Targeted Peptide-Mediated Delivery of Antisense Oligonucleotides to SMA Cells for SMN2 Gene Splicing Correction
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Antisense Oligonucleotides
2.3. Peptide Carrier
2.4. RNA Binding Assay
2.5. Flow Cytometry
2.6. Transfections
2.7. Toxicity Assay
2.8. Size and Zeta Potential Measurements of AON/Carrier Complexes
2.9. RNA Isolation and cDNA Synthesis
2.10. Semiquantitative and Quantitative Fluorescence (QF) RT-PCR
2.11. Immunocytochemistry
2.12. Statistical Methods
3. Results
3.1. Physicochemical Characterization of AON/Carrier Complexes
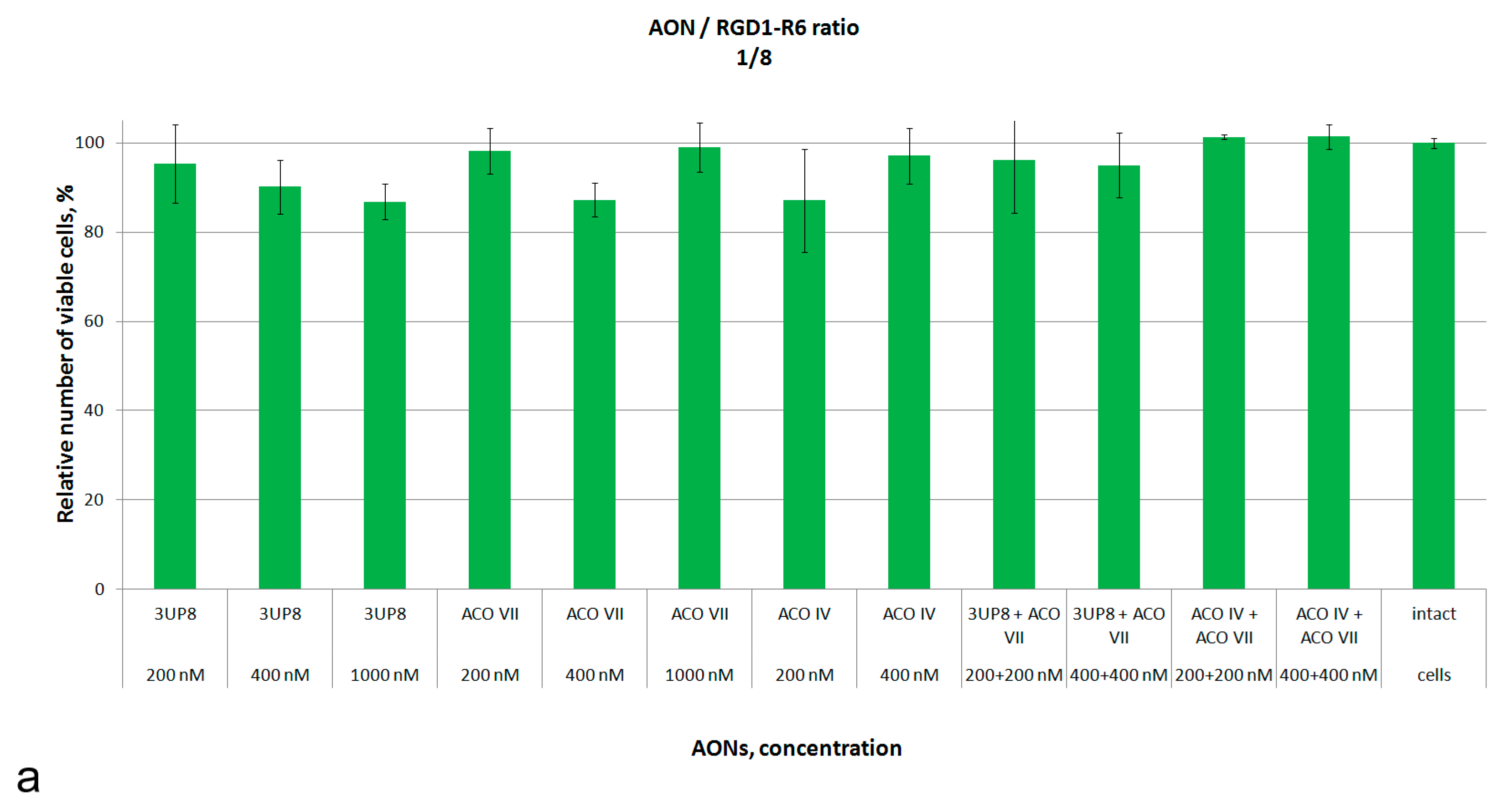
3.2. Assessment of Cytotoxic Properties of AON/Carrier Complexes
3.3. SMN2 Splicing Correction Efficiency of AON/RGD1-R6 Complexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.M.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Burnett, B.G.; Muñoz, E.; Tandon, A.; Kwon, D.Y.; Sumner, C.J.; Fischbeck, K.H. Regulation of SMN Protein Stability. Mol. Cell. Biol. 2009, 29, 1107–1115. [Google Scholar] [CrossRef]
- Wirth, B.; Brichta, L.; Hahnen, E. Spinal Muscular Atrophy: From Gene to Therapy. Semin. Pediatr. Neurol. 2006, 13, 121–131. [Google Scholar] [CrossRef]
- Prior, T.W.; Krainer, A.R.; Hua, Y.; Swoboda, K.J.; Snyder, P.C.; Bridgeman, S.J.; Burghes, A.H.M.; Kissel, J.T. A Positive Modifier of Spinal Muscular Atrophy in the SMN2 Gene. Am. J. Hum. Genet. 2009, 85, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates. Curr. Genom. 2018, 19, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Wadman, R.I.; Stam, M.; Gijzen, M.; Lemmink, H.H.; Snoeck, I.N.; Wijngaarde, C.A.; Braun, K.P.J.; Schoenmakers, M.A.G.C.; Van Den Berg, L.H.; Dooijes, D.; et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0–4. J. Neurol. Neurosurg. Psychiatry 2017, 88, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid. Ther. 2017, 27, 67–69. [Google Scholar] [CrossRef]
- Singh, R.N.; Ottesen, E.W.; Singh, N.N. The First Orally Deliverable Small Molecule for the Treatment of Spinal Muscular Atrophy. Neurosci. Insights 2020, 15, 263310552097398. [Google Scholar] [CrossRef]
- Hoy, S.M. Onasemnogene Abeparvovec: First Global Approval. Drugs 2019, 79, 1255–1262. [Google Scholar] [CrossRef]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta-Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef]
- Chaytow, H.; Huang, Y.-T.; Gillingwater, T.H.; Faller, K.M.E. The role of survival motor neuron protein (SMN) in protein homeostasis. Cell. Mol. Life Sci. 2018, 75, 3877–3894. [Google Scholar] [CrossRef]
- Liu, Q.; Dreyfuss, G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996, 15, 3555–3565. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H.M. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Mattis, V.B.; Rai, R.; Wang, J.; Chang, C.W.T.; Coady, T.; Lorson, C.L. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum. Genet. 2006, 120, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Yu, J.; Rose, F.F.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilal, H.; Maretina, M.; Egorova, A.; Glotov, A.; Kiselev, A. Assessment of Nuclear Gem Quantity for Evaluating the Efficacy of Antisense Oligonucleotides in Spinal Muscular Atrophy Cells. Methods Protoc. 2024, 7, 9. [Google Scholar] [CrossRef]
- Haché, M.; Swoboda, K.J.; Sethna, N.; Farrow-Gillespie, A.; Khandji, A.; Xia, S.; Bishop, K.M. Intrathecal Injections in Children with Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. J. Child Neurol. 2016, 31, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Mohr, F.; McMillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 2021, 74, 560–566. [Google Scholar] [CrossRef]
- Mirea, A.; Shelby, E.-S.; Axente, M.; Badina, M.; Padure, L.; Leanca, M.; Dima, V.; Sporea, C. Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I. J. Clin. Med. 2021, 10, 5540. [Google Scholar] [CrossRef]
- Osman, E.Y.; Washington, C.W.; Kaifer, K.A.; Mazzasette, C.; Patitucci, T.N.; Florea, K.M.; Simon, M.E.; Ko, C.P.; Ebert, A.D.; Lorson, C.L. Optimization of morpholino antisense oligonucleotides targeting the intronic repressor element1 in spinal muscular atrophy. Mol. Ther. 2016, 24, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.H.; Sun, J.; Krainer, A.R.; Hua, Y.; Prior, T.W. A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 2768–2780. [Google Scholar] [CrossRef]
- Pao, P.W.; Wee, K.B.; Yee, W.C.; Dwipramono, Z.A. Dual masking of specific negative splicing regulatory elements resulted in maximal exon 7 inclusion of SMN2 gene. Mol. Ther. 2014, 22, 854–861. [Google Scholar] [CrossRef]
- Maretina, M.; Il’ina, A.; Egorova, A.; Glotov, A.; Kiselev, A. Development of 2′-O-Methyl and LNA Antisense Oligonucleotides for SMN2 Splicing Correction in SMA Cells. Biomedicines 2023, 11, 3071. [Google Scholar] [CrossRef]
- Egorova, A.; Shtykalova, S.; Selutin, A.; Shved, N.; Maretina, M.; Selkov, S.; Baranov, V.; Kiselev, A. Development of iRGD-Modified Peptide Carriers for Suicide Gene Therapy of Uterine Leiomyoma. Pharmaceutics 2021, 13, 202. [Google Scholar] [CrossRef]
- Egorova, A.; Petrosyan, M.; Maretina, M.; Bazian, E.; Krylova, I.; Baranov, V.; Kiselev, A. iRGD-Targeted Peptide Nanoparticles for Anti-Angiogenic RNAi-Based Therapy of Endometriosis. Pharmaceutics 2023, 15, 2108. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, N.J.; Moffatt, S.; Fernyhough, P.; Humphries, M.J.; Streuli, C.H.; Tomlinson, D.R. Preconditioning injury-induced neurite outgrowth of adult rat sensory neurons on fibronectin is mediated by mobilisation of axonal α5 integrin. Mol. Cell. Neurosci. 2007, 35, 249–260. [Google Scholar] [CrossRef]
- Shida, M.; Mikami, T.; Tamura, J.; Kitagawa, H. Chondroitin sulfate-D promotes neurite outgrowth by acting as an extracellular ligand for neuronal integrin αVβ3. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Odnoshivkina, U.G.; Kuznetsova, E.A.; Petrov, A.M. 25-Hydroxycholesterol as a Signaling Molecule of the Nervous System. Biochemistry 2022, 87, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Long, Y.; Xu, C.; Wang, Y.; Qiu, Y.; Yu, Q.; Liu, Y.; Zhang, Q.; Gao, H.; Zhang, Z.; et al. Liposomes Combined an Integrin α v β 3 -Specific Vector with pH-Responsible Cell-Penetrating Property for Highly Effective Antiglioma Therapy through the Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2015, 7, 21442–21454. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- Zheleznyakova, G.Y.; Kiselev, A.V.; Vakharlovsky, V.G.; Rask-Andersen, M.; Chavan, R.; Egorova, A.A.; Schiöth, H.B.; Baranov, V.S. Genetic and expression studies of SMN2 gene in Russian patients with spinal muscular atrophy type II and III. BMC Med. Genet. 2011, 12, 96. [Google Scholar] [CrossRef]
- Singh, N.N.; Shishimorova, M.; Cao, L.C.; Gangwani, L.; Singh, R.N. A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy. RNA Biol. 2009, 6, 341–350. [Google Scholar] [CrossRef]
- Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Baranov, V.; Kiselev, A. Characterization of iRGD-Ligand Modified Arginine-Histidine-Rich Peptides for Nucleic Acid Therapeutics Delivery to αvβ3 Integrin-Expressing Cancer Cells. Pharmaceuticals 2020, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shubina, A.; Sokolov, D.; Selkov, S.; Baranov, V.; Kiselev, A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int. J. Pharm. 2016, 515, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Maretina, M.; Egorova, A.; Lanko, K.; Baranov, V.; Kiselev, A. Evaluation of Mean Percentage of Full-Length SMN Transcripts as a Molecular Biomarker of Spinal Muscular Atrophy. Genes 2022, 13, 1911. [Google Scholar] [CrossRef]
- Goyal, N.; Narayanaswami, P. Making sense of antisense oligonucleotides: A narrative review. Muscle Nerve 2018, 57, 356–370. [Google Scholar] [CrossRef]
- Federici, T.; Boulis, N.M. Gene-based treatment of motor neuron diseases. Muscle Nerve 2006, 33, 302–323. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, A.P.; Sleigh, J.N. Intramuscular Delivery of Gene Therapy for Targeting the Nervous System. Front. Mol. Neurosci. 2020, 13, 129. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Macasaet, R.; Liu, J.; Velasco, J.V.; Peligro, P.J.; Vallo, J.; Goldrich, N.; Lahoti, L.; Zhou, J.; et al. Infusion reactions to adeno-associated virus (AAV)-based gene therapy: Mechanisms, diagnostics, treatment and review of the literature. J. Med. Virol. 2023, 95, e29305. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Gao, X.; Chen, J. Harnessing the Capacity of Cell-Penetrating Peptides for Drug Delivery to the Central Nervous System. Curr. Pharm. Biotechnol. 2014, 15, 220–230. [Google Scholar] [CrossRef]
- Yao, H.; Wang, K.; Wang, Y.; Wang, S.; Li, J.; Lou, J.; Ye, L.; Yan, X.; Lu, W.; Huang, R. Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials 2015, 37, 345–352. [Google Scholar] [CrossRef]
- Shtykalova, S.; Deviatkin, D.; Freund, S.; Egorova, A.; Kiselev, A. Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications. Life 2023, 13, 903. [Google Scholar] [CrossRef]
- Wei, X.; Zhan, C.; Shen, Q.; Fu, W.; Xie, C.; Gao, J.; Peng, C.; Zheng, P.; Lu, W. A D-Peptide Ligand of Nicotine Acetylcholine Receptors for Brain-Targeted Drug Delivery. Angew. Chem. 2015, 127, 3066–3070. [Google Scholar] [CrossRef]
- Eskandari, S.; Rezayof, A.; Asghari, S.M.; Hashemizadeh, S. Neurobiochemical characteristics of arginine-rich peptides explain their potential therapeutic efficacy in neurodegenerative diseases. Neuropeptides 2023, 101, 102356. [Google Scholar] [CrossRef]
- Du, L.; Kayali, R.; Bertoni, C.; Fike, F.; Hu, H.; Iversen, P.L.; Gatti, R.A. Arginine-rich cell-penetrating peptide dramatically enhances AMO-mediated ATM aberrant splicing correction and enables delivery to brain and cerebellum. Hum. Mol. Genet. 2011, 20, 3151–3160. [Google Scholar] [CrossRef]
- Shabanpoor, F.; Hammond, S.M.; Abendroth, F.; Hazell, G.; Wood, M.J.A.; Gait, M.J. Identification of a Peptide for Systemic Brain Delivery of a Morpholino Oligonucleotide in Mouse Models of Spinal Muscular Atrophy. Nucleic Acid Ther. 2017, 27, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Hazell, G.; Shabanpoor, F.; Saleh, A.F.; Bowerman, M.; Sleigh, J.N.; Meijboom, K.E.; Zhou, H.; Muntoni, F.; Talbot, K.; et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2016, 113, 10962–10967. [Google Scholar] [CrossRef]
- Nguyen, Q.; Yokota, T. Antisense oligonucleotides for the treatment of cardiomyopathy in Duchenne muscular dystrophy. Am. J. Transl. Res. 2019, 11, 1202–1218. [Google Scholar] [PubMed]
- Kurano, T.; Kanazawa, T.; Iioka, S.; Kondo, H.; Kosuge, Y.; Suzuki, T. Intranasal Administration of N-acetyl-L-cysteine Combined with Cell-Penetrating Peptide-Modified Polymer Nanomicelles as a Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis. Pharmaceutics 2022, 14, 2590. [Google Scholar] [CrossRef] [PubMed]
- Lygoe, K.A.; Norman, J.T.; Marshall, J.F.; Lewis, M.P. αv integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 2004, 12, 461–470. [Google Scholar] [CrossRef] [PubMed]




| Name | Sequence | Target Site | Reference |
|---|---|---|---|
| 3UP8 | 5′-GCU GGC AG-3′ | ISS-N1 | [34] |
| ASO IV | 5′-UCA CUU UCA UAA UGC UGG-3′ | ISS-N1 | [25] |
| ASO VII | 5′-UUC AAC UUU CUA ACA UCU GA-3′ | ISS+100 | [25] |
| AON | Size (nm) ± S.D. | PdI ± S.D. | Zeta Potential (mV) ± S.D. |
|---|---|---|---|
| 3UP8 | 233.73 ± 1.68 | 0.135 ± 0.05 | 17.8 ± 0.82 |
| ASOIV | 113.83 ± 0.64 | 0.179 ± 0.01 | 20.33 ± 0.98 |
| ASOVII | 151.73 ± 8.56 | 0.379 ± 0.01 | 22.93 ± 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maretina, M.; Egorova, A.; Il’ina, A.; Krylova, N.; Donnikov, M.; Glotov, O.; Kiselev, A. Targeted Peptide-Mediated Delivery of Antisense Oligonucleotides to SMA Cells for SMN2 Gene Splicing Correction. Sci. Pharm. 2025, 93, 38. https://doi.org/10.3390/scipharm93030038
Maretina M, Egorova A, Il’ina A, Krylova N, Donnikov M, Glotov O, Kiselev A. Targeted Peptide-Mediated Delivery of Antisense Oligonucleotides to SMA Cells for SMN2 Gene Splicing Correction. Scientia Pharmaceutica. 2025; 93(3):38. https://doi.org/10.3390/scipharm93030038
Chicago/Turabian StyleMaretina, Marianna, Anna Egorova, Arina Il’ina, Nadezhda Krylova, Maxim Donnikov, Oleg Glotov, and Anton Kiselev. 2025. "Targeted Peptide-Mediated Delivery of Antisense Oligonucleotides to SMA Cells for SMN2 Gene Splicing Correction" Scientia Pharmaceutica 93, no. 3: 38. https://doi.org/10.3390/scipharm93030038
APA StyleMaretina, M., Egorova, A., Il’ina, A., Krylova, N., Donnikov, M., Glotov, O., & Kiselev, A. (2025). Targeted Peptide-Mediated Delivery of Antisense Oligonucleotides to SMA Cells for SMN2 Gene Splicing Correction. Scientia Pharmaceutica, 93(3), 38. https://doi.org/10.3390/scipharm93030038







