Identification of Novel Piperidine and Pyrrolidine Derivatives as Potent Inhibitors of Pancreatic Lipase-Based Molecular Docking and In Vitro Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking Studies
2.2. Pancreatic Lipase Activity Assay
2.3. Statistical Analyses
3. Results
3.1. Chemical Structures
3.2. Pancreatic Lipase Activity
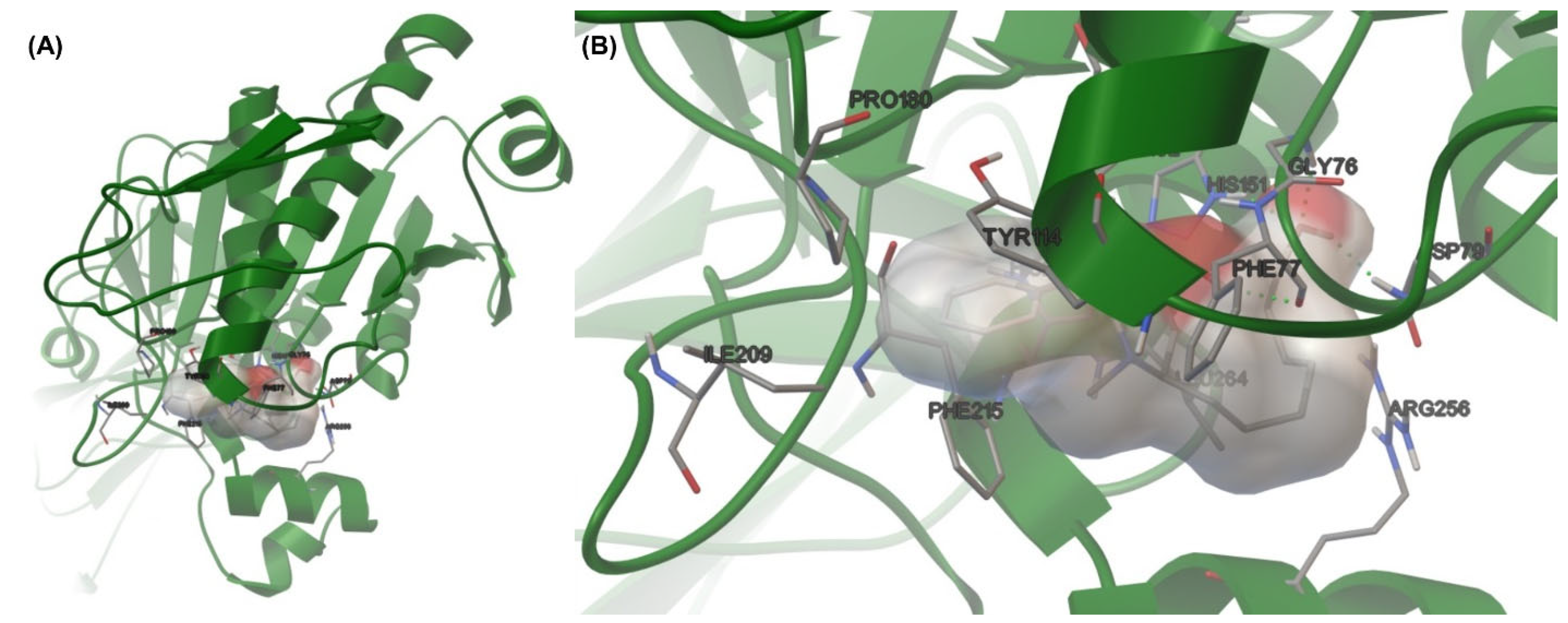
3.3. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; ElHafeez, S.A.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Toy, E.C.; Baker, B., III. The evaluation and treatment of the overweight patient. Prim. Care Update Ob. Gyns. 2000, 7, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Perrio, M.J.; Wilton, L.V.; Shakir, S.A. The safety profiles of orlistat and sibutramine: Results of prescription-event monitoring studies in England. Obesity 2007, 15, 2712–2722. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.S.; Raja, K.K.; Murugesan, S.; Kumar, B.K.; Rajagopal, G.; Thirunavukkarasu, S. Synthesis, biological evaluation, molecular docking, molecular dynamics and DFT studies of quinoline-fluoroproline amide hybrids. J. Mol. Struct. 2020, 1217, 128360. [Google Scholar] [CrossRef]
- Ganesan, M.S.; Raja, K.K.; Narasimhan, K.; Murugesan, S.; Kumar, B.K. Design, synthesis, α-amylase inhibition and in silico docking study of novel quinoline bearing proline derivatives. J. Mol. Struct. 2020, 1208, 127873. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in drug discovery: A versatile scaffold for novel biologically active compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Saify, Z.S.; Begum, N.; Ashraf, S.; Zarreen, T.; Saeed, S.G. Emerging phaaceutical applications of piperidine, pyrrolidine and it s derivaties. World J. Pharm. Res. 2014, 3, 2277–7105. [Google Scholar]
- Bhat, A.A.; Tandon, N.; Tandon, R. Pyrrolidine derivatives as Anti-diabetic agents: Current status and future prospects. ChemistrySelect 2022, 7, e202103757. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Sukpondma, Y.; Tadpetch, K.; Muanprasat, C.; Rukachaisirikul, V.; Inchai, J.; Vaddhanaphuti, C.S. Cholesterol-Lowering Effects of Asperidine B, a Pyrrolidine Derivative from the Soil-Derived Fungus Aspergillus sclerotiorum PSU-RSPG178: A Potential Cholesterol Absorption Inhibitor. Pharmaceuticals 2022, 15, 955. [Google Scholar] [CrossRef] [PubMed]
- Thongpat, K.; Holasut, P.; Ontawong, A.; Inchai, J.; Duangjai, A.; Rukachaisirikul, V.; Vaddhanaphuti, C.S.; Tadpetch, K. Synthesis and potential antidiabetic and lipid-lowering activities of putative asperidine B and its desmethyl analogue. Bioorg. Med. Chem. Lett. 2023, 93, 129437. [Google Scholar] [CrossRef] [PubMed]
- Tadpetch, K.; Thongpat, K.; Jeensrikong, N.; Permrungreang, N.; Holasut, P.; Rojanaverawong, W.; Saithong, T.; Tunkaew, K.; Vaddhanaphuti, C.S. Synthesis and lipid-lowering activities of preussin derivatives. Bioorg. Med. Chem. 2025, 129, 118336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.J.; Park, H.W.; Youn, S.H.; Choi, D.Y.; Shin, C.S. Development of inhibitors against lipase and α-glucosidase from derivatives of monascus pigment. FEMS Microbiol. Lett. 2007, 276, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.A.; Vereshchagin, A.N. Piperidine derivatives: Recent advances in synthesis and pharmacological applications. Int. J. Mol. Sci. 2023, 24, 2937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lathwal, E.; Saroha, B.; Kumar, G.; Bhardwaj, A.; Bishnoi, P.; Rani, M.; Raghav, N.; Kumar, R.; Kumar, S. Design, synthesis, molecular docking and biological studies of some novel pyrrolidine-triazole-aurone hybrids against digestive enzymes. Res. Chem. Intermed. 2024, 50, 1249–1271. [Google Scholar] [CrossRef]
- Banerjee, R.; Lipscomb, J.D. Small-molecule tunnels in metalloenzymes viewed as extensions of the active site. Acc. Chem. Res. 2021, 54, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saroha, B.; Lathwal, E.; Kumar, G.; Arya, P.; Raghav, N.; Kumar, R.; Kumar, S. Synthetic and Biological Studies of Some Pyrrolidine-Tethered Novel Aurones against Digestive Enzymes. Curr. Org. Chem. 2023, 27, 1821–1832. [Google Scholar] [CrossRef]
- Chahinian, H.; Sias, B.; Carriere, F. The C-Terminal Domain of Pancreatic Lipase Functional and Structural Analogies with C2 Domains. Curr. Protein Pept. Sci. 2000, 1, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Egloff, M.P.; Marguet, F.; Buono, G.; Verger, R.; Cambillau, C.; van Tilbeurgh, H. The 2.46. ANG. resolution structure of the pancreatic lipase-colipase complex inhibited by a C11 alkyl phosphonate. Biochemistry 1995, 34, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Guerciolini, R. Mode of action of orlistat. International journal of obesity and related metabolic disorders. Int. J. Obes. Relat. Metab. Disord. 1997, 21, S12–S23. [Google Scholar] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-based nanoparticles: Advancements and challenges in tumor therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef] [PubMed]
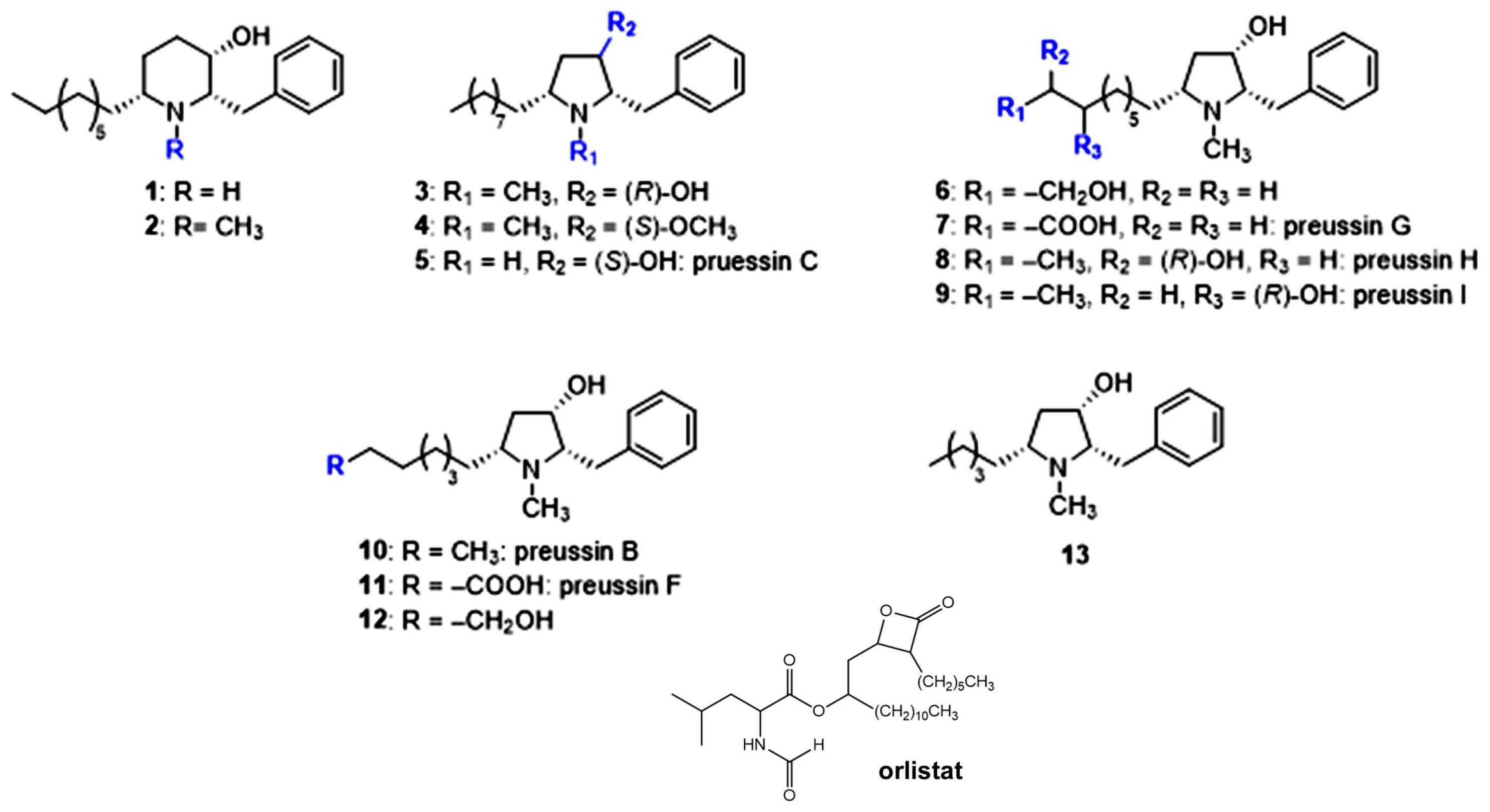

| Compounds | Pancreatic Lipase Inhibition (IC50; mg/mL) | Compounds | Pancreatic Lipase Inhibition (IC50; mg/mL) |
|---|---|---|---|
| Orlistat | 0.0005 ± 0.0005 | 7 | 0.329 ± 0.001 |
| 1 | >10 | 8 | 0.655 ± 0.001 |
| 2 | >10 | 9 | 0.591 ± 0.001 |
| 3 | 4.045 ± 0.003 | 10 | 0.362 ± 0.001 |
| 4 | 0.769 ± 0.001 | 11 | 1.837 ± 0.001 |
| 5 | 1.314 ± 0.001 | 12 | 0.143 ± 0.001 |
| 6 | 0.846 ± 0.001 | 13 | 0.226 ± 0.001 |
| Compounds | Binding Energy (kcal/mol) | % Member in Cluster | H-Bond Interaction |
|---|---|---|---|
| Orlistat | −5.72 | 8 | - |
| 1 | −6.59 | 32 | O-H---O=C (Phe77) |
| 2 | −6.27 | 33 | - |
| 3 | −7.42 | 57 | - |
| 4 | −7.20 | 28 | - |
| 5 | −6.87 | 21 | O-H---O=C (Phe77) |
| 6 | −6.19 | 16 | - |
| 7 | −6.79 | 20 | C=O---H-N (His263) |
| 8 | −7.43 | 15 | H-O---H-N (Gly76) O-H---O=C (Asp79) H-O---H-N (His151) |
| 9 | −7.20 | 12 | H-O---H-N (Gly76) O-H---O=C (Asp79) H-O---H-N (His151) |
| 10 | −7.39 | 50 | H-O---H-N (Phe77) H-O---H-O (Ser152) |
| 11 | −6.78 | 27 | C=O---H-N (Gly76) C=O---H-N (His151) |
| 12 | −8.24 | 32 | H-O---H-N (Gly76) O-H---O=C (Phe77) O-H---O=C (Asp79) H-O---H-N (His151) |
| 13 | −7.24 | 83 | H-O---H-N (Phe77) H-O---H-O (Ser152) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangjai, A.; Tadpetch, K.; Rukachaisirikul, V.; Vaddhanaphuti, C.S.; Utsintong, M. Identification of Novel Piperidine and Pyrrolidine Derivatives as Potent Inhibitors of Pancreatic Lipase-Based Molecular Docking and In Vitro Testing. Sci. Pharm. 2025, 93, 43. https://doi.org/10.3390/scipharm93030043
Duangjai A, Tadpetch K, Rukachaisirikul V, Vaddhanaphuti CS, Utsintong M. Identification of Novel Piperidine and Pyrrolidine Derivatives as Potent Inhibitors of Pancreatic Lipase-Based Molecular Docking and In Vitro Testing. Scientia Pharmaceutica. 2025; 93(3):43. https://doi.org/10.3390/scipharm93030043
Chicago/Turabian StyleDuangjai, Acharaporn, Kwanruthai Tadpetch, Vatcharin Rukachaisirikul, Chutima S. Vaddhanaphuti, and Maleeruk Utsintong. 2025. "Identification of Novel Piperidine and Pyrrolidine Derivatives as Potent Inhibitors of Pancreatic Lipase-Based Molecular Docking and In Vitro Testing" Scientia Pharmaceutica 93, no. 3: 43. https://doi.org/10.3390/scipharm93030043
APA StyleDuangjai, A., Tadpetch, K., Rukachaisirikul, V., Vaddhanaphuti, C. S., & Utsintong, M. (2025). Identification of Novel Piperidine and Pyrrolidine Derivatives as Potent Inhibitors of Pancreatic Lipase-Based Molecular Docking and In Vitro Testing. Scientia Pharmaceutica, 93(3), 43. https://doi.org/10.3390/scipharm93030043





