; Fayez, A.M.; Alnajjar, R.; Al-Karmalawy, A.A.; Abdelmonem, A.R.; Mohsen, E. Cognitive-Enhancing Effect of Cordia dichotoma Fruit on Scopolamine-Induced Cognitive Impairment in Rats: Metabolite Profiling, in Vivo, and in Silico Investigations. RSC Adv. 2024, 14, 40267–40286. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, R.; Zhang, Y.; Li, R.; Oduro, P.K.; Si, D.; Han, L.; Huang, Y.; Pan, G. An Integrated Strategy for the Systematic Chemical Characterization of Salvianolate Lyophilized Injection Using Four Scan Modes Based on the Ultra-High Performance Liquid Chromatography-Triple Quadrupole-Linear Ion Trap Mass Spectrometry. J. Pharm. Biomed. Anal. 2022, 215, 114769. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Kon’kova, N.G.; Zakharenko, A.M.; Golokhvast, K.S. Polyphenols of Perilla frutescens of the Family Lamiaceae Identified by Tandem Mass Spectrometry. Vavilov J. Genet. Breed. 2022, 26, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Cirigliano, A.; Fabani, M.; Lima, B.; Alberti, S.; Kramer, F.; Tapia, A.; Cabrera, G.; Palermo, J.; Sánchez, M. Antioxidant Neolignans from Cordia americana. Planta Medica 2013, 79, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Kılınc, H.; D’Urso, G.; Paolillo, A.; Alankus, O.; Piacente, S.; Masullo, M. LC-MS and NMR Based Plant Metabolomics: A Comprehensive Phytochemical Investigation of Symphytum anatolicum. Metabolites 2023, 13, 1051. [Google Scholar] [CrossRef]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of Seasonal Variation on Thymus longicaulis C. Presl Chemical Composition and Its Antioxidant and Anti-Inflammatory Properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Kuwabara, H.; Hoshiyama, H. Identification of Sucrose Diesters of Aryldihydronaphthalene-Type Lignans from Trigonotis peduncularis and the Nature of Their Fluorescence. J. Nat. Prod. 2008, 71, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-L.; Xu, J.-J.; Zhong, K.-R.; Shang, Z.-P.; Wang, F.; Wang, R.-F.; Zhang, L.; Zhang, J.-Y.; Liu, B. Analysis of Non-Volatile Chemical Constituents of Menthae haplocalycis Herba by Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- March, R.E.; Lewars, E.G.; Stadey, C.J.; Miao, X.-S.; Zhao, X.; Metcalfe, C.D. A Comparison of Flavonoid Glycosides by Electrospray Tandem Mass Spectrometry. Int. J. Mass Spectrom. 2006, 248, 61–85. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Nowak, K.; Radwan, S.; Włodarczyk, M.; Kuś, P.M.; Susniak, K.; Korona-Głowniak, I. Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts. Molecules 2024, 29, 437. [Google Scholar] [CrossRef]
- Ahn, E.M.; Asamenew, G.; Kim, H.W.; Lee, S.H.; Yoo, S.-M.; Cho, S.-M.; Cha, Y.-S.; Kang, M.-S. Anti-Obesity Effects of Petasites japonicus (Meowi) Ethanol Extract on RAW 264.7 Macrophages and 3T3-L1 Adipocytes and Its Characterization of Polyphenolic Compounds. Nutrients 2020, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ruslay, S.; Abas, F.; Shaari, K.; Zainal, Z.; Maulidiani; Sirat, H.; Israf, D.A.; Lajis, N.H. Characterization of the Components Present in the Active Fractions of Health Gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC–DAD–ESIMS. Food Chem. 2007, 104, 1183–1191. [Google Scholar] [CrossRef]
- Parejo, I.; Jáuregui, O.; Viladomat, F.; Bastida, J.; Codina, C. Characterization of Acylated Flavonoid-O-glycosides and Methoxylated Flavonoids from Tagetes maxima by Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2801–2810. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules 2021, 26, 6493. [Google Scholar] [CrossRef]
- Wakui, V.G.; De Oliveira, V.M.; Keng Queiroz Júnior, L.H.; Alves De Oliveira, C.M.; Kato, L. Metabolic Profiling of Lomatozona artemisiifolia Baker Plants Grown in Vitro and Collected from Nature Using Molecular Networking and Chemometric Analysis. Nat. Prod. Res. 2024, 38, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U. Negative Atmospheric Pressure Chemical Ionisation Low-Energy Collision Activation Mass Spectrometry for the Characterisation of Flavonoids in Extracts of Fresh Herbs. J. Chromatogr. A 2000, 902, 369–379. [Google Scholar] [CrossRef]
- Rak, G.; Fodor, P.; Abrankó, L. Three-Step HPLC–ESI-MS/MS Procedure for Screening and Identifying Non-Target Flavonoid Derivatives. Int. J. Mass Spectrom. 2010, 290, 32–38. [Google Scholar] [CrossRef]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural Characterization of Flavonoid Glycosides from Leaves of Wheat (Triticum aestivum L.) Using LC/MS/MS Profiling of the Target Compounds. J. Mass. Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.-Q.; Guo, J.-X.; Yang, Y.; Xu, L.; Wang, J.; Yang, F.; Xu, Z.-B.; Huang, Y.-F.; Shi, W.; Lu, X.; et al. Elucidating the Chemical Interaction Effects of Herb Pair Danshen-Chuanxiong and Its Anti-Ischemic Stroke Activities Evaluation. J. Ethnopharmacol. 2024, 318, 117058. [Google Scholar] [CrossRef] [PubMed]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.D.M. Metabolic Profiling of the Oil of Sesame of the Egyptian Cultivar ’Giza 32’ Employing LC-MS and Tandem MS-Based Untargeted Method. Foods 2021, 10, 298. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics Driven Analysis of Six Nigella Species Seeds via UPLC-qTOF-MS and GC–MS Coupled to Chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef]
- Lu, A.-J.; Lu, Y.-L.; Tan, D.-P.; Qin, L.; Ling, H.; Wang, C.-H.; He, Y.-Q. Identification of Pyrrolizidine Alkaloids in Senecio Plants by Liquid Chromatography-Mass Spectrometry. J. Anal. Methods Chem. 2021, 2021, 1957863. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Zengin, G.; Sinan, K.I.; Ak, G.; Sadeer, N.B.; Angeloni, S.; Mustafa, A.M.; Caprioli, G.; Maggi, F.; Cakilcioglu, U.; et al. Two Medicinal Plants (Alkanna trichophila and Convolvulus galaticus) from Turkey: Chemical Characterization and Biological Perspectives. Chem. Biodivers. 2021, 18, e2100356. [Google Scholar] [CrossRef]
- Yusufbeyoğlu, S.; Baldemir Kılıç, A. An Overview of the Chemical Composition, Biological Activities and Traditional Uses of Genus Alkanna Tausch. J. Fac. Pharm. Ank. Univ. 2025, 49, 21. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Vallverdú-Queralt, A.; Pérez, M.; Jáuregui, O.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Metabolomics Technologies for the Identification and Quantification of Dietary Phenolic Compound Metabolites: An Overview. Antioxidants 2021, 10, 846. [Google Scholar] [CrossRef]
- Mädge, I.; Gehling, M.; Schöne, C.; Winterhalter, P.; These, A. Pyrrolizidine Alkaloid Profiling of Four Boraginaceae Species from Northern Germany and Implications for the Analytical Scope Proposed for Monitoring of Maximum Levels. Food Addit. Contam. Part A 2020, 37, 1339–1358. [Google Scholar] [CrossRef]
- Riedl, H. Boraginaceae. In Flora Malesiana-Series 1, Spermatophyta; Springer Nature: Berlin, Germany, 1997; Volume 13, pp. 43–144. [Google Scholar]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Suo, M.-R.; Yang, J.-S.; Liu, Q.-H. Lignan Oligosaccharide Esters from Eritrichium rupestre. J. Nat. Prod. 2006, 69, 682–684. [Google Scholar] [CrossRef]
- Davies, K.M. (Ed.) Plant Pigments and Their Manipulation. In Annual Plant Reviews; Blackwell: Oxford, UK, 2004; p. 97. ISBN 978-1-4051-1737-1. [Google Scholar]
- Liu, X.; Liu, Y.; Xu, X.; Huang, W.; Yan, Y.; Wang, Y.; Tian, W.; Mo, T.; Cui, X.; Li, J.; et al. Molecular characterization and structure basis of a malonyltransferase with both substrate promiscuity and catalytic regiospecificity from Cistanche tubulosa. Acta Pharm. Sin. B 2024, 14, 2333–2348. [Google Scholar] [CrossRef]
- Sendri, N.; Bhandari, P. Anthocyanins: A Comprehensive Review on Biosynthesis, Structural Diversity, and Industrial Applications. Phytochem. Rev. 2024, 23, 1913–1974. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and Their Glycosides, Including Anthocyanins. Nat. Prod. Rep. 2008, 25, 555. [Google Scholar] [CrossRef] [PubMed]
- Tatsuzawa, F.; Toki, K.; Ohtani, Y.; Kato, K.; Saito, N.; Honda, T.; Mii, M. Floral Pigments from the Blue Flowers of Nemophila Menziesii ‘Insignis Blue’ and the Purple Flower of Its Variants. J. Jpn. Soc. Hortic. Sci. 2014, 83, 259–266. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Acylated Flavonoids from Spiraea Genus as Inhibitors of α-Amylase. Russ. J. Bioorganic Chem. 2018, 44, 876–886. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Q.-S.; Wang, G.-S. Flavonoid Glycosides and Their Derivatives from the Herbs of Scorzonera austriaca Wild. Molecules 2016, 21, 803. [Google Scholar] [CrossRef]
- Opiyo, S.A. A Review of Chemical Compounds and Bioactivity of Conyza Species. IOSR J. Appl. Chem. 2023, 16, 36–48. [Google Scholar]
- Olennikov, D.N.; Kartashova, M.E.; Velichko, V.V.; Kruglov, D.S. New Flavonoids from Nonea rossica and tournefortia Sibirica. Chem. Nat. Compd. 2022, 58, 1021–1025. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Christinat, N.; Badoud, F. From Structural Alerts to Signature Fragment Alerts: A Case Study on Pyrrolizidine Alkaloids. Chem. Res. Toxicol. 2023, 36, 213–229. [Google Scholar] [CrossRef]
- Ruan, J.; Li, N.; Xia, Q.; Fu, P.P.; Peng, S.; Ye, Y.; Lin, G. Characteristic Ion Clusters as Determinants for the Identification of Pyrrolizidine Alkaloid N-oxides in Pyrrolizidine Alkaloid–Containing Natural Products Using HPLC–MS Analysis. J. Mass. Spectrom. 2012, 47, 331–337. [Google Scholar] [CrossRef]
- Dreger, M.; Stanisławska, M.; Krajewska-Patan, A.; Mielcarek, S.; Mikołajczak, P.Ł.; Buchwald, W. Pyrrolizidine Alkaloids—Chemistry, Biosynthesis, Pathway, Toxicity, Safety and Perspectives of Medicinal Usage. Herba Pol. 2009, 55, 127–147. [Google Scholar]
- Larcher, R.; Nardin, T. Suspect Screening of Glycoalkaloids in Plant Extracts Using Neutral Loss—High Resolution Mass Spectrometry. J. Chromatogr. A 2019, 1596, 59–68. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Italy the Biological Pathways of Alzheimer Disease: A Review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Behl, T.; Sachdeva, M. Key Milestones in the Diabetes Research: A Comprehensive Update. Obes. Med. 2020, 17, 100183. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Lee, S.; Kang, K. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-O-Glucoside and Quercetin 3-O-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]


| No. | Identified Compounds | Rt (min) | Relative Abundance (%) | Precursor Ion | Molecular Formula | Error (ppm) | MS/MS | Reference |
|---|---|---|---|---|---|---|---|---|
| HYDROXYCINNAMIC ACIDS | ||||||||
| Coumaroyl derivatives | ||||||||
| 1. | Coumaric acid * | 7.79 | 0.09 | 163.0387 | C9H8O3 | −5.02 | 119.05 | [41] |
| 2. | Coumaroyl-glyceric acid | 7.34 | 0.14 | 251.0544 | C12H12O6 | −4.63 | 163.04/145.03/119.05/105.02 | [42] |
| 3. | Coumaroyl- threonic/erythronic acid | 6.92 | 0.18 | 281.0649 | C13H14O7 | −4.72 | 163.04/135.03/119.05/75.01 | [43] |
| 4. | Coumaroyl malic acid methyl ester | 9.45 | 0.15 | 293.0655 | C14H14O7 | −2.14 | 163.04/145.03/133.05/119.04 | [44] |
| 5. | Coumaroyl-threonic/erythronic acid methyl ester | 6.47 | 0.09 | 295.0805 | C14H16O7 | −4.33 | 193.04/163.04/149.03/133.05/119.05 | [45] |
| 6. | Coumaric acid hexoside | 4.89 | 0.05 | 325.0911 | C15H18O8 | −3.82 | 179.05/163.04/145.02/135.04/119.05 | [46] |
| Feruloyl derivatives | ||||||||
| 7. | Feruloyl malic acid methyl ester | 10.7 | 0.21 | 323.0758 | C15H16O8 | −2.76 | 193.04/175.04/149.06/134.04 | [47] |
| Caffeoyl derivatives | ||||||||
| Monomers | ||||||||
| 8. | Caffeic acid * | 4.23 | 0.57 | 179.0332 | C9H8O4 | −6.89 | 135.05 | [41] |
| 9. | Methyl caffeate | 15.4 | 0.19 | 193.0493 | C10H10O4 | −4.06 | 161.02/133.03 | [48] |
| 10. | Danshensu * | 2.27 | 0.42 | 197.0460 | C9H10O5 | 5.08 | 179.03/135.04/123.05/93.03/72.99 | [49] |
| 11. | Caffeoyl-glyceric acid | 4.5 | 2.28 | 267.0484 | C12H12O7 | −7.78 | 179.04/161.02/135.04/105.02/75.01 | [50] |
| 12. | Caffeoyl-threonic/erythronic acid | 2.95 | 0.24 | 297.0628 | C13H14O8 | 6.25 | 179.03/161.02/135.03/75.01 | [50] |
| 13. | Caffeoyl malic acid methyl ester | 4.93 | 0.45 | 309.0605 | C14H14O8 | −1.75 | 179.03/161.02/135.04/119.05 | [51] |
| 14. | Caffeoyl-methyl threonic/erythronic acid | 3.89 | 0.29 | 311.0768 | C14H16O8 | 0.35 | 179.03/163.04/149.05/119.05 | [52] |
| Dimers | ||||||||
| 15. | Nepetoidin B (Isomer I) | 32.62 | 0.33 | 313.0710 | C17H14O6 | −0.68 | 161.02/151.04/133.03/123.04 | [53] |
| 16. | Nepetoidin B (Isomer II) | 31.84 | 0.20 | 313.0717 | C17H14O6 | 1.56 | 161.02/151.04/133.03/123.04 | [53] |
| 17. | Caffeoyl-4-hydroxyphenyllactic acid | 23.98 | 0.19 | 343.0823 | C18H16O7 | 1.52 | 197.04/179.03/161.02/145.03/135.04/72.99 | [54] |
| 18. | Rosmarinic acid * | 18.52 | 14.3 | 359.0778 | C18H15O8 | 3.08 | 197.05/179.03/161.02 | [41] |
| 19. | Rosmarinic acid methyl ester | 25.63 | 0.21 | 373.0928 | C19H18O8 | 1.23 | 197.04/175.04/135.04 | [50] |
| 20. | Danshen suan C (Salvianic acid C) | 4.5 | 0.16 | 377.0857 | C18H18O9 | −4.13 | 198.05/179.03/161.02/135.04 | [55] |
| 21. | Rosmarinic acid hexoside Ι | 12.77 | 0.15 | 521.1277 | C24H26O13 | −3.48 | 359.09/197.04/161.03 | [54] |
| 22. | Rosmarinic acid hexoside ΙΙ | 14.08 | 0.18 | 521.1280 | C24H26O13 | −2.91 | 359.09/197.05/179.03/135.04 | [54] |
| Trimers | ||||||||
| 23. | Salvianolic acid C * | 28.52 | 0.85 | 491.0973 | C26H20O10 | −1.06 | 311.06/295.06/185.02/135.04 | [49] |
| 24. | Salvianolic acid A * | 21.73 | 1.09 | 493.1117 | C26H22O10 | −3.59 | 295.06/185.02/109.03 | [54] |
| 25. | Yunnaneic acid D | 9.21 | 0.71 | 539.1171 | C27H24O12 | −3.43 | 359.07/297.07/197.04/161.02/135.04 | [49] |
| 26. | Yunnaneic acid I | 19.42 | 0.18 | 541.1339 | C27H26O12 | −1.30 | 343.08/329.07/299.09/267.06/197.04/135.04 | [56] |
| 27. | Monomethyl lithospermate | 29.12 | 0.10 | 551.1195 | C28H24O12 | 1.00 | 519.08/353.07/339.05/321.04/295.05/197.04 | [57] |
| 28. | Salvianolic acid K | 11.54 | 0.14 | 555.1151 | C27H24O13 | 2.22 | 467.13/359.07/313.07/269.08/197.04/135.04 | [58] |
| 29. | Yunnaneic acid E * | 11.5 | 0.74 | 571.1079 | C27H24O14 | −1.54 | 527.12/439.14/285.07/241.09/197.04 | [49] |
| 30. | Yunnaneic acid F * | 11.22 | 0.32 | 597.1224 | C29H26O14 | −3.40 | 491.14/329.10/311.09/267.10/197.04/179.03/135.04 | [49] |
| Tetramers | ||||||||
| 31. | Sebesteniod E | 27.66 | 0.18 | 671.1394 | C35H28O14 | −1.01 | 520.08/473.08/321.03/169.91 | [56] |
| 32. | Lithospermic acid B * | 27.75 | 0.30 | 717.1428 | C36H30O16 | −3.85 | 519.09/321.04 | [50] |
| 33. | Trigonotin B/Rupestrin A | 19.11 | 0.19 | 733.1950 | C34H38O18 | −4.08 | 689.21/367.07/323.09/193.01/161.04/125.02 | [59] |
| 34. | Sodium rabdosiin * | 27.62 | 0.28 | 739.1259 | C36H29NaO16 | −2.17 | 559.08/515.10/335.06/291.06 | [12] |
| 35. | Sodium lithospermate B | 18.89 | 0.23 | 741.1400 | C36H31O16Na | −4.26 | 579.10/381.06/219.02/197.04/179.03/135.04 | [60] |
| 36. | Sodium salvianolic acid E | 17.6 | 0.27 | 741.1428 | C36H31O16Na | −0.48 | 561.10/381.06/161.02 | [60] |
| 37. | Trigonotin A/Rupestrin C | 13.64 | 0.32 | 763.2083 | C35H40O19 | −0.33 | 633.03/500.10/369.10 | [59] |
| 38. | Pulmonarioside C/Echiumin E | 15.44 | 0.22 | 983.2814 | C47H52O23 | −0.73 | 837.23/793.23/629.18/469.13/367.08/145.03 | [45] |
| 39. | Pulmonarioside C/Echiumin E | 20.41 | 0.13 | 983.2816 | C47H52O23 | −0.52 | 837.22/793.21/469.13/367.08 | [45] |
| FLAVONOIDS AND FLAVONOID GLYCOSIDES | ||||||||
| Kaempferol derivatives | ||||||||
| 40. | Kaempferol | 32.73 | 0.04 | 285.0396 | C15H10O6 | −1.10 | 285.04/255.03/227.03/151.00 | [61] |
| 41. | Kaempferol-methylether * | 33.24 | 0.05 | 299.0548 | C16H12O6 | −2.55 | 284.03/255.03/227.03 | [62] |
| 42. | Kaempferol-O-hexoside I * | 14.99 | 0.94 | 447.0929 | C21H20O11 | 0.37 | 447.09/284.03/151.00 | [61] |
| 43. | Kaempferol-O-hexoside II * | 16.2 | 7.71 | 447.0930 | C21H20O11 | 0.59 | 447.09/284.03/151.00 | [61] |
| 44. | Kaempferol-O-acetyl-hexoside I * | 22.74 | 0.58 | 489.1037 | C23H22O12 | 0.82 | 285.04/284.03/227.03 | [63] |
| 45. | Kaempferol-O-acetyl-hexoside II * | 24.91 | 0.08 | 489.1046 | C23H22O12 | 2.66 | 285.04/255.03 | [63] |
| 46. | Kaempferol diacetyl-deoxyhexoside | 28.87 | 0.15 | 515.1177 | C25H24O12 | −2.43 | 285.04/255.02/179.03 | [64] |
| 47. | Kaempferol-O-malonyl hexoside | 20.56 | 0.08 | 533.0938 | C24H22O14 | 1.26 | 489.10/285.04 | [50] |
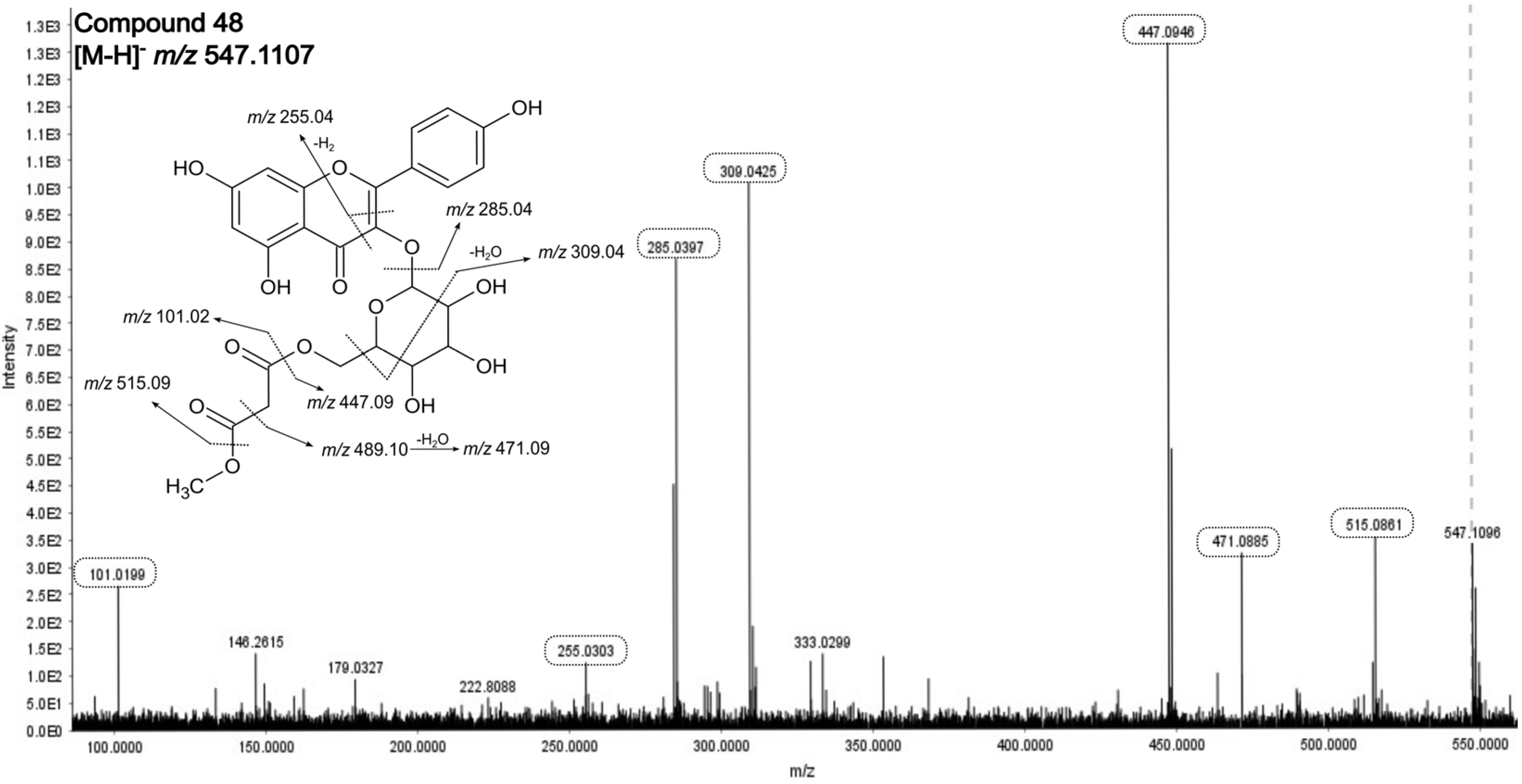
| 48. | Kaempferol-O- (malonyl methyl ester)-hexoside | 24.79 | 0.09 | 547.1107 | C25H24O14 | −2.91 | 515.09/489.09/471.09/447.09/309.04/285.04 | |
| 49. | Kaempferol-triacetyl hexoside | 22.96 | 0.09 | 573.1255 | C27H26O14 | 1.87 | 489.10/368.99/285.04/125.02 | |
| 50. | Kaempferol-O-hexose-deoxyhexose I * | 13.09 | 0.09 | 593.1490 | C27H30O15 | −2.77 | 431.10/285.04/284.03/255.03 | [41] |
| 51. | Kaempferol-O- hexose-deoxyhexose II * | 14.95 | 0.34 | 593.1500 | C27H30O15 | −1.09 | 447.08/285.04/284.04/255.03/227.03 | [41] |
| 52. | Kaempferol-O-caffeoyl-hexoside | 25.51 | 0.09 | 609.1264 | C30H26O14 | 3.23 | 447.09/323.08/285.04/179.03 | [65] |
| Quercetin derivatives | ||||||||
| 53. | Quercetin * | 28.66 | 0.07 | 301.0346 | C15H10O7 | −0.76 | 178.99/151.00/107.02 | [66] |
| 54. | Quercetin-O-hexoside * | 12.18 | 6.96 | 463.0863 | C21H20O12 | −2.92 | 300.03/301.03/151.00 | [61] |
| 55. | Quercetin-O-acetyl-hexoside I * | 16.66 | 3.16 | 505.0996 | C23H22O13 | 3.83 | 300.03 | [63] |
| 56. | Quercetin-O-acetyl-hexoside II * | 18.48 | 0.11 | 505.1005 | C23H22O13 | 2.74 | 446.09/360.08/300.03/151.00 | [63] |
| 57. | Quercetin-O-malonyl-hexoside | 16.7 | 0.30 | 549.0902 | C24H22O15 | 3.92 | 505.10/463.10/301.03 | [63] |
| 58. | Rutin * | 9.85 | 0.07 | 609.1438 | C27H30O16 | −2.89 | 300.03 | [66] |
| Other flavonoid derivatives | ||||||||
| 59. | Dimethoxy-dihydroxy-flavone * | 36.68 | 0.12 | 313.0717 | C17H14O6 | 1.56 | 298.05/283.02 | [67] |
| 60. | Isorhamnetin | 30.08 | 0.18 | 315.0508 | C16H12O7 | 1.02 | 300.03/271.03/255.03 | [68] |
| 61. | Dimethoxy-trihydroxy-flavone * | 34.87 | 0.15 | 329.0654 | C17H14O7 | −2.21 | 314.04/299.02/285.03/271.02 | [67] |
| 62. | Trimethoxy-dihydroxy-flavone * | 35.2 | 0.13 | 343.0818 | C18H16O7 | 0.06 | 328.06/313.03/298.01/285.03/270.01 | [67] |
| 63. | Dimethoxy-tetrahydroxyflavone | 29.78 | 0.06 | 345.0605 | C17H14O8 | −1.57 | 330.04/315.01/287.01 | [67] |
| 64. | Myricetin-O-hexoside | 6.39 | 0.22 | 479.0810 | C21H20O13 | −3.27 | 317.02/316.02/271.03/178.99/151.01 | [69] |
| 65. | Dimethoxy-trihydroxy-flavone-O-hexoside | 31.1 | 0.08 | 491.1165 | C23H24O12 | −4.99 | 429.11/329.06/314.04/135.04 | [70] |
| 66. | Trihydroxy-trimethoxyflavone-O-hexoside * | 27.83 | 0.29 | 521.1287 | C24H26O13 | −1.57 | 359.07/225.86 | [65] |
| COUMARINS | ||||||||
| 67. | Hydroxy coumarin | 18.52 | 0.62 | 161.0243 | C9H6O3 | 2.68 | 133.03 | [54] |
| ORGANIC ACIDS | ||||||||
| 68. | malic acid * | 1.8 | 0.25 | 133.0150 | C4H6O5 | 9.79 | 115.01/73.00/71.02 | [71] |
| 69. | Azelaic acid | 13.84 | 0.10 | 187.0962 | C9H16O4 | −4.46 | 125.10/123.08/98.08 | [72] |
| 70. | Citric acid | 2.13 | 0.57 | 191.0202 | C6H8O7 | 5.35 | 129.02/111.01/87.01/85.03 | [71] |
| 71. | Gluconic/galactonic acid * | 1.8 | 0.25 | 195.0507 | C6H12O7 | 1.14 | 162.03/105.02/99.01/87.00/75.01/71.01 | [72] |
| FATTY ACIDS | ||||||||
| 72. | Trihydroxy-octadecenoic acid | 32.38 | 0.15 | 329.2319 | C18H34O5 | −2.73 | 331.25/229.14/211.13/171.10 | [57] |
| 73. | Hydroxy-octadecadienoic acid | 38.84 | 0.14 | 295.2267 | C18H32O3 | −2.10 | 183.14 | [73] |
| 74. | Trihydroxy-octadecadienoic acid | 31.02 | 0.13 | 327.2167 | C18H32O5 | −1.37 | 327.21/229.14/211.13/171.10 | [57] |
| 75. | Hydroxy-octadecatrienoic acid | 38.04 | 0.12 | 293.2110 | C18H30O3 | −2.28 | 275.20/235.17/223.17/183.10 | [73] |
| 76. | Hydroxy-octadecenoic acid * | 39.65 | 0.08 | 297.2432 | C18H34O3 | 0.77 | 297.24/279.23/171.10 | [40] |
| No. | Identified Compounds | Rt (min) | Relative Abundance (%) | Precursor Ions | Type | Molecular Formula | Δppm | MS/MS | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PA1. | Trachelanthamidine/Isotretronecanol | 11.62 | 5.26 | 142.1223 | TRA | C8H15NO | −2.4 | 124.11/108.08 | [74] |
| PA2. | Platynecine * | 4.6 | 1.26 | 158.1166 | PLAT | C8H15NO2 | −6.04 | 140.11/122.09/105.07 | [17] |
| PA3. | 9-sarracinoyl-trachelanthamidine/isotretronecanol | 16.96 | 0.74 | 240.1594 | TRA | C13H21NO3 | −0.08 | 142.12/124.11/108.08 | |
| PA4. | Retronecine-pentoside | 3.91 | 0.57 | 288.1435 | RET (monoester) | C13H21NO6 | −0.57 | 156.10/138.09/120.08/108.08 | |
| PA5. | Trachelanthamidine/Isotretronecanol-hexoside | 8.95 | 8.16 | 304.1758 | TRA | C14H25NO6 | 1.1 | 142.12 | |
| PA6. | Leptanthine | 12.41 | 1.41 | 316.1751 | RET (monoester) | C15H25NO6 | −1.15 | 298.16/254.14/156.10/139.10/138.09/122.09/120.08/108.08 | [17] |
| PA7. | Leptanthine-N-oxide | 4.9 | 1.70 | 332.1709 | RETNO (monoester) | C15H25NO7 | 1.57 | 172.09/155.09/138.08/111.06 | [17] |
| PA8. | Dihydroxy triangularine * | 13.4 | 7.41 | 370.1859 | RET (diester) | C18H27NO7 | −0.35 | 341.12/326.16/288.14/238.14/220.13/138.09/120.08 | [17] |
| PA9. | Dihydroxy triangularine N-oxide * | 13.1 | 6.45 | 386.1796 | RETNO (diester) | C18H27NO8 | −3.48 | 254.14/220.13/154.08/136.07 | [17] |
| PA10. | 7-senecioyl-9-(3-acetoxy-2-hydroxy-2-methylbutyryl) retronecine N-oxide * | 14.29 | 1.11 | 412.196 | RETNO (diester) | C20H29NO8 | −1.44 | 238.14/138.09/120.08/103.05 | [17] |
| Phytochemical Assays | AC * |
|---|---|
| TPC (mg GAE/g of extract) | 74.45 ± 4.37 |
| TFC (mg RE/g of extract) | 46.66 ± 0.11 |
| Antioxidant Assays | AC * |
|---|---|
| DPPH• (mg TE/g of extract) | 227.01 ± 2.15 |
| ABTS•+ (mg TE/g of extract) | 450.91 ± 11.42 |
| FRAP (mg TE/g of extract) | 365.59 ± 0.61 |
| CUPRAC (mg TE/g of extract) | 462.42 ± 7.95 |
| Phosphomolybdenum (mmol TE/g of extract) | 2.11 ± 0.02 |
| Metal chelating (mg EDTAE/g of extract) | 7.91 ± 0.53 |
| Enzyme Inhibitory Assays | AC * |
|---|---|
| AChE inhibition (mg GALAE/g of extract) | 1.43 ± 0.10 |
| BChE inhibition (mg GALAE/g of extract) | 0.38 ± 0.14 |
| α-amylase inhibition (mmol ACAE/g of extract) | 0.47 ± 0.01 |
| α-glucosidase inhibiton (mmol ACAE/g of extract) | 6.65 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panou, E.; Tsafantakis, N.; Zengin, G.; Graikou, K.; Ganos, C.; Fokialakis, N.; Chinou, I. Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis. Sci. Pharm. 2025, 93, 45. https://doi.org/10.3390/scipharm93030045
Panou E, Tsafantakis N, Zengin G, Graikou K, Ganos C, Fokialakis N, Chinou I. Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis. Scientia Pharmaceutica. 2025; 93(3):45. https://doi.org/10.3390/scipharm93030045
Chicago/Turabian StylePanou, Evgenia, Nikolaos Tsafantakis, Gokhan Zengin, Konstantia Graikou, Christos Ganos, Nikolas Fokialakis, and Ioanna Chinou. 2025. "Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis" Scientia Pharmaceutica 93, no. 3: 45. https://doi.org/10.3390/scipharm93030045
APA StylePanou, E., Tsafantakis, N., Zengin, G., Graikou, K., Ganos, C., Fokialakis, N., & Chinou, I. (2025). Untargeted Metabolomic Profiling and Bioactivity Insights into Alkanna corcyrensis. Scientia Pharmaceutica, 93(3), 45. https://doi.org/10.3390/scipharm93030045







