In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Methods
2.2.1. Essential Oil Preparation
2.2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.2.3. In Silico Assays of the Potential Inhibition of Indoleamine 2,3-dioxygenase and Hydroxymethylglutaryl-CoA
Molecular Docking
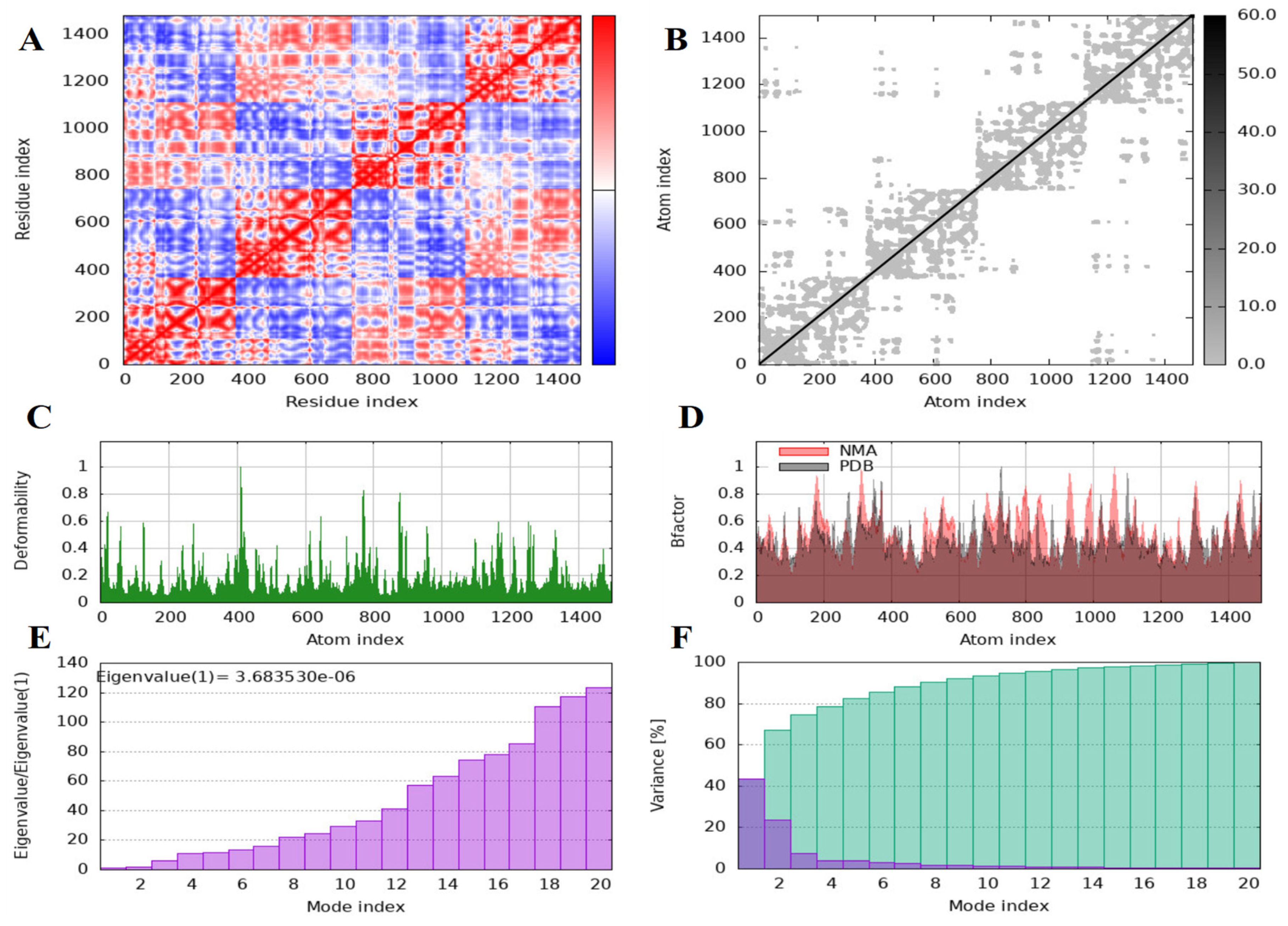
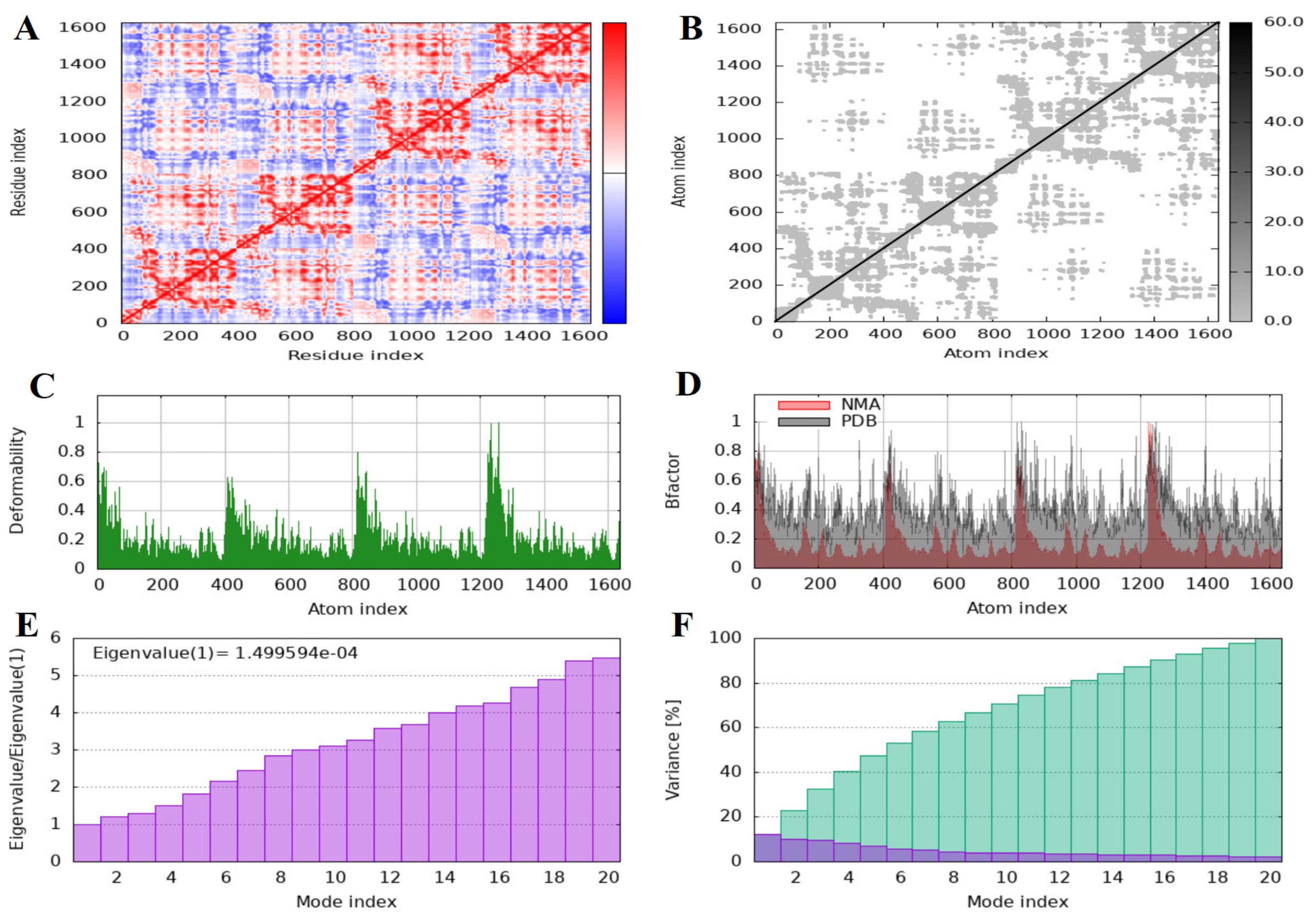
Normal Mod Analysis
2.2.4. Fabrication of EO Nanocapsules
2.2.5. Measurement of Particle Size, Polydispersity Index, and Zeta Potential
2.2.6. Encapsulation Efficiency Determination
2.2.7. Antioxidant DPPH and FRAP Assays
DPPH Free Radical Scavenging Activity Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
2.2.8. Release of the EO at Gastrointestinal pH
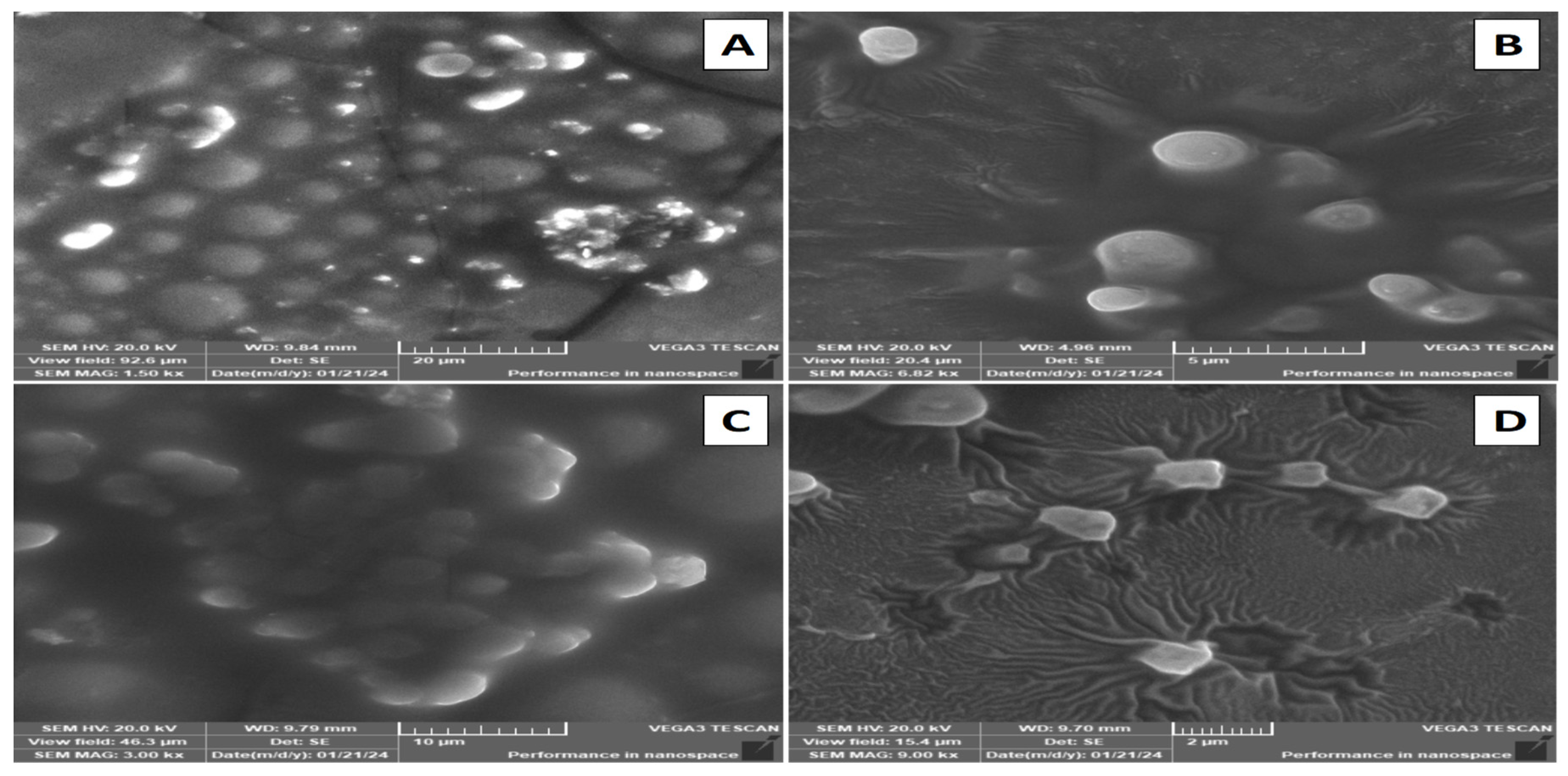
2.2.9. Scanning Electron Microscopy (SEM)
2.2.10. Bioassays
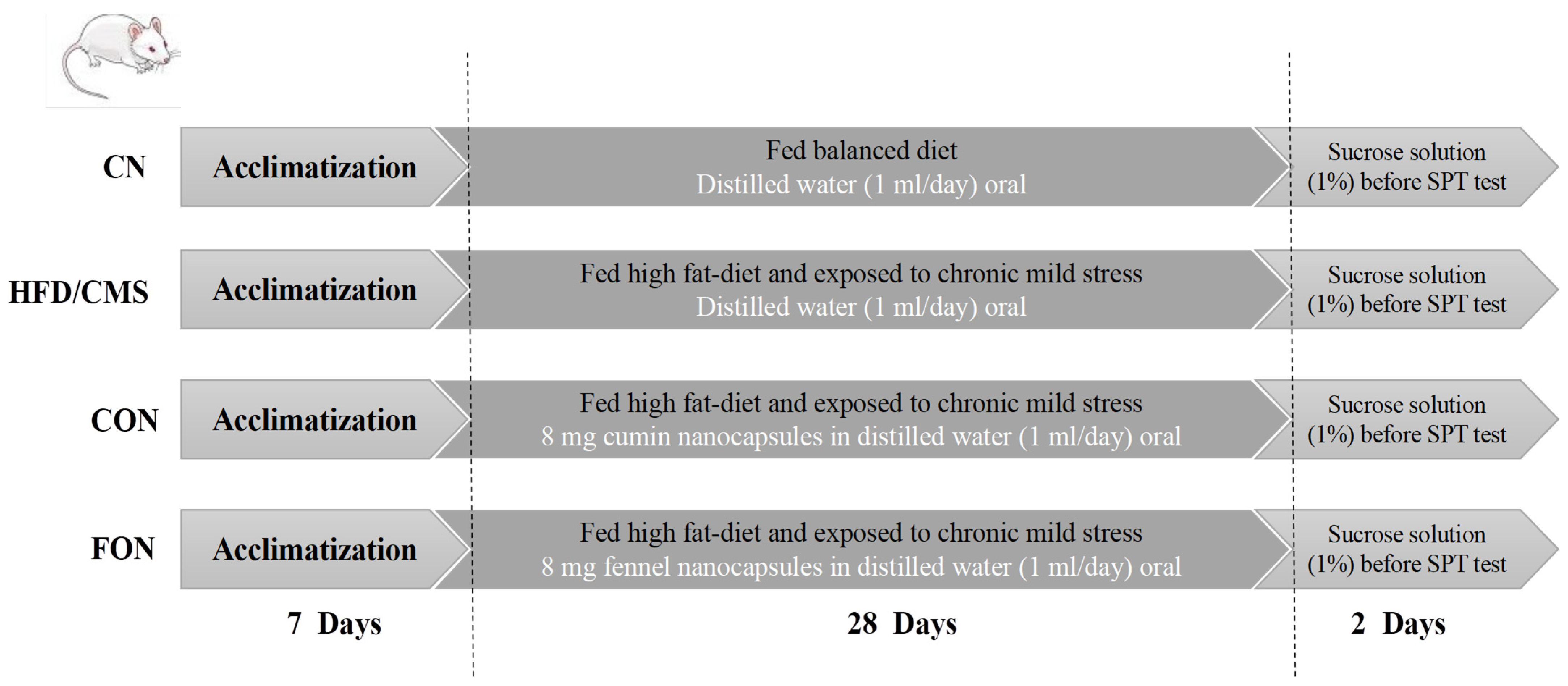
Experimental Animals
Diets
Chronic Mild Stress (CMS)
The Design of the Animal Experiment
Sucrose Preference Test
Blood Collection
Tissues Collection
Biochemical Analysis of the Brain and Serum
Statistical Analysis
3. Results and Discussion
3.1. The Composition of Cumin and Fennel EOs
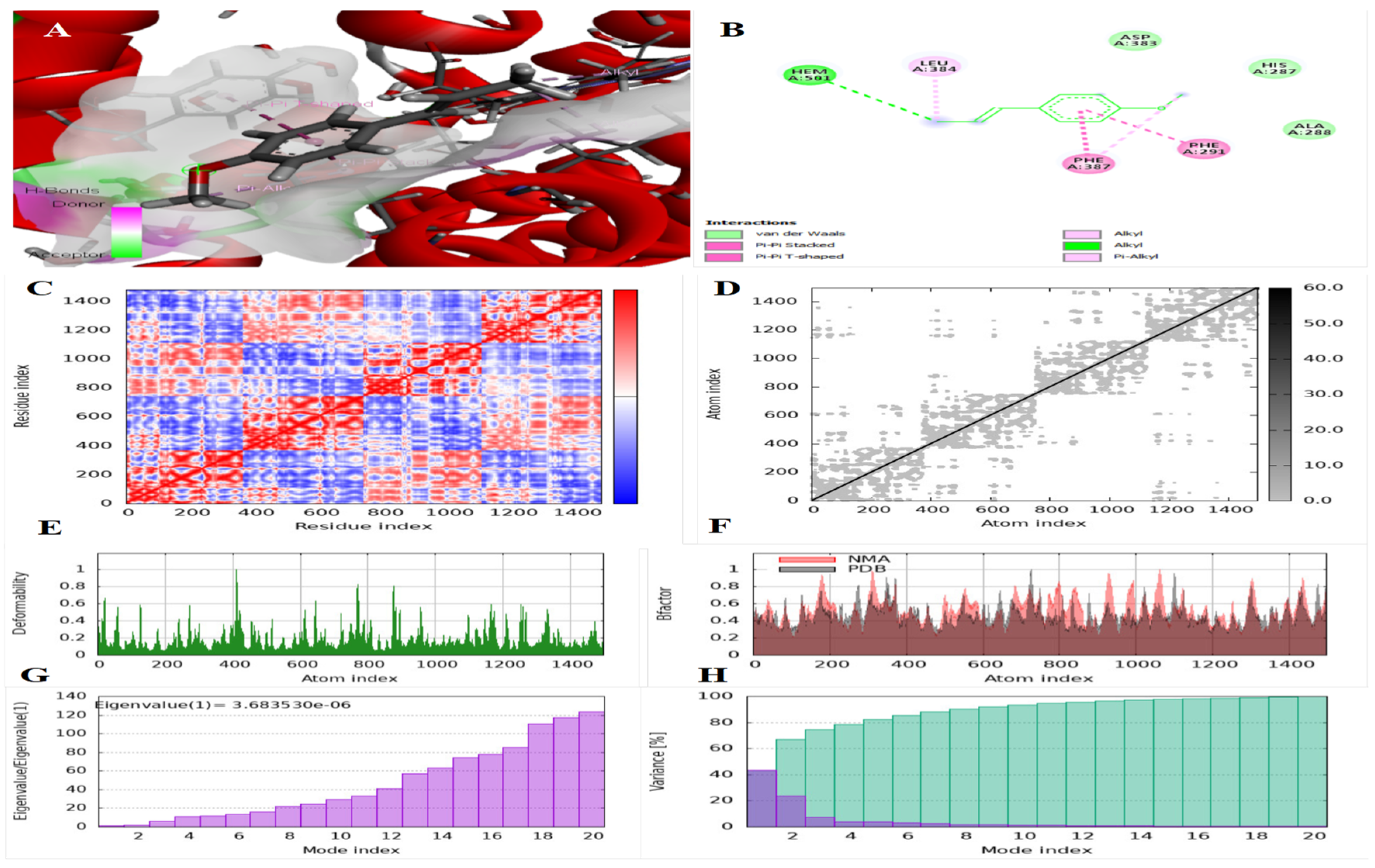
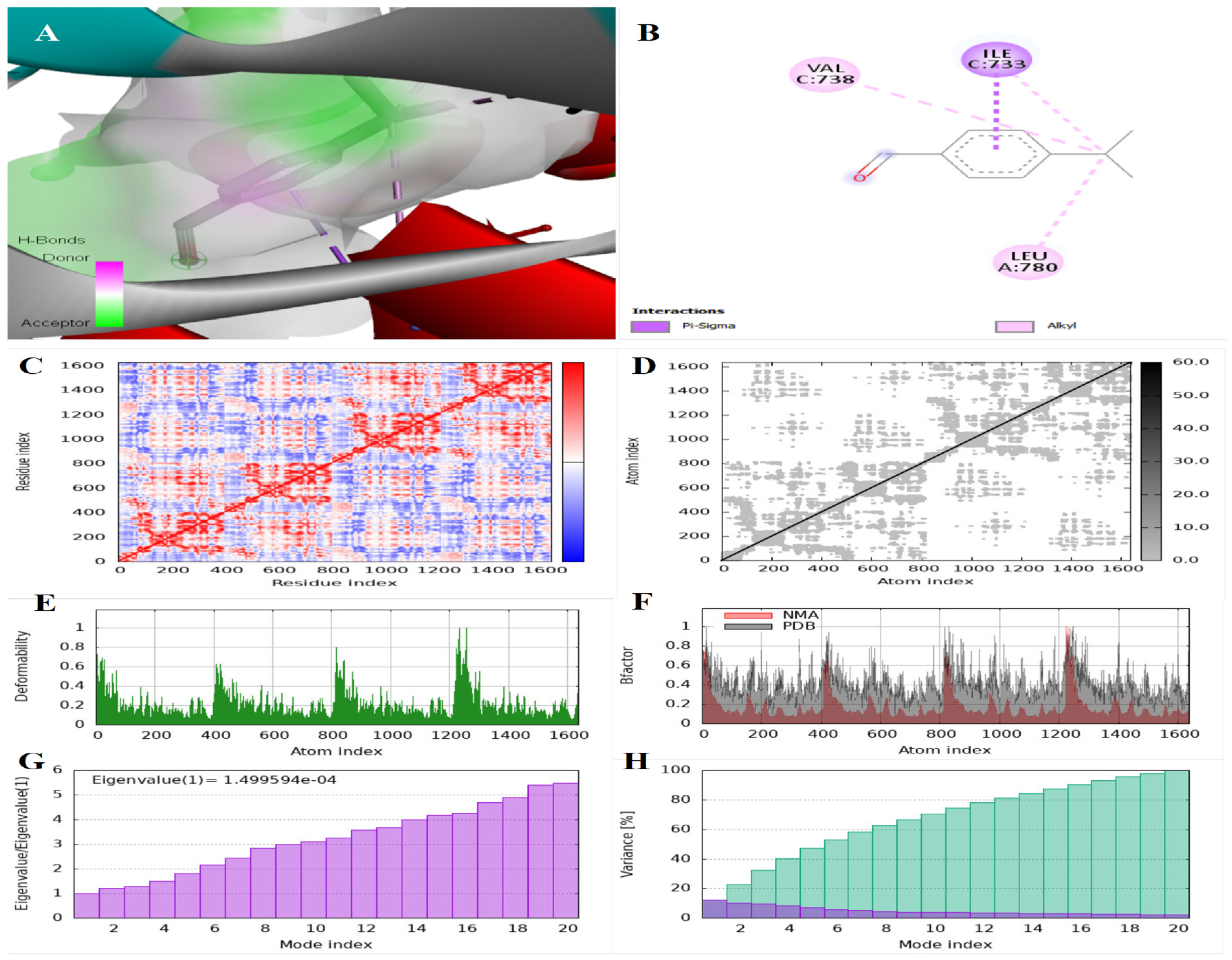
3.2. Findings of the In Silico Studies
3.3. The Particle Size, Polydispersity Index, and Zeta Potential of Cumin and Fennel EO Nanoparticles
3.4. In Vitro Antioxidant Activity of Cumin and Fennel EO Nanoparticles
3.5. Release of the EO at Gastrointestinal pH
3.6. Scanning Electron Microscopy (SEM)
3.7. In Vivo Study Findings
3.7.1. The Effect of Cumin and Fennel EO Nanoparticles on Sucrose Preference
3.7.2. The Effect of Cumin and Fennel EO Nanoparticles on Growth Performance
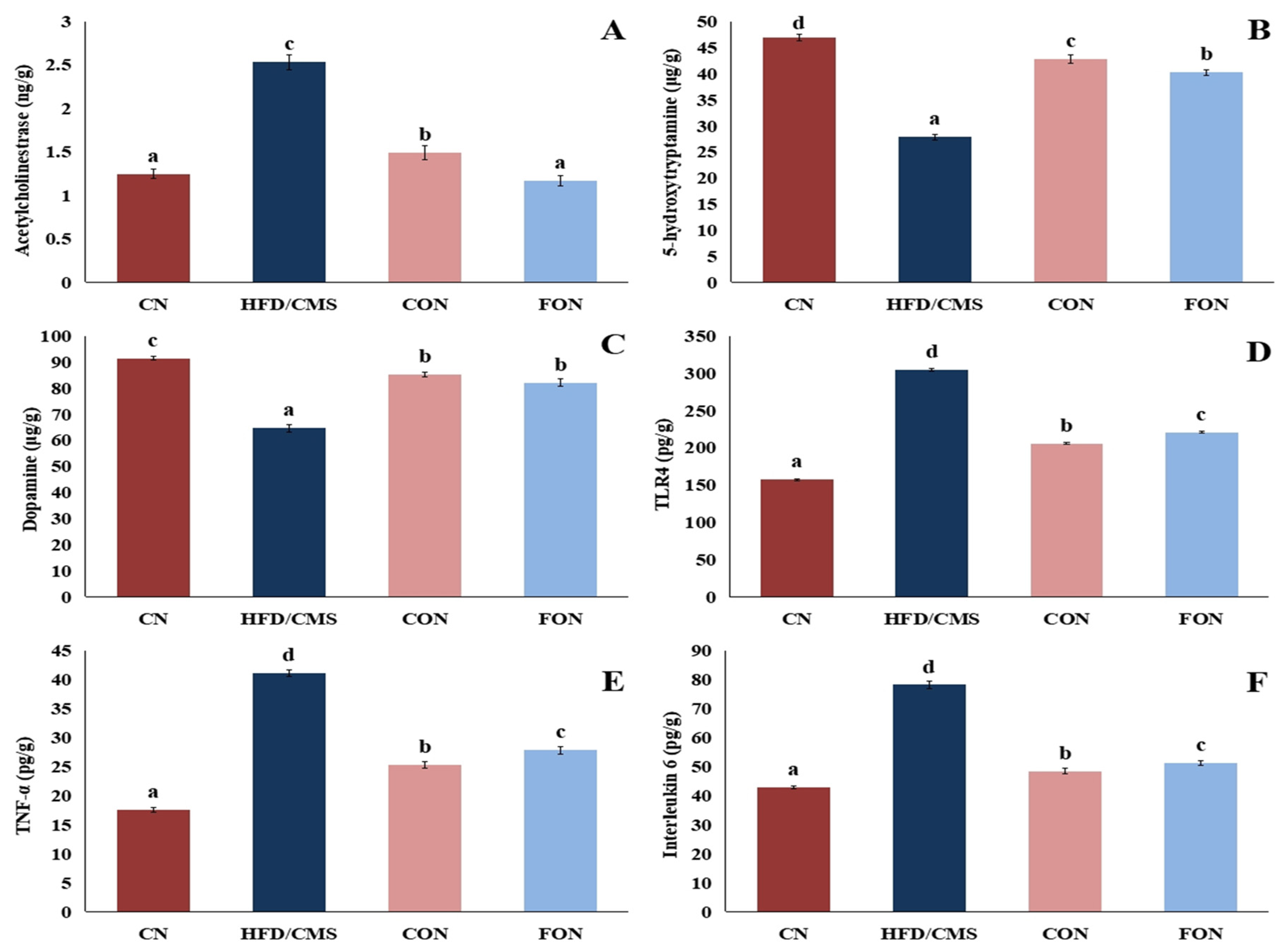
3.7.3. The Effect of Cumin and Fennel EO Nanoparticles on Neurotransmitters and Inflammatory Markers in the Brain
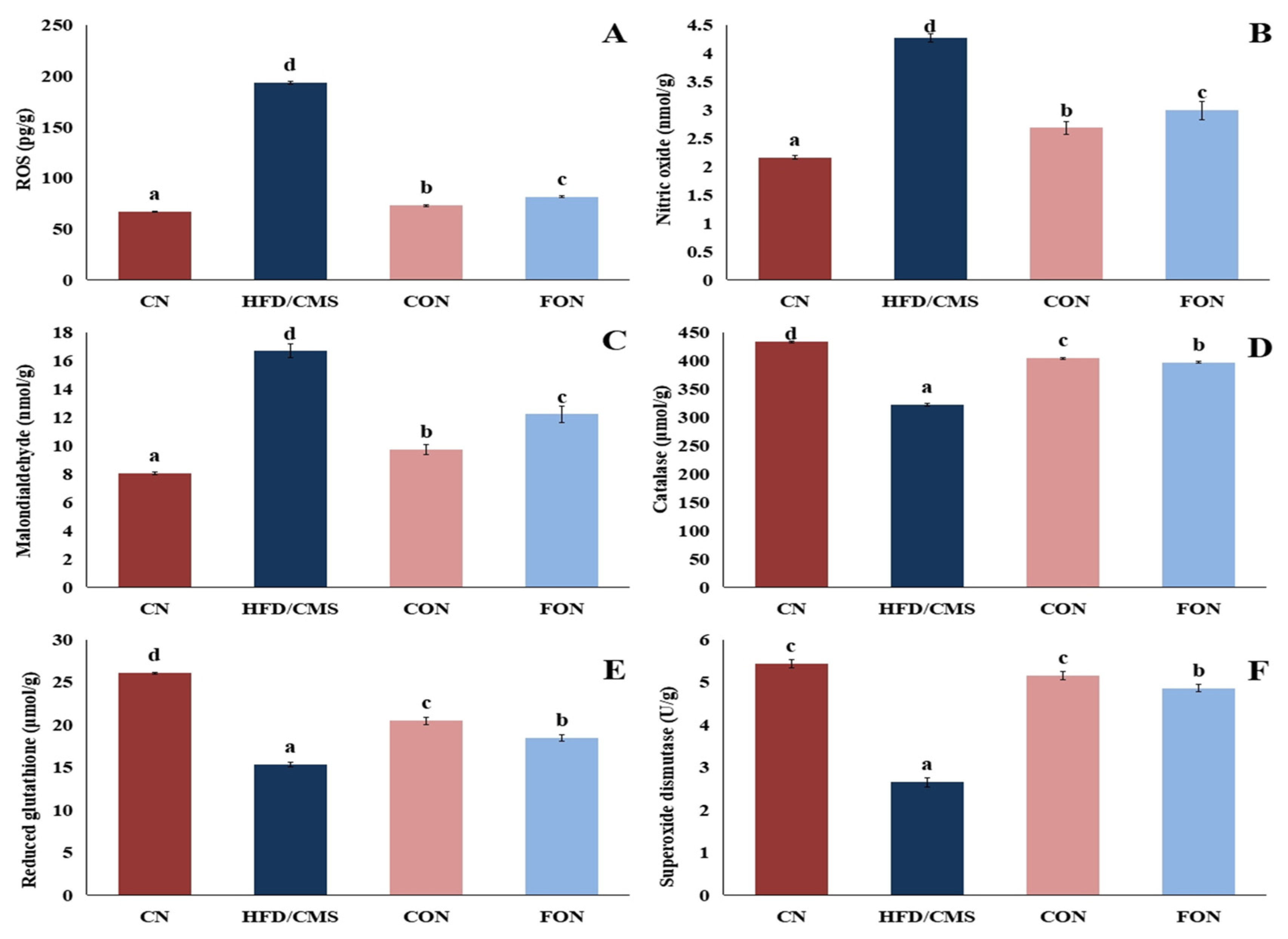
3.7.4. The Effect of Cumin and Fennel EO Nanoparticles on Oxidant and Antioxidant Markers in the Brain
3.7.5. The Effect of Cumin and Fennel EO Nanoparticles on the Lipid Profile
3.7.6. The Effect of Cumin and Fennel EO Nanoparticles on Liver and Kidney Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, H.M.; Stevenson, R.J.; Tan, L.S.Y.; Ehrenfeld, L.; Byeon, S.; Attuquayefio, T.; Gupta, D.; Lim, C.K. Kynurenic acid as a biochemical factor underlying the association between Western-style diet and depression: A cross-sectional study. Front. Nutr. 2022, 9, 945538. [Google Scholar] [CrossRef]
- Gao, L.N.; Yan, M.; Zhou, L.; Wang, J.; Sai, C.; Fu, Y.; Liu, Y.; Ding, L. Puerarin Alleviates Depression-Like Behavior Induced by High-Fat Diet Combined with Chronic Unpredictable Mild Stress via Repairing TLR4- Induced Inflammatory Damages and Phospholipid Metabolism Disorders. Front. Pharmacol. 2021, 12, 767333. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary between Brain and Periphery. Antioxid. Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Lützhøft, D.O.; Bækgård, C.; Wimborne, E.; Straarup, E.M.; Pedersen, K.M.; Swann, J.R.; Pedersen, H.D.; Kristensen, K.; Morgills, L.; Nielsen, D.S.; et al. High fat diet is associated with gut microbiota dysbiosis and decreased gut microbial derived metabolites related to metabolic health in young Göttingen Minipigs. PLoS ONE 2024, 19, e0298602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qi, C.; Zheng, J.; Sun, M.; Jin, L.; Sun, J. The Antidepressant Effect of Deoiled Sunflower Seeds on Chronic Unpredictable Mild Stress in Mice Through Regulation of Microbiota–Gut–Brain Axis. Front. Nutr. 2022, 9, 908297. [Google Scholar] [CrossRef]
- Korinek, M.; Handoussa, H.; Tsai, Y.H.; Chen, Y.Y.; Chen, M.H.; Chiou, Z.W.; Fang, Y.; Chang, F.R.; Yen, C.H.; Hsieh, C.F.; et al. Anti-Inflammatory and Antimicrobial Volatile Oils: Fennel and Cumin Inhibit Neutrophilic Inflammation via Regulating Calcium and MAPKs. Front. Pharmacol. 2021, 12, 674095. [Google Scholar] [CrossRef]
- Tang, D.; Liang, Q.; Zhang, M.; Li, M.; Zhang, Q.; Zhang, S.; Ai, L.; Wu, C. Anti-depression effectiveness of essential oil from the fruits of Zanthoxylum bungeanum maxim. on chronic unpredictable mild stress-induced depression behavior in mice. Front. Pharmacol. 2022, 13, 999962. [Google Scholar] [CrossRef]
- Fonseca, E.C.; Ferreira, L.R.; Figueiredo, P.L.; Maia, C.D.; Setzer, W.N.; Da Silva, J.K. Antidepressant Effects of Essential Oils: A Review of the Past Decade (2012–2022) and Molecular Docking Study of Their Major Chemical Components. Int. J. Mol. Sci. 2022, 24, 9244. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, W.; Yu, G.; Li, Z.; She, Y.; Lee, M. Three-stage microwave extraction of cumin (Cuminum cyminum L.) Seed essential oil with natural deep eutectic solvents. Ind. Crops Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Iram, N.; Edwin, E.C. Chemical composition, essential oil characterization and antibacterial activity of cumin (Cuminum cyminum). Pak. Biomed. J. 2022, 5, 86–90. [Google Scholar] [CrossRef]
- Nikkhah, A.; Nikkhah, H.; Shahbazi, A.; Zarin, M.K.Z.; Beykal, I.D.; Ebadi, M.T.; Fakhroleslam, M.; Beykal, B. Cumin and eucalyptus essential oil standardization using fractional distillation: Data-driven optimization and techno-economic analysis. Food Bioprod. Process. 2024, 143, 90–101. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Bi, Y.; Miao, R.; Zhang, Z.; Su, H. Anti-Inflammatory Effects of Cumin Essential Oil by Blocking JNK, ERK, and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 474509. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Mahmoodnia, L.; Tahmasebi, P.; Memarzadeh, M.R.; Sedehi, M.; Beigi, M.; Fallah, A.A. Effect of cumin (Cuminum cyminum) essential oil supplementation on metabolic profile and serum leptin in pre-diabetic subjects: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2018, 47, 416–422. [Google Scholar] [CrossRef]
- Haque, M.R.; Ansari, S.H. Aromatic aldehyde compound cuminaldehyde protects nonalcoholic fatty liver disease in rats feeding high fat diet. Hum. Exp. Toxicol. 2019, 38, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Imbabi, T.; Sabeq, I.; Osman, A.; Mahmoud, K.; Amer, S.A.; Hassan, A.M.; Kostomakhin, N.; Habashy, W.; Easa, A.A. Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality. Antioxidants 2021, 10, 1797. [Google Scholar] [CrossRef]
- Barakat, H.; Alkabeer, I.A.; Althwab, S.A.; Alfheeaid, H.A.; Alhomaid, R.M.; Almujaydil, M.S.; Almuziree, R.S.A.; Bushnaq, T.; Mohamed, A. Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats. Antioxidants 2023, 12, 325. [Google Scholar] [CrossRef]
- Hong, S.J.; Yoon, S.; Jo, S.M.; Jeong, H.; Youn, M.Y.; Kim, Y.J.; Kim, J.K.; Shin, E.C. Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients 2022, 14, 741. [Google Scholar] [CrossRef]
- Neetu, J.S.; Sunil, K.; Rana, A.C. Antidepressant activity of methanolic extract of Foeniculum vulgare (Fennel) fruits in experimental animal models. Int. J. Pharm. Sci. Res. 2013, 3, 065–070. [Google Scholar] [CrossRef]
- Singh, S.P. A comprehensive review on pharmacological activity of Foeniculum vulgare. Glob. J. Pharm. Sci. 2019, 7, 23–27. [Google Scholar] [CrossRef]
- Jayari, A.; Donsì, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of Thyme Essential Oils: Formulation, Characterization, Storage Stability, and Biological Activity. Foods 2022, 11, 1858. [Google Scholar] [CrossRef]
- Luo, S.; Xu, K.; Xiang, S.; Chen, J.; Chen, C.; Guo, C.; Tong, Y.; Tong, L. High-resolution structures of inhibitor complexes of human indoleamine 2,3-dioxygenase 1 in a new crystal form. Acta Crystallogr. F Struct. Biol. Commun. 2018, 74, 717–724. [Google Scholar] [CrossRef]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.M.; Mahross, M.H.; Gad-Elkareem, M.A.; Rudrapal, M.; Gogoi, N.; Chetia, D.; Aouadi, K.; Snoussi, M.; Kadri, A. Toward a treatment of antibacterial and antifungal infections: Design, synthesis and in vitro activity of novel arylhydrazothiazolylsulfonamides analogues and their insight of DFT, docking and molecular dynamic simulations. J. Mol. Struct. 2021, 1243, 130862. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Aqeel, S.M.; Cui, H.; Lin, L. Nanoencapsulation of Mandarin Essential Oil: Fabrication, Characterization, and Storage Stability. Foods 2021, 11, 54. [Google Scholar] [CrossRef]
- Pudžiuvelytė, L.; Petrauskaitė, E.; Stabrauskienė, J.; Bernatonienė, J. Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals 2025, 18, 963. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Singh, V.K.; Prakash, B.; Dubey, N.K. Nanoencapsulated Illicium verum Hook.f. essential oil as an effective novel plant-based preservative against aflatoxin B1 production and free radical generation. Food Chem. Toxicol. 2018, 111, 102–113. [Google Scholar] [CrossRef]
- Asres, Y.; Hymete, A.; Admassu, H.; Ayalew, A. Application of microencapsulated essential oil extracted from Thymus schimperi for its antibacterial and antioxidant activity on minced meat. Discov. Appl. Sci. 2025, 7, 485. [Google Scholar] [CrossRef]
- Djihad, N.; Naima, F.O.; Petronilho, S.; Hamid, S.; Bedjou, F.N.E.; Coimbra, M.A. Microencapsulation of Citrus limon essential oil by complex coacervation and release behavior of terpenic and derived volatile compounds. Food Hydrocoll. 2024, 152, 109830. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Fouda, K.; Zaghloul, A.H.; Abdel-Salam, A.M. Potential antidiabetic activity of probiotic and Garcinia kola-yoghurt and its role in regulation of male fertility-stimulating hormones in high-fat diet/low dose streptozotocin-treated rats. Food Prod. Process. Nutr. 2024, 6, 55. [Google Scholar] [CrossRef]
- Lippi, S.L.P. Chronic Mild Unpredictable Stress and High-Fat Diet Given during Adolescence Impact Both Cognitive and Noncognitive Behaviors in Young Adult Mice. Brain Sci. 2021, 11, 260. [Google Scholar] [CrossRef]
- Moubarz, G.; Embaby, M.A.; Doleib, N.M.; Taha, M.M. Effect of dietary antioxidant supplementation (Cuminum cyminum) on bacterial susceptibility of diabetes-induced rats. Cent. Eur. J. 2016, 2, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Asaad, G.F.; Redai, A.Q.; Hakami, A.O.; Ghazwani, F.Y.; Nomier, Y.; Alshahrani, S. Potential analgesic and anti-inflammatory effect of Cuminum cyminum and Borago officinalis in rats and mice. Asian J. Pharm. Clin. Res. 2020, 13, 216–218. [Google Scholar] [CrossRef][Green Version]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stresss-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Hosney, M.; Bassiony, H.; Hassanein, S.S.; Soliman, A.M.; Fahmy, S.R.; Gaafar, K. Sodium pentobarbital dosages for exsanguination affect biochemical, molecular and histological measurements in rats. Sci. Rep. 2020, 10, 378. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Fouda, K.; Salama, A.; Akl, E.M. Peanut meal-derived bioactive compounds: Extraction, co-extrusion encapsulation and neuroprotection against aluminum-induced Alzheimer’s disease via in silico and in vivo studies. Phytomed. Plus 2024, 4, 100588. [Google Scholar] [CrossRef]
- Moradi, A.; Davati, N.; Emamifar, A. Effects of Cuminum cyminum L. essential oil and its nanoemulsion on oxidative stability and microbial growth in mayonnaise during storage. Food Sci. Nutr. 2023, 11, 4781–4793. [Google Scholar] [CrossRef]
- Stefănescu, R.; Osz, B.E.; Pintea, A.; Laczkó-Zöld, E.; Tero-Vescan, A.; Vari, C.E.; Fulop, E.; Blas, I.; Vancea, S. Fennel Essential Oil as a Complementary Therapy in the Management of Diabetes. Pharmaceutics 2023, 15, 2657. [Google Scholar] [CrossRef]
- Saleem, U.; Shehzad, A.; Shah, S.; Raza, Z.; Shah, M.A.; Bibi, S.; Chauhdary, Z.; Ahmad, B. Antiparkinsonian activity of Cucurbita pepo seeds along with possible underlying mechanism. Metab. Brain Dis. 2021, 36, 1231–1251. [Google Scholar] [CrossRef]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.G.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr–/– Mice: Impact on Cognitive Function. J. Alzheimer’s Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, N.; Zhang, X.; Han, X.; Zhai, X.; Lu, Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab. Brain Dis. 2018, 33, 1787–1800. [Google Scholar] [CrossRef]
- Shah, M.A.; Muhammad, H.; Mehmood, Y.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. Superoxide Scavenging and Antiglycation Activity of Rhinacanthins-rich Extract Obtained from the Leaves of Rhinacanthus nasutus. Pharmacogn. Mag. 2017, 13, 652–658. [Google Scholar] [CrossRef]
- Noshad, M.; Behbahani, B.A.; Nikfarjam, Z.; Zargari, F. Antimicrobial activity between Coriandrum sativum seed and Cuminum cyminum essential oils against foodborne pathogens: A multi-ligand molecular docking simulation. LWT 2023, 185, 115217. [Google Scholar] [CrossRef]
- Sumera; Anwer, F.; Waseem, M.; Fatima, A.; Malik, N.; Ali, A.; Zahid, S. Molecular Docking and Molecular Dynamics Studies Reveal Secretory Proteins as Novel Targets of Temozolomide in Glioblastoma Multiforme. Molecules 2022, 27, 7198. [Google Scholar] [CrossRef] [PubMed]
- Shakibay Senobari, Z.; Masoumian Hosseini, M.; Teimouri, M.B.; Rezayan, A.H.; Samarghandian, S.; Hekmat, A. Chromone-embedded peptidomimetics and furopyrimidines as highly potent SARS-CoV-2 infection inhibitors: Docking and MD simulation study. BMC Res. Notes 2023, 16, 224. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Ockun, M.A.; Uner, B.; Tas, C.; Ivanauskas, L. Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules. Molecules 2021, 26, 6304. [Google Scholar] [CrossRef]
- García-Armenta, E.; Picos-Corrales, L.A.; Gutiérrez-López, G.F.; Gutiérrez-Dorado, R.; Perales-Sánchez, J.X.K.; García-Pinilla, S.; Reynoso-García, F.; Martínez-Audelo, J.M.; Armenta-Manjarrez, M.A. Preparation of surfactant-free emulsions using amaranth starch modified by reactive extrusion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125550. [Google Scholar] [CrossRef]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Liu, L.; Cao, Y.C.; Zhu, H. Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp. Cyprinus carpio. Aquaculture 2020, 518, 734781. [Google Scholar] [CrossRef]
- Shah, M.A.; Jakkawanpitak, C.; Sermwittayawong, D.; Panichayupakaranant, P. Rhinacanthins-rich Extract Enhances Glucose Uptake and Inhibits Adipogenesis in 3T3-L1 Adipocytes and L6 Myotubes. Pharmacogn. Mag. 2018, 13, S817–S821. [Google Scholar] [CrossRef]
- Van, C.K.; Nguyen, P.T.N.; Nguyen, T.T.T.; Bach, L.G. Microencapsulation of Citrus latifolia peel essential oil by spray-drying using maltodextrin: Characterization, antimicrobial activities, and release profile. LWT 2024, 197, 115825. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. The Effects of High-fat-diet Combined with Chronic Unpredictable Mild Stress on Depression-like Behavior and Leptin/LepRb in Male Rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, R.; Jayamurali, D.; Manoharan, N.; Govindarajalu, S.N. Neuroprotective effect of bromelain on BDNF-TRKB signalling pathway in chronic unpredictable stress-induced depression model. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 21. [Google Scholar] [CrossRef]
- Asadi, K.; Abbasi-Maleki, S.; Sadeghi Hashjin, G. Antidepressant-like effect of Cuminum cyminum essential oil on the forced swim and tail suspension tests in male mice. J. Shahrekord Univ. Med. Sci. 2020, 22, 167–172. [Google Scholar] [CrossRef]
- Haque, M.; Ansari, H. Anti-Obesity Effect of Arq Zeera and Its Main Components Thymol and Cuminaldehyde in High Fat Diet Induced Obese Rats. Drug Res. 2018, 68, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-Anethole Ameliorates Obesity via Induction of Browning in White Adipocytes and Activation of Brown Adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Maleki, S.G. Antidepressant-like effects of Foeniculum vulgare essential oil and potential involvement of dopaminergic and serotonergic systems on mice in the forced swim test. PharmaNutrition 2021, 15, 100241. [Google Scholar] [CrossRef]
- Hassanzadeh, S.A.; Abbasi-Maleki, S.; Mousavi, Z. Anti-depressive-like effect of monoterpene trans-anethole via monoaminergic pathways. Saudi J. Biol. Sci. 2022, 29, 3255–3261. [Google Scholar] [CrossRef]
- Rostami-Faradonbeh, N.; Amini-Khoei, H.; Zarean, E.; Bijad, E.; Lorigooini, Z. Anethole as a promising antidepressant for maternal separation stress in mice by modulating oxidative stress and nitrite imbalance. Sci. Rep. 2024, 14, 7766. [Google Scholar] [CrossRef]
- Azmi, N.H.; Ismail, N.; Imam, M.U.; Ooi, D.J.; Oslan, S.N.H. Modulation of High-Fat Diet-Induced Brain Oxidative Stress by Ferulate-Rich Germinated Brown Rice Ethyl Acetate Extract. Molecules 2022, 27, 4907. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What Does Plasma CRP Tell Us about Peripheral and central Inflammation in Depression. Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tian, Z.; Zhou, H.; Zhang, Y.; Chen, X.; Cui, Y.; Aifeire, A.; Zhang, X.; Wei, Z.; Shen, P.; et al. Elucidating the effects of cumin (Cuminum cyminum) fruit and stem as feed additives on growth, antioxidant capacity, liver and intestinal health, and gut microbiome of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2023, 31, 101687. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, L.; Wu, W.Z. Trans-Anethole Alleviates DSS-Induced Ulcerative Colitis by Remodeling the Intestinal Flora to Regulate Immunity and Bile Acid Metabolism. Mediat. Inflamm. 2023, 2023, 4188510. [Google Scholar] [CrossRef]
- de Morais, S.V.; Calado, G.P.; Carvalho, R.C.; Garcia, J.B.S.; de Queiroz, T.M.; Cantanhede Filho, A.J.; Lopes, A.J.O.; Cartágenes, M.d.S.d.S.; Domingues, G.R.d.S. Impact of Cuminaldehyde and Indomethacin Co-Administration on Inflammatory Responses in MIA-Induced Osteoarthritis in Rats. Pharmaceuticals 2024, 17, 630. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Li, Q.; Tong, Y.; Yang, Z.; Wang, T. Trans-anethole ameliorates LPS-induced inflammation via suppression of TLR4/NF-κB pathway in IEC-6 cells. Int. Immunopharmacol. 2022, 108, 108872. [Google Scholar] [CrossRef]
- Chen, Q.; Gan, Z.; Zhao, J.; Wang, Y.; Zhang, S.; Li, J.; Ni, Y. In vitro comparison of antioxidant capacity of cumin (Cuminum cyminum L.) oils and their main components. LWT-Food Sci. Technol. 2014, 55, 632–637. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants 2022, 11, 535. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Li, Y.; Ma, J. Prevalence and correlates of severe anxiety in patients with first hospitalization for major depressive disorder combined with dyslipidemia: A large sample cross-sectional study. Front. Psychiatry 2024, 14, 1289614. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Li, P.; Liu, S.; Zeng, Y. Chronic unpredictable mild stress combined with a high-fat diets aggravates atherosclerosis in rats. Lipids Health Dis. 2014, 13, 77. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, H.; Zhuang, N.; Yin, X.; Zhu, Z.; Yuan, Y.; Yin, X.; Wang, Y.; Cheung, E.F.C.; Chan, R.C.K.; et al. The role of lipoprotein profile in depression and cognitive performance: A network analysis. Sci. Rep. 2020, 10, 20704. [Google Scholar] [CrossRef]
- Han, A.L. Association between lipid ratio and depression: A cross-sectional study. Sci. Rep. 2022, 12, 6190. [Google Scholar] [CrossRef]
- Lin, P.Y.; Chang, A.Y.; Lin, T.K. Simvastatin treatment exerts antidepressant-like effect in rats exposed to chronic mild stress. Pharmacol. Biochem. Behav. 2014, 124, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Samadi-Noshahr, Z.; Hadjzadeh, M.A.; Moradi-Marjaneh, R.; Khajavi-Rad, A. The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci. Nutr. 2021, 9, 1121–1131. [Google Scholar] [CrossRef]
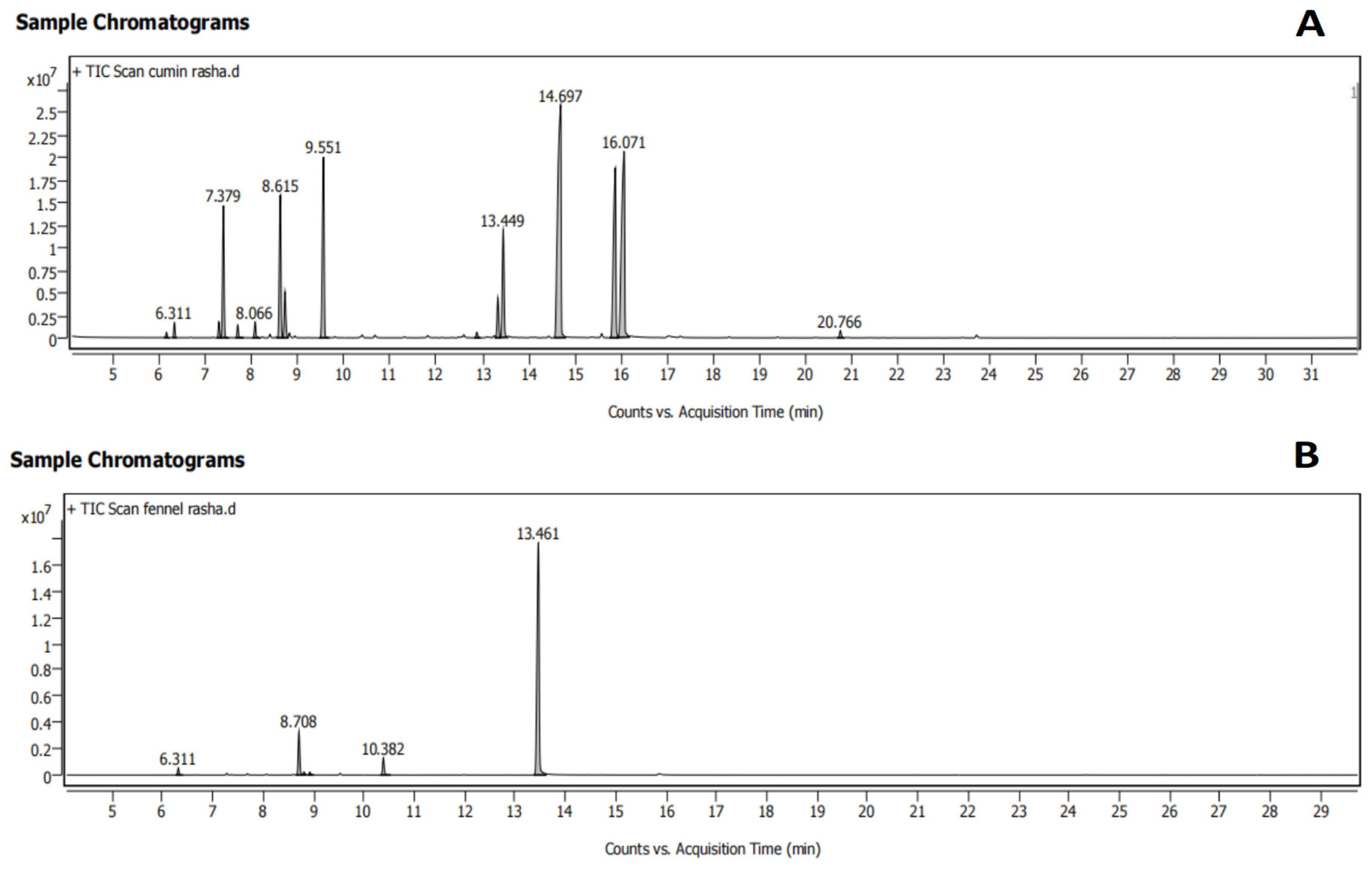









| Retention Time (min) | Compound | Chemical Formula | Molecular Weight (g/mol) | % |
|---|---|---|---|---|
| 6.143 | α-Thujene | C10H16 | 136.23 | 0.22 |
| 6.311 | α-Pinene | C10H16 | 136.23 | 0.64 |
| 7.281 | β-Phellandrene | C10H16 | 136.23 | 0.72 |
| 7.379 | β-Pinene | C10H16 | 136.23 | 6.67 |
| 7.685 | β-Myrcene | C10H16 | 136.23 | 0.58 |
| 8.066 | α-Phellandrene | C10H16 | 136.23 | 0.74 |
| 8.615 | p-Cymene | C10H14 | 134.22 | 7.94 |
| 8.713 | D-Limonene | C10H16 | 136.23 | 2.41 |
| 9.551 | γ-Terpinene | C10H16 | 136.23 | 11.02 |
| 12.877 | Terpinen-4-ol | C10H18O | 154.25 | 0.3 |
| 13.334 | 3-p-Menthen-7-al | C10H16O | 152.23 | 2.18 |
| 13.449 | Estragole | C10H12O | 148.2 | 6.49 |
| 14.697 | Cuminaldehyde | C10H12O | 148.2 | 26.48 |
| 15.880 | α-Terpinen-7-al | C10H14O | 150.22 | 13.87 |
| 16.071 | γ-Terpinen-7-al | C10H14O | 150.22 | 19.32 |
| 20.766 | Acoradiene | C15H22O | 218.33 | 0.42 |
| Retention Time (min) | Compound | Chemical Formula | Molecular Weight (g/mol) | % |
|---|---|---|---|---|
| 6.311 | α-Pinene | C10H16 | 136.23 | 1.5 |
| 8.708 | D-Limonene | C10H16 | 136.23 | 11.16 |
| 8.806 | Eucalyptol | C10H18O | 154.25 | 0.73 |
| 8.921 | trans-β-Ocimene | C10H16 | 136.23 | 0.68 |
| 10.382 | L-Fenchone | C10H16O | 152.23 | 4.48 |
| 13.461 | Trans-Anethole | C10H12O | 148.2 | 81.46 |
| Protein | Ligand | Binding Affinity (kcal/mol) | Interactions | |
|---|---|---|---|---|
| Hydrogen Bonds | Hydrophobic Interaction | |||
| IDO | Cuminaldehyde | −6.3 | (1) at HEM 501 residue | (2) at PHE 291 and PHE 387 residues |
| Terpinen-7-al | −9.9 | (1) at HEM 501 residue | (2) at PHE 291 and PHE 387 residues | |
| Trans-anethole | −5.9 | (1) at HEM 501 residue | (3) at PHE 291, PHE 387, and LEU 384 residues | |
| Indoximod | −6.2 | (1) at ARG 231 residue | - | |
| HMG-CoA | Cuminaldehyde | −5.9 | (3) at ILE 733, VAL 738, and LEU 780 residues | - |
| Terpinen-7-al | −8.5 | (1) at GLU 730 residue | (2) at ILE 733 and LEU 780 | |
| Trans-anethole | −5.7 | (5) at THR 557, THR 758, GLU 559, ASN 755, and ASP 767 residues | (5) at ILE 536, ILE 762, LUE 562, ALA 768, and ALA 769 residues | |
| Fluvastatin | −8.2 | (3) at TYR 514, ARG 515, and TYR 533 residues | (4) at TYR 511, PRO 513, TYR 517, and PRO 813 residues | |
| CON | FON | |
|---|---|---|
| Particle size (nm) | 193.7 ± 77.30 | 269.3 ± 59.80 |
| Zeta potential (mV) | −35.5 ± 2.34 | −22.8 ± 1.55 |
| PDI | 0.159 ± 0.00 | 0.049 ± 0.00 |
| EE (%) | 89.36 ± 1.11 | 86.42 ± 0.94 |
| DPPH (mg ascorbic acid equivalents/g nanocapsules) | 68.36 ± 1.23 | 64.17 ± 1.35 |
| FRAP (mg ascorbic acid equivalents/g nanocapsules) | 57.23 ± 0.21 | 55.33 ± 0.17 |
| CN | HFD/CMS | CON | FON | |
|---|---|---|---|---|
| Initial body weight (g) | 35.40 a ± 0.72 | 35.10 a ± 0.92 | 35.40 a ± 1.14 | 35.10 a ± 0.91 |
| Final body weight (g) | 69.20 a ± 1.47 | 76.80 b ± 1.73 | 69.60 a ± 1.13 | 71.40 a ± 1.49 |
| Body weight gain (g) | 33.80 a ± 0.93 | 41.70 b ± 1.05 | 34.20 a ± 0.70 | 36.30 a ± 0.84 |
| Daily food intake (g) | 13.94 | 13.23 | 13.56 | 13.47 |
| CN | HFD/CMS | CON | FON | |
|---|---|---|---|---|
| AST (U/L) | 46.78 b ± 0.61 | 73.20 c ± 1.41 | 48.70 b ± 1.11 | 42.00 a ± 0.75 |
| ALT (U/L) | 27.66 a ± 0.41 | 63.80 c ± 1.07 | 36.90 b ± 0.94 | 38.30 b ± 1.03 |
| LDH (U/L) | 240.80 a ± 0.85 | 381.70 d ± 1.58 | 259.00 b ± 1.32 | 278.90 c ± 1.75 |
| Urea (mg/dL) | 26.50 a ± 0.34 | 37.20 d ± 0.49 | 29.50 b ± 0.69 | 33.20 c ± 0.83 |
| Creatinine (mg/dL) | 0.35 a ± 0.01 | 0.81 c ± 0.02 | 0.47 b ± 0.02 | 0.51 b ± 0.02 |
| Albumin (g/dL) | 4.26 d ± 0.06 | 2.18 a ± 0.05 | 4.01 c ± 0.07 | 3.70 b ± 0.10 |
| ALP (U/L) | 125.90 a ± 0.78 | 159.10 d ± 1.69 | 130.70 b ± 0.75 | 143.20 c ± 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda, K.; Mohamed, R.S. In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress. Sci. Pharm. 2025, 93, 37. https://doi.org/10.3390/scipharm93030037
Fouda K, Mohamed RS. In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress. Scientia Pharmaceutica. 2025; 93(3):37. https://doi.org/10.3390/scipharm93030037
Chicago/Turabian StyleFouda, Karem, and Rasha S. Mohamed. 2025. "In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress" Scientia Pharmaceutica 93, no. 3: 37. https://doi.org/10.3390/scipharm93030037
APA StyleFouda, K., & Mohamed, R. S. (2025). In Silico and In Vivo Studies Reveal the Potential Preventive Impact of Cuminum cyminum and Foeniculum vulgare Essential Oil Nanocapsules Against Depression-like States in Mice Fed a High-Fat Diet and Exposed to Chronic Unpredictable Mild Stress. Scientia Pharmaceutica, 93(3), 37. https://doi.org/10.3390/scipharm93030037





