Steroidal Oximes and Cervical Cancer: An In Silico Mechanistic Pathway Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Construction
2.2. Target Selection
2.3. In Silico Studies
2.3.1. Protein Preparation
2.3.2. Ligand Preparation
2.3.3. Molecular Docking
3. Results
3.1. Structural Similarity Analysis
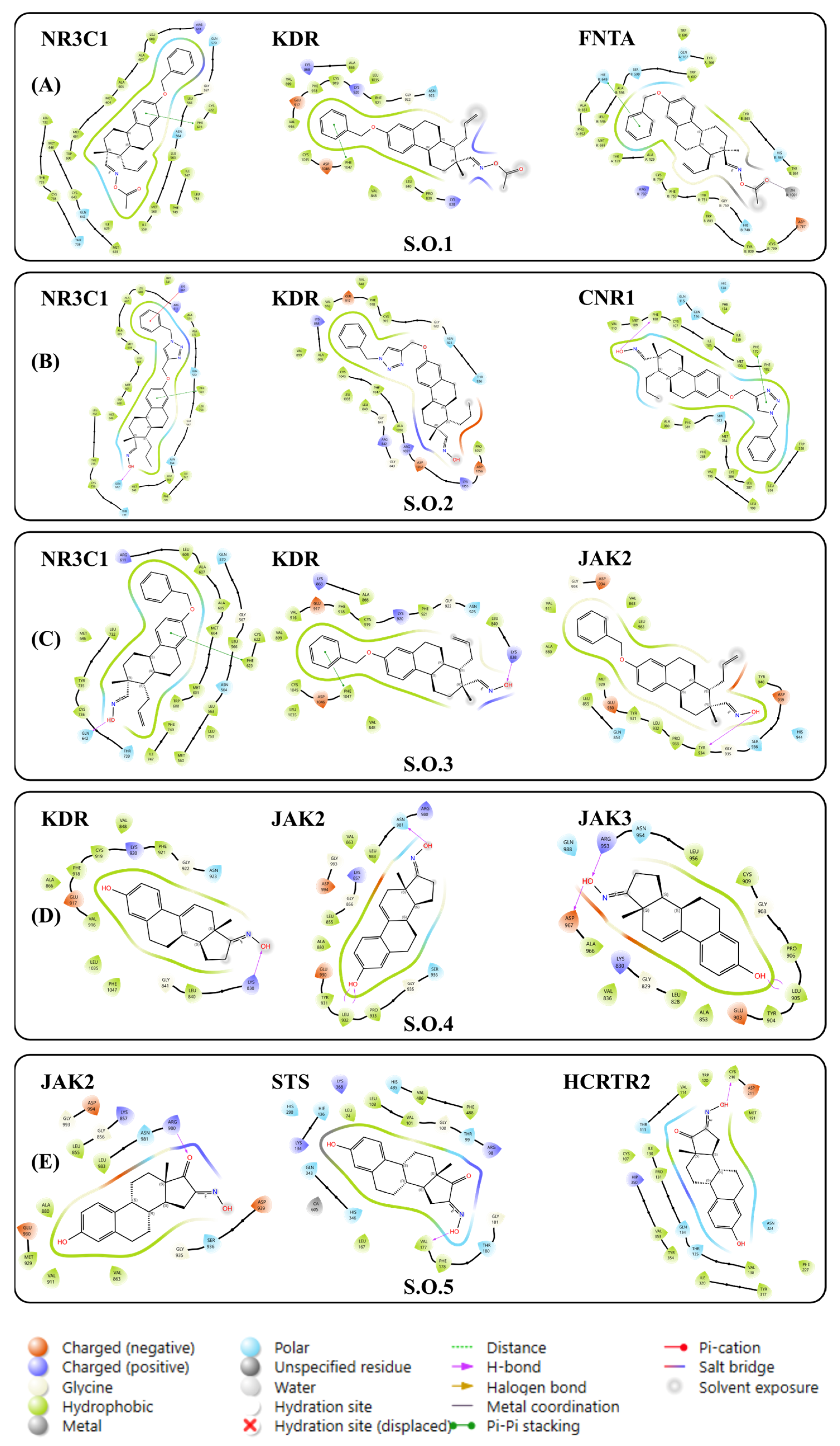
3.2. Molecular Docking Analyses
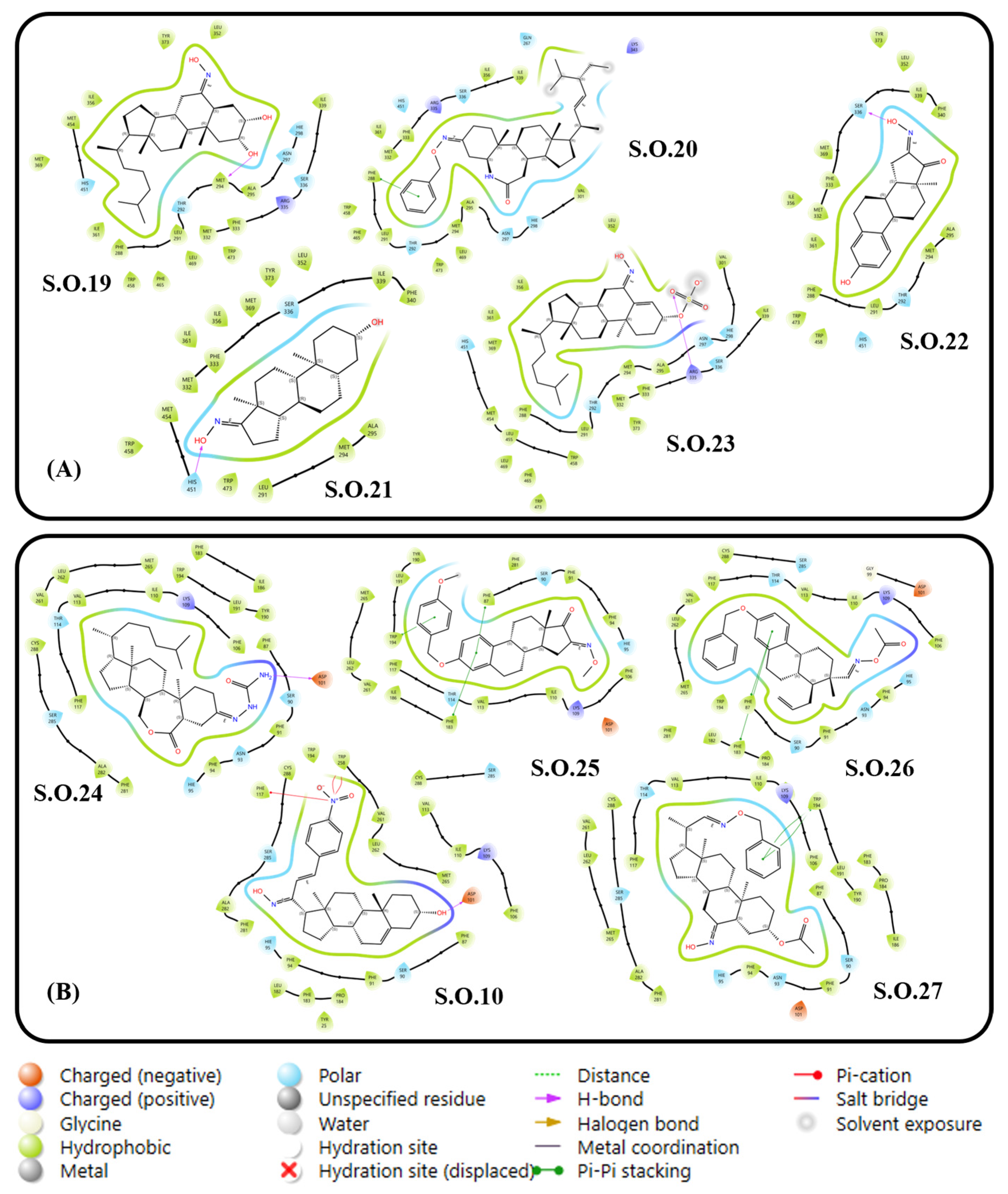
3.2.1. Molecular Docking via Linear Interactions Between Steroidal Oximes
3.2.2. Molecular Docking of Steroidal Oximes with Greater Interactions
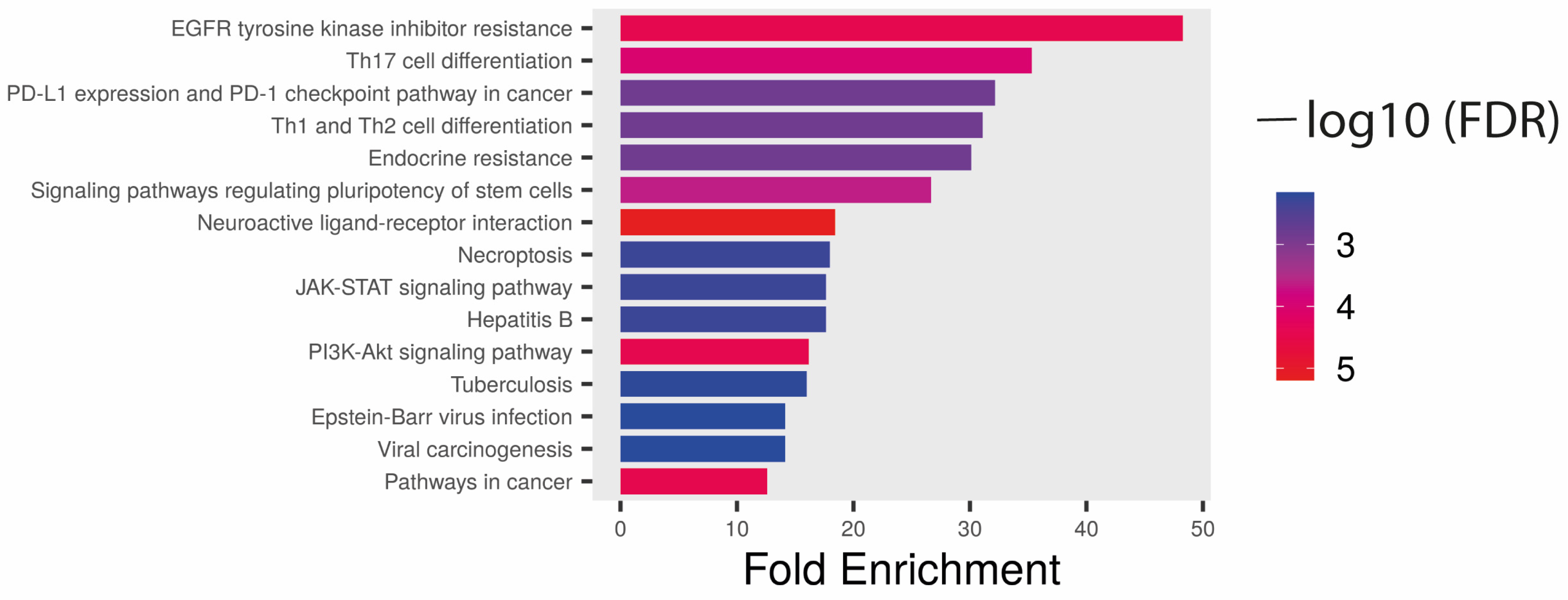
3.3. Target Selection via Ligand–Target Interactions and Structural Requirements
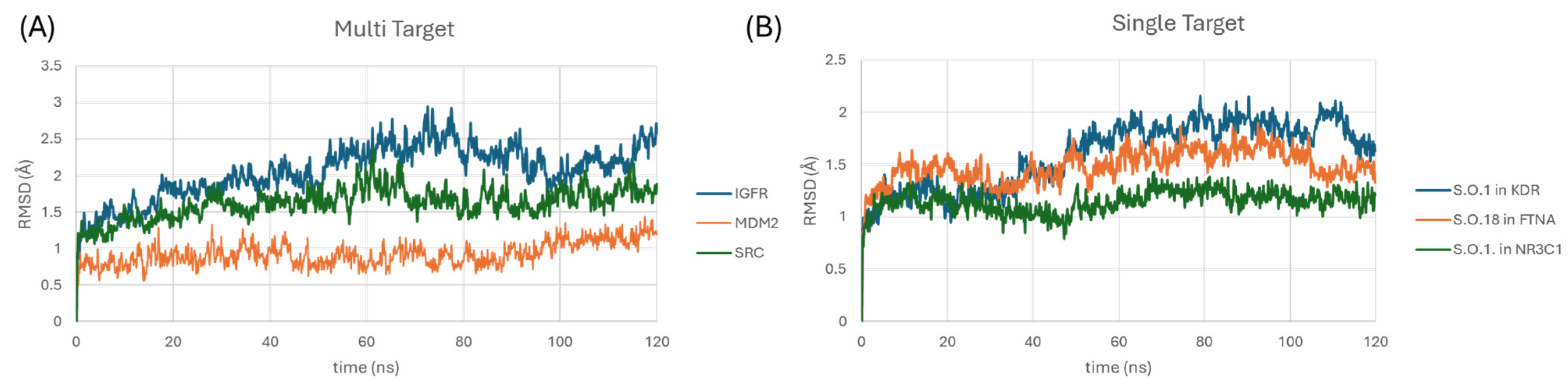
3.4. ADME and Molecular Dynamic Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagaraja, M.; Narendra, H.; Venkataramana, B.; Kalawat, U. HPV genotype prevalence in Indian women with cervical disease and estimation of the potential impact of HPV vaccines on prevention of cervical cancer. Indian J. Med. Microbiol. 2022, 42, 73–78. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, 191–203. [Google Scholar] [CrossRef]
- Ferrara, P.; Dallagiacoma, G.; Alberti, F.; Gentile, L.; Bertuccio, P.; Odone, A. Prevention, diagnosis and treatment of cervical cancer: A systematic review of the impact of COVID-19 on patient care. Prev. Med. 2022, 164, 107264. [Google Scholar] [CrossRef]
- Monteiro, J.C.; Tsutsumi, M.Y.; de Carvalho, D.O. Prevalence, Diversity, and Risk Factors for Cervical HPV Infection in Women Screened for Cervical Cancer in Belém, Pará, Northern Brazil. Pathogens 2022, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Vora, C.; Gupta, S. Targeted therapy in cervical cancer. ESMO Open 2018, 3, e000462. [Google Scholar] [CrossRef]
- Linhares Moreira, A.S.; Cunha, T.M. Esteves S, Cervical cancer recurrence—Can we predict the type of recurrence? Diagn. Interv. Radiol. 2020, 26, 403–410. [Google Scholar] [CrossRef]
- Gonçalves, B.M.F.; Mendes, V.I.S.; Silvestre, S.M.; Salvador, J.A.R. Design, synthesis, and biological evaluation of new arjunolic acid derivatives as anticancer agents. RSC Med. Chem. 2023, 2, 313–331. [Google Scholar] [CrossRef]
- Swedan, H.K.; Kassab, A.E.; Gedawy, E.M.; Elmeligie, S.E. Design, synthesis, and biological evaluation of novel ciprofloxacin derivatives as potential anticancer agents targeting topoisomerase II enzyme. J. Enzyme Inhib. Med. Chem. 2023, 38, 118–137. [Google Scholar] [CrossRef]
- Khan, B.A.; Hamdani, S.S.; Jalil, S.; Irshad, S.; Riaz, M.; Ashraf, S.; Hussain, G.; Ali, S.; Shafiq, M.; Iqbal, J.; et al. Synthesis and Evaluation of Novel S-alkyl Phthalimide- and S-benzyl-oxadiazole-quinoline Hybrids as Inhibitors of Monoamine Oxidase and Acetylcholinesterase. Pharmaceuticals 2022, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Laamari, Y.; Bimoussa, A.; Fawzi, M.; Merzouk, S.; Lamiri, A.; Touzani, R.; Khouchlaa, A.; Elmsellem, H.; Aouf, N. Synthesis, crystal structure and evaluation of anticancer activities of some novel heterocyclic compounds based on thymol. J. Mol. Struct. 2023, 1278, 134906. [Google Scholar] [CrossRef]
- Raju, R.; Chidambaram, K.; Chandrasekaran, B.; Bayan, M.F.; Kumar Maity, T.; Alkahtani, A.M.; Chandramoorthy, H.C. Synthesis, pharmacological evaluation, and molecular modeling studies of novel isatin hybrids as potential anticancer agents. J. Saudi Chem. Soc. 2023, 27, 101598. [Google Scholar] [CrossRef]
- Carrasco-Carballo, A.; Mendoza-Lara, D.F.; Rojas-Morales, J.A.; Alatriste, V.; Merino-Montiel, P.; Luna, F.; Sandoval, J. In silico Study of Coumarins Derivatives with Potential Use in Systemic Diseases. Biointerface Res. Appl. Chem. 2022, 13, 240. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Bhushan, S.; Aggarwal, V.; Aggarwal, D.; Chugh, V.; Choudhary, R.; Shukla, N.; Beniwal, S.; Varol, M.; et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl. Oncol. 2023, 27, 101596. [Google Scholar] [CrossRef] [PubMed]
- Schanknecht, E.; Bachari, A.; Nassar, N.; Piva, T.; Mantri, N. Phytochemical Constituents and Derivatives of Cannabis sativa; Bridging the Gap in Melanoma Treatment. Int. J. Mol. Sci. 2023, 24, 859. [Google Scholar] [CrossRef]
- Grzes, P.A.; Sawicka, A.; Niemirowicz-Laskowska, K.; Wielgat, P.; Sawicka, D.; Car, H.; Jastrzebska, I. Metal-promoted synthesis of steroidal ethynyl selenides having anticancer activity. J. Steroid Biochem. Mol. Biol. 2023, 227, 106232. [Google Scholar] [CrossRef] [PubMed]
- Ilovaisky, A.I.; Scherbakov, A.M.; Merkulova, V.M.; Chernoburova, E.I.; Shchetinina, M.A.; Andreeva, O.E.; Salnikova, D.I.; Zavarzin, I.V.; Terentév, A.O. Secosteroid–quinoline hybrids as new anticancer agents. J. Steroid Biochem. Mol. Biol. 2023, 228, 106245. [Google Scholar] [CrossRef]
- Cobos-Ontiveros, L.A.; Romero-Hernández, L.L.; Mastranzo-Sánchez, E.B.; Colín-Lozano, B.; Puerta, A.; Padrón, J.M.; Merino-Montiel, P.; Vega Baez, J.L.; Montiel-Smith, S. Synthesis, antiproliferative evaluation and in silico studies of a novel steroidal spiro morpholinone. Steroids 2023, 192, 109173. [Google Scholar] [CrossRef]
- Lee, M.M.-L.; Chan, B.D.; Wong, W.-Y.; Leung, T.-W.; Qu, Z.; Huang, J.; Zhu, L.; Lee, C.-S.; Chen, S.; Tai, W.C.-S. Synthesis Evaluation of Novel Anticancer Compounds Derived from the Natural Product Brevilin, A. ACS Omega 2020, 5, 14586–14596. [Google Scholar] [CrossRef]
- Gomes, A.R.; Pires, A.S.; Roleira, F.M.F.; Tavares-da-Silva, E.J. The Structural Diversity and Biological Activity of Steroid Oximes. Molecules 2023, 28, 1690. [Google Scholar] [CrossRef]
- Cui, J.-G.; Fan, L.; Huang, L.-L.; Liu, H.-L.; Zhou, A.-M. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (I). Steroids 2009, 74, 62–72. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Yildiz, U. Synthesis, Structural Analysis and Antiproliferative Activity of Nitrogen-Containing Hetero Spirostan Derivatives: Oximes, Heterocyclic Ring-Fused and Furostanes. ChemistrySelect 2022, 7, e202200439. [Google Scholar] [CrossRef]
- Hernández-Vázquez, J.M.V.; López-Muñoz, H.; Escobar-Sánchez, M.L.; González-Esquinca, A.R.; Alvarado-Hernández, D.L.; Rojas-Molina, A.; Zentella-Dehesa, A.; Larrea, F.; Pérez-Rojas, J.M.; Aguilar-Rojas, A. Apoptotic, necrotic, and antiproliferative activity of diosgenin and diosgenin glycosides on cervical cancer cells. Eur. J. Pharmacol. 2020, 871, 172942. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Cheng, S.; Du, J.; Chen, Y.; Yang, Y.; Liu, Y. Diosgenin induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Rep. 2015, 33, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Tsukayama, I.; Mega, T.; Hojo, N.; Toda, K.; Kawakami, Y.; Takahashi, Y.; Suzuki-Yamamoto, T. Diosgenin suppresses COX-2 and mPGES-1 via GR and improves LPS-induced liver injury in mouse. Prostaglandins Other Lipid Mediat. 2021, 156, 106580. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, K.-L.; Hsieh, T.-C.; Chang, T.-Y.; Lin, W.-H.; Hsu, J.T.-A. Diosgenin, a Plant-Derived Sapogenin, Exhibits Antiviral Activity in Vitro against Hepatitis C Virus. J. Nat. Prod. 2011, 74, 580–584. [Google Scholar] [CrossRef]
- Dong, M.; Meng, Z.; Kuerban, K.; Liu, L.; Liu, J.; Wang, X.; Gao, X.; Yan, Y.; Zhang, C.; Jiang, X.; et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Gomes, A.R.; Pires, A.S.; Abrantes, A.M.; Marques, I.A.; Gonçalves, A.C.; Gaspar, D.; Robalo, M.P.; Silvestre, S.; Oliveira, N.G.; Botelho, M.F. Design, synthesis, and antitumor activity evaluation of steroidal oximes. Bioorg Med. Chem. 2021, 46, 116360. [Google Scholar] [CrossRef] [PubMed]
- Jach, R. Expression of VEGF VEGF-C and VEGFR-2 in in situ and invasive SCC of cervix. Front. Biosci. 2010, 2, 411–423. [Google Scholar] [CrossRef]
- Khan, A.U.; Avecillia, F.; Malik, N.; Khan, M.S.; Khan, M.S.; Mushtaque, M. Theoretical and experimental studies of 3β-acetoxy-5α-cholestan-6-one oxime. J. Mol. Struct. 2016, 1122, 100–110. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Chen, S.; Wang, H.; He, X.; Zhou, Q.; Chen, L.; Jiang, S.; Chen, C. Synthesis and Evaluation of Some New Aza-B-homocholestane Derivatives as Anticancer Agents. Mar. Drugs 2014, 12, 1715–1731. [Google Scholar] [CrossRef]
- Martínez-Pascual, R.; Meza-Reyes, S.; Vega-Baez, J.L.; Merino-Montiel, P.; Padrón, J.M.; Mendoza, Á.; Montiel-Smith, S. Novel synthesis of steroidal oximes and lactams and their biological evaluation as antiproliferative agents. Steroids 2017, 122, 24–33. [Google Scholar] [CrossRef]
- Vágvölgyi, M.; Martins, A.; Kulmány, Á.; Molnár, J.; Hohmann, J.; Zupkó, I.; Spengler, G. Nitrogen-containing ecdysteroid derivatives vs. multi-drug resistance in cancer: Preparation and antitumor activity of oximes, oxime ethers and a lactam. Eur. J. Med. Chem. 2018, 144, 730–739. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry V: Synthesis of hybrid molecules based on steroid oximes and (5Z,9Z)-tetradeca-5,9-dienedioic acid as potential anticancer agents. Steroids 2018, 138, 14–20. [Google Scholar] [CrossRef]
- Acharya, P.C.; Bansal, R. Synthesis of androstene oxime-nitrogen mustard bioconjugates as potent antineoplastic agents. Steroids 2017, 123, 73–83. [Google Scholar] [CrossRef]
- Nikolić, A.R.; Kuzminac, I.Z.; Jovanović-Šanta, S.S.; Jakimov, D.S.; Aleksić, L.D.; Sakač, M.N. Anticancer activity of novel steroidal 6-substituted 4-en-3-one D-seco dinitriles. Steroids 2018, 135, 101–107. [Google Scholar] [CrossRef]
- Bu, M.; Cao, T.; Li, H.; Wang, X.; Chen, H.; Ren, Y.; Ma, L.; Zhang, Y. Synthesis and biological evaluation of novel steroidal 5α,8α-epidioxyandrost-6-ene-3β-ol-17-(O-phenylacetamide) oxime derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 3856–3861. [Google Scholar] [CrossRef]
- Chowdhury, P.; Das, A.; Goswami, P. Synthesis of some new steroidal [16α,17α-d]-isoxazolines. Steroids 2005, 70, 494–498. [Google Scholar] [CrossRef]
- Pokhrel, M.; Ma, E. Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs. Molecules 2011, 16, 9868–9885. [Google Scholar] [CrossRef]
- Canário, C.; Matias, M.; Brito, V.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. New Estrone Oxime Derivatives: Synthesis, Cytotoxic Evaluation and Docking Studies. Molecules 2021, 26, 2687. [Google Scholar] [CrossRef]
- Grandi, G.; Del Savio, M.C.; Facchinetti, F. The paradigm of norgestimate: A third-generation testosterone-derivative progestin with a peripheral anti-androgenic activity and the lowest risk of venous thromboembolism. Expert Rev. Clin. Pharmacol. 2021, 14, 211–224. [Google Scholar] [CrossRef]
- Salvador, J.-P.; Sanchez-Baeza, F.; Marco, M.-P. Preparation of Antibodies for the Designer Steroid Tetrahydrogestrinone and Development of an Enzyme-Linked Immunosorbent Assay for Human Urine Analysis. Anal. Chem. 2007, 79, 3734–3740. [Google Scholar] [CrossRef]
- Bodnár, B.; Mernyák, E.; Szabó, J.; Tóth, G.; Valkó, L.; Farkas, Á.; Berkecz, R.; Greiner, I.; Kása, P.; Madarász, D.; et al. Synthesis and in vitro investigation of potential antiproliferative monosaccharide–d-secoestrone bioconjugates. Bioorg. Med. Chem. Lett. 2017, 27, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, J.J.; Penov Gaši, K.M.; Jakimov, D.S.; Klisurić, O.R.; Jovanović-Šanta, S.S.; Sakač, M.N.; Aleksić, L.D.; Djurendić, E.A. Synthesis, structural analysis and antitumor activity of novel 17α-picolyl and 17(E)-picolinylidene A-modified androstane derivatives. Bioorg. Med. Chem. 2015, 23, 1557–1568. [Google Scholar] [CrossRef]
- Mekky, H.; Al-Sabahi, J.; Abdel-Kreem, M.F.M. Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of Catharanthus roseus. S. Afr. J. Bot. 2018, 114, 29–31. [Google Scholar] [CrossRef]
- Krstić, N.M.; Bjelaković, M.S.; Žižak, Ž.; Pavlović, M.D.; Juranić, Z.D.; Pavlović, V.D. Synthesis of some steroidal oximes, lactams, thiolactams and their antitumor activities. Steroids 2007, 72, 406–414. [Google Scholar] [CrossRef]
- Berényi, Á.; Minorics, R.; Iványi, Z.; Tóth, G.; Greiner, I.; Szabó, J.; Farkas, Á.; Kelemen, Z. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar] [CrossRef]
- Berényi, Á.; Frotscher, M.; Marchais-Oberwinkler, S.; Hahner, D.; Barna, J.; Zsila, F.; Erdélyi, M.; Kelemen, Z.; Kuhn, W. Direct antiproliferative effect of nonsteroidal 17β-hydroxysteroid dehydrogenase type 1 inhibitors in vitro. J. Enzyme Inhib. Med. Chem. 2013, 4, 695–703. [Google Scholar] [CrossRef]
- Bansal, R.; Acharya, P.C. Man-Made Cytotoxic Steroids: Exemplary Agents for Cancer Therapy. Chem. Rev. 2014, 114, 6986–7005. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lin, Q.; Gan, C.; Yao, Q.; Su, W.; Huang, Y. Synthesis and cytotoxic activity of some 4,6-diaza-A,B-dihomo-steroid bilactams. Steroids 2014, 79, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, L.; Zhao, D.; He, X.; Zhang, Z.; Chen, S.; Huang, Y.; Jiang, S.; Chen, C. Synthesis, characterization and antitumor activities of some steroidal derivatives with side chain of 17-hydrazone aromatic heterocycle. Steroids 2015, 95, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lin, Q.; Huang, Y.; Chen, S.; He, X.; Jiang, S.; Chen, C. Design, synthesis and antiproliferative evaluation of some B-homo steroidal lactams. Med. Chem. Res. 2015, 24, 2906–2915. [Google Scholar] [CrossRef]
- Gan, C.; Cui, J.; Huang, Y.; Jia, L.; Wei, W. Synthesis and antiproliferative activity of some steroidal lactone compounds. Steroids 2012, 77, 255–259. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Chen, S.; Gan, C.; Zhou, A. Synthesis and antiproliferative activity of some steroidal lactams. Steroids 2011, 76, 1346–1350. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Li, Y.; Fan, L.; Jiao, Y.; Su, S. Syntheses and antiproliferative activity of some sulfated hydroximinosterols. Med. Chem. Res. 2013, 22, 409–414. [Google Scholar] [CrossRef]
- Latif, A.D.; Gonda, T.; Vágvölgyi, M.; Kulmány, Á.; Molnár, J.; Zupkó, I.; Hohmann, J.; Szabó, I.E.; Szendrei, L.; Tóth, G. Synthesis and In Vitro Antitumor Activity of Naringenin Oxime and Oxime Ether Derivatives. Int. J. Mol. Sci. 2019, 20, 2184. [Google Scholar] [CrossRef]
- Semeikin, A.V.; Fedotcheva, T.A.; Levina, I.S.; Kirsanova, S.A.; Naumova, I.B.; Voloshin, A.I.; Kurochkina, N.A.; Bakunina, I.Y. Synthesis and Cytostatic Activity of some Pregna-D′-Pentaranes on HeLa Cell Culture. Pharm. Chem. J. 2014, 48, 363–367. [Google Scholar] [CrossRef]
- Yao, J.; Ye, W.; Liu, J.; Liu, J.; Wang, C. Synthesis and cytotoxicity of (3β)-3-acetyloxy-5(6)-androsten-7-one oxime and 3,5(6)-androstadien-7-one oxime. Med. Chem. Res. 2014, 23, 1839–1843. [Google Scholar] [CrossRef]
- Rega, M.; Jiménez, C.; Rodríguez, J. 6E-Hydroximinosteroid homodimerization by cross-metathesis processes. Steroids 2007, 72, 729–735. [Google Scholar] [CrossRef]
- Cui, J.; Huang, L.; Fan, L.; Zhou, A. A facile and efficient synthesis of some (6E)-hydroximino-4-en-3-one steroids, steroidal oximes from Cinachyrella spp. sponges. Steroids 2008, 73, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Choi, T. An Efficient 4 beta-Hydroxylation of Steroidal 5-en-3 beta-ols and 1,4-Conjugation of Steroidal 4-en-3-ones Using SeO2 Oxidation. Bull. Korean Chem. Soc. 2009, 30, 245–248. [Google Scholar]
- Banday, A.H.; Akram, S.M.M.; Shameem, S.A. Benzylidine pregnenolones and their oximes as potential anticancer agents: Synthesis and biological evaluation. Steroids 2014, 84, 64–69. [Google Scholar] [CrossRef]
- Palmer, R.A.; Lisgarten, D.R.; Cockcroft, J.K.; Simpson, P. Crystal and Molecular Structure and DFT Calculations of the Steroidal Oxime 6E-Hydroximino-androst-4-ene-3,17-dione (C19H25NO3) a Molecule with Antiproliferative Activity. J. Chem. Crystallogr. 2019, 49, 29–36. [Google Scholar] [CrossRef]
- Bordet, T.; Buisson, B.; Michaud, M.; Garcia, M.; Leblanc, A.; Schmitt, C.; Camu, W.; Vallat, J.-M.; Pouget, J.; Miquel, M.-C.; et al. Identification and Characterization of Cholest-4-en-3-one, Oxime (TRO19622), a Novel Drug Candidate for Amyotrophic Lateral Sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Nuñez, L.; Peixinho, S.; Jiménez, C. Isolation and synthesis of the first natural 6-hydroximino 4-en-3-one- steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Ajduković, J.J.; Jakimov, D.S.; Rárová, L.; Penov Gaši, K.M.; Vujčić, Z.Ž.; Stanojković, M.D.; Đorđević, S.P.; Bugarčić, Ž.D. Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: Synthesis and evaluation of cytotoxic effects. RSC Adv. 2021, 11, 37449–37461. [Google Scholar] [CrossRef]
- Kovganko, N.B.; Chernov, Y.u.G. Novel synthesis of (24R,6E)-24-ethylcholest-6-hydroxyimino-4-en-3-one, a steroidal oxime from Cinachyrella spp. sponges. Chem. Nat. Compd. 2000, 36, 189–191. [Google Scholar] [CrossRef]
- Krstic, N.; Bjelakovic, M.; Dabovic, M.; Lorenc, L.; Pavlovic, V. Photochemical and Beckmann rearrangement of (Z)-cholest-4-en-6-one oxime. J. Serbian Chem. Soc. 2004, 69, 413–420. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Ma, E. Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes. Molecules 2011, 17, 355–368. [Google Scholar] [CrossRef]
- Huang, Y.; Su, S.; Jia, L.; Gan, C.; Lin, Q.; Kong, E.; Cui, J. Synthesis and Antiproliferative Evaluation of Some Steroidal Oxime Ether. Chin. J. Org. Chem. 2014, 34, 1816–1828. [Google Scholar] [CrossRef]
- Sikharulidze, M.I.; Nadaraia NSh Kakhabrishvili, M.L.; Barbakadze, N.N.; Mulkidzhanyan, K.G. Synthesis and biological activity of several steroidal oximes. Chem. Nat. Compd. 2010, 46, 493–494. [Google Scholar] [CrossRef]
- Richmond, V.; Careaga, V.P.; Sacca, P.; Calvo, J.C.; Maier, M.S. Synthesis and cytotoxic evaluation of four new 6E-hydroximinosteroids. Steroids 2014, 84, 7–10. [Google Scholar] [CrossRef]
- Holland, H.L.; Kumaresan, S.; Tan, L.; Njar, V.C.O. Synthesis of 6-hydroximino-3-oxo steroids, a new class of aromatase inhibitor. J. Chem. Soc. Perkin 1992, 1, 585–587. [Google Scholar] [CrossRef]
- Deive, N.; Rodríguez, J.; Jiménez, C. Synthesis of Cytotoxic 6 E -Hydroximino-4-ene Steroids: Structure/Activity Studies. J. Med. Chem. 2001, 44, 2612–2618. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small moleculas. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Lan, C.; Shen, J.; Wang, Y.; Wang, J.; Luo, J.; Qian, K.; Wei, J.; Wang, L.; Shi, Y.; Lin, L.; et al. Camrelizumab Plus Apatinib in Patients with Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J. Clin. Oncol. 2020, 38, 4095–4106. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Prasad, C.B.; Singh, D.; Pandey, L.K.; Pradhan, S.; Singh, S.; Narayan, G. VEGFa/VEGFR2 autocrine and paracrine signaling promotes cervical carcinogenesis via β-catenin and snail. Int. J. Biochem. Cell Biol. 2022, 142, 106122. [Google Scholar] [CrossRef] [PubMed]
- Zamo, A.; Chiarle, R.; Piva, R.; Schlessinger, K.; Brennan, C.; Cerutti, A.; Inghirami, G.; Levy, D.E. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Li, Y.; Liu, M.; Ma, S.; Guan, X.-Y. TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 2019, 152, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Liao, P.-J.; Liu, Y.; Zeng, Y.; Chen, J.; Liu, J.; Chen, H.; Zheng, X.; Xiong, X.; Chen, L.; et al. Protein tyrosine phosphatase, receptor type B is a potential biomarker and facilitates cervical cancer metastasis via epithelial-mesenchymal transition. Bioengineered 2021, 12, 5739–5748. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, C.; Liao, Y.; Liu, Y.; Wang, Q.; Zhang, J.; Xu, Z.; Li, J.; Chen, Y.; Wu, Q.; et al. High expression of PTPRM predicts poor prognosis and promotes tumor growth and lymph node metastasis in cervical cancer. Cell Death Dis. 2020, 11, 687. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, Z.-H.; Chen, Z.; Tan, D. SULF2 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells through the ERK/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e8901. [Google Scholar] [CrossRef]
- Shin, S.; Im, H.-J.; Kwon, Y.-J.; Lee, S.; Choi, J.; Park, J.-H.; Kim, Y.-S.; Lee, H.; Kim, S.-Y. Human steroid sulfatase induces Wnt/β-catenin signaling and epithelial-mesenchymal transition by upregulating Twist1 and HIF-1α in human prostate and cervical cancer cells. Oncotarget 2017, 8, 61604–61617. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Z.; Di, Y.; Jaen, J.C.; Labelle, M.; Ma, J.; Miao, S.; Sudom, A.; Tang, L.; Tomooka, C.S.; et al. Discovery and initial SAR of arylsulfonylpiperazine inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). Bioorg. Med. Chem. Lett. 2008, 18, 3513–3516. [Google Scholar] [CrossRef]
- Contassot, E.; Tenan, M.; Schnüriger, V.; Pelte, M.-F.; Dietrich, P.-Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188. [Google Scholar] [CrossRef]
- Hou, T.; Xiao, J.; Zhang, H.; Gu, H.; Feng, Y.; Li, J. Phosphorylated c-Src is a novel predictor for recurrence in cervical squamous cell cancer patients. Int. J. Clin. Exp. Pathol. 2013, 6, 1121–1127. [Google Scholar]
- Wang, Z.; Dong, J.; Tian, W.; Qiao, S.; Wang, H. Role of TRPV1 ion channel in cervical squamous cell carcinoma genesis. Front. Mol. Biosci. 2022, 9, 980262. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-T.; Liu, X.-F.; Yang, W.-T.; Zheng, P.-S. REX1 promotes EMT-induced cell metastasis by activating the JAK2/STAT3-signaling pathway by targeting SOCS1 in cervical cancer. Oncogene 2019, 38, 6940–6957. [Google Scholar] [CrossRef]
- Askandar, B.; Ekaputra, V.G.; Iskandar, T.M. Comparison of VDR Expression and Blood Vitamin D 1.25 (OH)2 Level between Cervical Cancer Patients and Normal Women. Indones. J. Cancer 2020, 14, 80–85. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, S.; Tian, S.; Wang, Z. Morphine stimulates cervical cancer cells and alleviates cytotoxicity of chemotherapeutic drugs via opioid receptor-dependent and -independent mechanisms. Pharmacol. Res. Perspect. 2022, 10, e01016. [Google Scholar] [CrossRef]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Huet, R.; Fraga, F.; Mourino, A.; Moreno, F.; Khandekar, S.; Thérond, P.; Bassard, J.-E.; Lancelot, J.-C.; Ringe, D.; Bourguet, W. Design, chemical synthesis, functional characterization and crystal structure of the sidechain analogue of 1,25-dihydroxyvitamin D3. Protein Data Bank 2011. [Google Scholar] [CrossRef]
- Edman, K.; Ahlgren, R.; Bengtsson, M.; Kjellgren, J.; Nilsson, J.; Nestor, C.; Stiernström, E.; Wladis, A.; Henriksson, R.; Larsson, L.; et al. The discovery of potent and selective non-steroidal glucocorticoid receptor modulators, suitable for inhalation. Bioorg. Med. Chem. Lett. 2014, 24, 2571–2577. [Google Scholar] [CrossRef]
- Matsui, Y.; Yamaguchi, T.; Yamazaki, T.; Arai, S.; Shimizu, M.; Toyoshima, Y.; Umehara, K.; Nishimura, A.; Matsuura, N.; Yoshida, K.; et al. Discovery and structure-guided optimization of tert-butyl 6-(phenoxymethyl)-3-(trifluoromethyl)benzoates as liver X receptor agonists. Bioorg. Med. Chem. Lett. 2015, 25, 3914–3920. [Google Scholar] [CrossRef]
- Boggon, T.J.; Li, Y.; Manley, P.W.; Eck, M.J. Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood 2005, 106, 996–1002. [Google Scholar] [CrossRef]
- Huang, X.; Wang, B.; Shen, H.; Huang, D.; Shi, G. Farnesoid X receptor functions in cervical cancer via the p14ARF-mouse double minute 2-p53 pathway. Mol. Biol. Rep. 2022, 49, 3617–3625. [Google Scholar] [CrossRef]
- Song, H.; Park, H.; Park, G.; Lee, D.; Lee, J.; Lee, H.; Park, S.; Lee, S. Corticotropin-releasing factor induces immune escape of cervical cancer cells by downregulation of NKG2D. Oncol. Rep. 2014, 32, 425–430. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yu, J.; Sun, W.; Wang, Z.; Zhang, J.; Zhang, W.; Wang, W.; Wang, H.; Liu, S.; Wei, W.; et al. A comprehensive pancancer analysis reveals the potential value of RAR-related orphan receptor C (RORC) for cancer immunotherapy. Front. Genet. 2022, 13, 969476. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Mobarec, J.C.; Kolb, P.; Rosenbaum, D.M. Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature 2015, 519, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.P.; Beyer, S.; Schröder, L.; Wollenberg, B.; Weber, M.; Wurster, I.; Thiel, F.; Bartel, C.; Ludwig, K.; Richter, H.; et al. Glucocorticoid receptor in cervical cancer: An immunhistochemical analysis. Arch. Gynecol. Obstet. 2019, 299, 203–209. [Google Scholar] [CrossRef]
- Mernyák, E.; Fiser, G.; Szabó, J.; Bodnár, B.; Berényi, Á.; Berkecz, R.; Tóth, G.; Greiner, I.; Szakonyi, Z.; Marchais-Oberwinkler, S.; et al. Synthesis and in vitro antiproliferative evaluation of d-secooxime derivatives of 13β- and 13α-estrone. Steroids 2014, 89, 47–55. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-4: MacroModel; Schrödinger, LLC: New York, NY, USA, 2021.
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021.
- Bossi, R.T.; Saccardo, M.B.; Ardini, E.; Menichincheri, M.; Rusconi, L.; Magnaghi, P.; Orsini, P.; Avanzi, N.; Borgia, A.L.; Nesi, M.; et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry 2010, 49, 6813–6825. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal structure of the human cannabinoid receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef]
- Hollenstein, K.; Kean, J.; Bortolato, A.; Cheng, R.K.Y.; Doré, A.S.; Jazayeri, A.; Cooke, R.M.; Weir, M.; Marshall, F.H. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 2013, 499, 438–443. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Textor, L.C.; Melo, D.C.; Nascimento, A.S.; Skaf, M.S.; Polikarpov, I. An alternative conformation of ERβ bound to estradiol reveals H12 in a stable antagonist position. Sci. Rep. 2017, 7, 3509. [Google Scholar] [CrossRef]
- Bell, I.M.; Gallicchio, S.N.; Abrams, M.; Beese, L.S.; Beshore, D.C.; Bhimnathwala, H.; Bogusky, M.J.; Buser, C.A.; Culberson, J.C.; Davide, J.; et al. 3-Aminopyrrolidinone Farnesyltransferase Inhibitors: Design of Macrocyclic Compounds with Improved Pharmacokinetics and Excellent Cell Potency. J. Med. Chem. 2002, 45, 2388–2409. [Google Scholar] [CrossRef]
- Velaparthi, U.; Wittman, M.; Liu, P.; Stoffan, K.; Zimmermann, K.; Sang, X.; Carboni, J.; Li, A.; Attar, R.; Gottardis, M.; et al. Discovery and initial SAR of 3-(1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-ones as inhibitors of insulin-like growth factor 1-receptor (IGF-1R). Bioorg. Med. Chem. Lett. 2007, 17, 2317–2321. [Google Scholar] [CrossRef]
- Zak, M.; Mendonca, R.; Balazs, M.; Barrett, K.; Bergeron, P.; Blair, W.S.; Chang, C.; Deshmukh, G.; DeVoss, J.; Dragovich, P.S.; et al. Discovery and optimization of C-2 methyl imidazopyrrolopyridines as potent and orally bioavailable JAK1 inhibitors with selectivity over JAK2. J. Med. Chem. 2012, 55, 6176–6193. [Google Scholar] [CrossRef] [PubMed]
- Baffert, F.; Régnier, C.H.; De Pover, A.; Gosselin, G.; Bretones, P.; Bonnet, M.; Chapuis, N.; Clément, A.; Delwail, A.; Fabbro, D.; et al. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol. Cancer Ther. 2010, 9, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Ben-Bassat, A.; Giladi, M.; Haitin, Y. Structure of KCNH2 cyclic nucleotide-binding homology domain reveals a functionally vital salt-bridge. J. Gen. Physiol. 2020, 152, e201912505. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.L.; Solowiej, J.; Kania, R. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef]
- Lundquist, J.T.; Harnish, D.C.; Kim, C.Y.; Sloop, K.W.; Patil, R.; Tilahun, M.; Nguyen, T.; Kang, H.J.; Driscoll, J.S.; Halladay, J.S.; et al. Improvement of physiochemical properties of the tetrahydroazepinoindole series of farnesoid X receptor (FXR) agonists: Beneficial modulation of lipids in primates. J. Med. Chem. 2010, 53, 1774–1787. [Google Scholar] [CrossRef] [PubMed]
- Claff, T.; Yu, J.; Blais, V.; Basran, J.; Borsodi, A.; Clemons, N.; Cox, S.; Elmore, C.S.; Gebhart, C.; Gutierrez, D.; et al. Elucidating the active δ-opioid receptor crystal structure with peptide and small-molecule agonists. Sci. Adv. 2019, 5, eaax9115. [Google Scholar] [CrossRef] [PubMed]
- Puius, Y.A.; Zhao, Y.; Sullivan, M.; Lawrence, D.S.; Mazzarella, R.M.; Schindler, J.F.; Linder, M.E.; Lawrence, D.S.; Dixon, J.E. Identification of a second aryl phosphate-binding site in protein-tyrosine phosphatase 1B: A paradigm for inhibitor design. Proc. Natl. Acad. Sci. USA 1997, 94, 13420–13425. [Google Scholar] [CrossRef]
- Fujita-Sato, S.; Ito, S.; Isobe, T.; Tanimoto, Y.; Senda, M.; Kawai, S.; Kiyonari, H.; Hata, Y.; Hirayama, Y.; Kuroda, K.; et al. Structural basis of digoxin that antagonizes RORγt receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J. Biol. Chem. 2011, 286, 31409–31417. [Google Scholar] [CrossRef]
- Xu, W.; Harrison, S.C.; Eck, M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 1997, 385, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Guzman, F.G.; Higashiyama, T.; Pangborn, W.; Schutt, C.E.; Garavito, R.M. Structure of human estrone sulfatase suggests functional roles of membrane association. J. Biol. Chem. 2003, 278, 22989–22997. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Nikolaev, Y.A.; Petrenko, A.S.; Filippov, D.V.; Konstantinov, A.A.; Arseniev, A.S. Extracellular cap domain is an essential component of the TRPV1 gating mechanism. Nat. Commun. 2021, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2022-4: Glide; Schrödinger, LLC: New York, NY, USA, 2021.
- Ha, N.T.; Lee, C.H. Roles of Farnesyl-Diphosphate Farnesyltransferase 1 in Tumour and Tumour Microenvironments. Cells 2020, 9, 2352. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, C.S. Combined effect of protein kinase B inhibitor or extracellular signal-regulated kinase inhibitor against farnesyltransferase inhibition-induced apoptosis in SiHa cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 291–303. [Google Scholar] [CrossRef]
- Hemmat, N.; Mokhtarzadeh, A.; Aghazadeh, M.; Jadidi-Niaragh, F.; Baradaran, B.; Bannazadeh Baghi, H. Role of microRNAs in epidermal growth factor receptor signaling pathway in cervical cancer. Mol. Biol. Rep. 2020, 47, 4553–4568. [Google Scholar] [CrossRef]
- Jaye, M.C.; Krawiec, J.A.; Campobasso, N.; Smallwood, A.; Qiu, C.; Lu, Q.; Kerrigan, J.J.; De Los Frailes Alvaro, M.; Laffitte, B.; Liu, W.S.; et al. Discovery of Substituted Maleimides as Liver X Receptor Agonists and Determination of a Ligand-Bound Crystal Structure. J. Med. Chem. 2005, 48, 5419–5422. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, L.; Hernández-Linares, M.; Escobar, M.; Pérez-Morales, J.; Sánchez-Monroy, V. Antiproliferative, Cytotoxic, and Apoptotic Activity of Steroidal Oximes in Cervicouterine Cell Lines. Molecules 2016, 21, 1533. [Google Scholar] [CrossRef]
- Manzerra, P.; Behrens, M.M.; Canzoniero, L.M.T.; Sensi, S.L.; Kaczmarek, L.K.; Weiss, J.H. Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 11055–11061. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ebihara, M.; Oishi, S.; Fujimoto, Y.; Okada, T.; Imamura, T. Histamine H1 receptor antagonists selectively kill cisplatin-resistant human cancer cells. Sci. Rep. 2021, 11, 1492. [Google Scholar] [CrossRef]
- Omar, A.M.; Aljahdali, A.S.; Safo, M.K.; Mohamed, G.A.; Ibrahim, S.R.M. Docking and Molecular Dynamic Investigations of Phenylspirodrimanes as Cannabinoid Receptor-2 Agonists. Molecules 2022, 28, 44. [Google Scholar] [CrossRef]
- Rosales-López, A.; López-Castillo, G.N.; Sandoval-Ramírez, J.; Terán, J.L.; Carrasco-Carballo, A. Correlation between molecular docking and the stabilizing interaction of HOMO-LUMO: Spirostans in CHK1 and CHK2, an in silico cancer approach. Int. J. Mol. Sci. 2024, 25, 8588. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, X.; Mumtahana, F.; Li, Y.; Zhang, Q.; Zhang, Y.; Zhang, J.; Wang, Z.; Wang, S. The existence of Th22, pure Th17 and Th1 cells in CIN and Cervical Cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer 2015, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef]
- She, S.; Zhao, Y.; Kang, B.; Chen, M.; Liu, J.; Zhang, X.; Li, H.; Wang, Y.; Sun, Q.; Liu, Y.; et al. Combined inhibition of JAK1/2 and DNMT1 by newly identified small-molecule compounds synergistically suppresses the survival and proliferation of cervical cancer cells. Cell Death Dis. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Tüzmen, Ş.; Hostetter, G.; Watanabe, A.; Seifert, A.; Cho, W.C.S.; Azmi, A.S.; Elsayed, A.M.A.; Xu, L.; Sun, Z. Characterization of Farnesyl Diphosphate Farnesyl Transferase 1 (FDFT1) Expression in Cancer. Pers. Med. 2019, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Jan, Y.-H.; Liu, Y.-P.; Wang, J.-Y.; Chen, T.-C.; Kuo, P.-L.; Huang, C.-C.; Tsai, J.-P.; Chen, C.-H.; Lai, M.-D. Squalene Synthase Induces Tumor Necrosis Factor Receptor 1 Enrichment in Lipid Rafts to Promote Lung Cancer Metastasis. Am. J. Respir. Crit. Care Med. 2014, 190, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Yun, H.; Kim, J.; Chung, J.-Y.; Kim, J.-H.; Cho, H. Overexpression of glucocorticoid receptor promotes the poor progression and induces cisplatin resistance through p38 MAP kinase in cervical cancer patients. Am. J. Cancer Res. 2022, 12, 3437–3454. [Google Scholar]
- Nayarisseri, A.; Abdalla, M.; Joshi, I.; Alharbi, N.S.; Taha, M.; Alharbi, A.; Al-Rashida, M.; Alqudah, A.M. Potential inhibitors of VEGFR1, VEGFR2, and VEGFR3 developed through Deep Learning for the treatment of Cervical Cancer. Sci. Rep. 2024, 14, 13251. [Google Scholar] [CrossRef]
- Qiu, H.; Li, J.; Liu, Q.; Tang, M.; Wang, Y. Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel. Cell Cycle 2018, 17, 1235–1244. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, M.Y.; Lee, D.H.; Kwon, S.Y.; Kim, Y.J.; Kim, S.Y.; Kim, Y.K.; Jeong, J.Y.; Lee, J.H.; Choi, J.H. O-linked N-acetylglucosamine transferase enhances secretory clusterin expression via liver X receptors and sterol response element binding protein regulation in cervical cancer. Oncotarget 2018, 9, 4625–4636. [Google Scholar] [CrossRef]
- Morales-Rodríguez, M.; Paniagua-García, L.; Narayanan, J.; Cano-Sarabia, M.; Aldalur, J.; Medina, L.; López-Fernández, L.; Santillán, D. Insulin-like growth factor axis: A potential nanotherapy target for resistant cervical cancer tumors (Review). Oncol. Lett. 2023, 25, 128. [Google Scholar] [CrossRef]
- Durzyńska, J. IGF axis and other factors in HPV-related and HPV-unrelated carcinogenesis (Review). Oncol. Rep. 2014, 32, 2295–2306. [Google Scholar] [CrossRef]
- Kuebler, U.; Fischer, S.; Mernone, L.; Breymann, C.; Abbruzzese, E.; Ehlert, U. Is stress related to the presence and persistence of oncogenic human papillomavirus infection in young women? BMC Cancer 2021, 21, 419. [Google Scholar] [CrossRef]
- Rai, R.; Nahar, M.; Jat, D.; Gupta, N.; Mishra, S.K. A systematic assessment of stress insomnia as the high-risk factor for cervical cancer and interplay of cervicovaginal microbiome. Front. Cell Infect. Microbiol. 2022, 12, 1042663. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Yuan, X.; Zhang, Q.; Tang, Y.; Tang, Y.; Huang, H.; Tang, Y.; Chen, Q. IU1 suppresses proliferation of cervical cancer cells through MDM2 degradation. Int. J. Biol. Sci. 2020, 16, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, H.; Shi, H.; Zhang, S.; Cui, J.; Zhao, Y.; Zhang, J.; Liu, X.; Yan, C.; Ma, L.; et al. A Designed Peptide Targets Two Types of Modifications of p53 with Anti-cancer Activity. Cell Chem. Biol. 2018, 25, 761–774. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Bi, X.; Yang, M.; Sun, J.; Wang, Y.; Liu, Y.; Zhang, X.; Zhang, S.; Zhao, H.; et al. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways. Cell Death Dis. 2021, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, J.; Zhou, L.; Liu, M.; Lu, W.; Wang, Y.; Li, X.; Zhang, Y.; Wang, Y.; Zhu, L.; et al. c-Src Promotes Tumorigenesis and Tumor Progression by Activating PFKFB3. Cell Rep. 2020, 30, 4235–4249. [Google Scholar] [CrossRef]
- Hacking, S.M.; Yakirevich, E.; Wang, Y. From Immunohistochemistry to New Digital Ecosystems: A State-of-the-Art Biomarker Review for Precision Breast Cancer Medicine. Cancers 2022, 14, 3469. [Google Scholar] [CrossRef]
- Huang, X.; Wang, B.; Chen, R.; Peng, J.; Xu, M.; Xu, Z.; Wang, C.; Liu, Y.; Zhang, Z.; Guo, C. The Nuclear Farnesoid X Receptor Reduces p53 Ubiquitination and Inhibits Cervical Cancer Cell Proliferation. Front. Cell Dev. Biol. 2021, 9, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, J.; Zhao, T.; Wang, H.; Lai, G. Over-expression of cannabinoid receptor 2 induces the apoptosis of cervical carcinoma Caski cells. Chin. J. Cell Mol. Immunol. 2015, 31, 758–762. [Google Scholar]
- Sheng, B.; Wang, W.; Xia, D.; Qu, X. Panobinostat (LBH589) combined with AM1241 induces cervical cancer cell apoptosis through autophagy pathway. BMC Pharmacol. Toxicol. 2023, 24, 45. [Google Scholar] [CrossRef]
- Wahnou, H.; Hmimid, F.; Errami, A.; Nait-Irahal, I.; Limani, Y.; Oudghiri, M. Integratin ADMET, enrichment analysis and molecular docking approah to elucidate the mechanism of Artemisia herba alba for the treatment of inflammatory bowel disease associated arthritis. J. Toxicol. Environ. Health Part A 2024, 87, 836–854. [Google Scholar] [CrossRef]
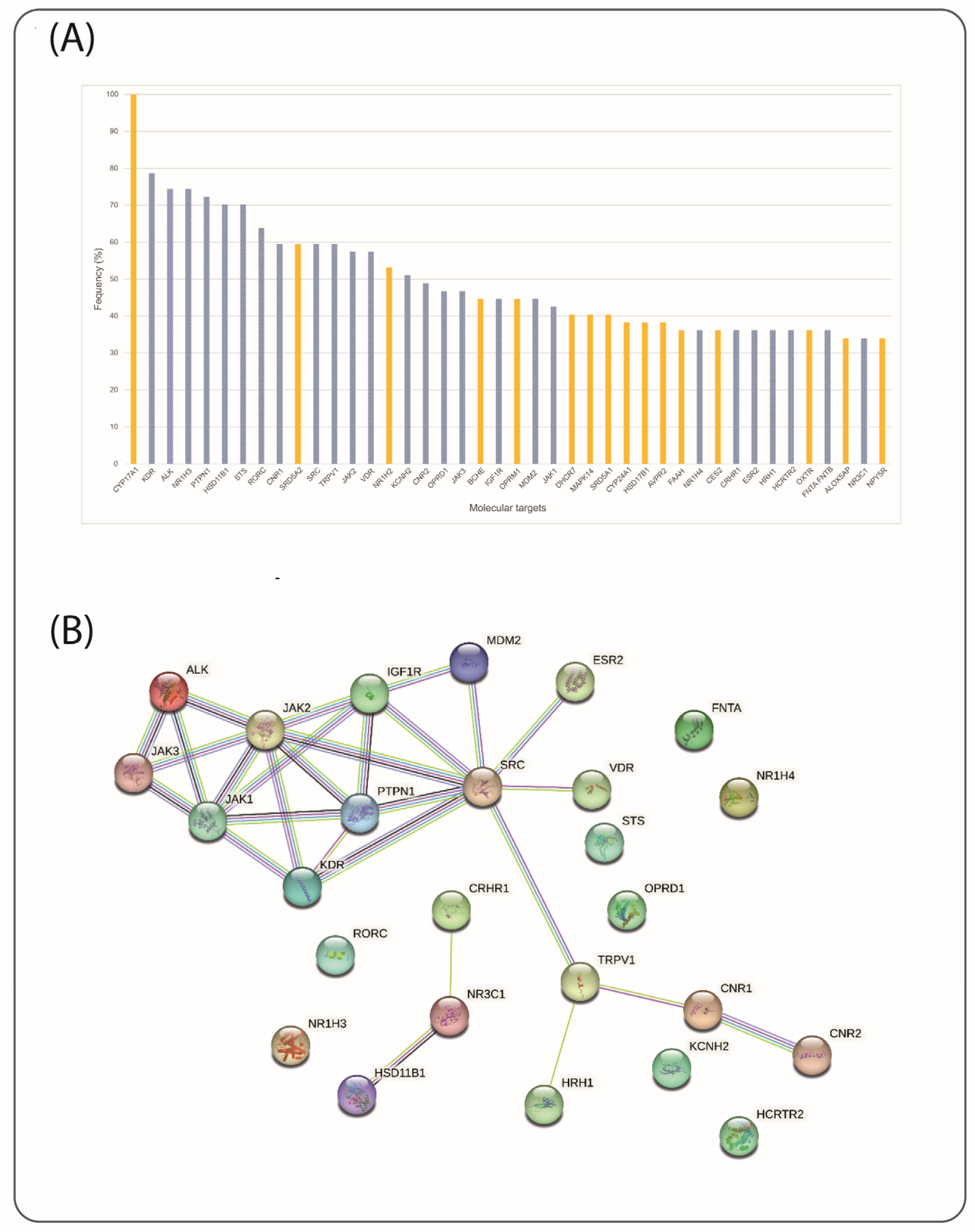



| Target | UniProt ID | Reported Data on Cervical Cancer (CC) | Reference |
|---|---|---|---|
| ALK | Q9UM73 | Induces cell migration and cancer survival. Together with IGF-1R, ALK can activate pathways related to cancer progression (Akt and Stat3). | [79,80,81] |
| CNR1 | P21554 | Present in CC cell lines (HeLa, CaSki, C33A). Its overexpression suggests a protective effect. | [82] |
| CNR2 | P34972 | CBR2 overexpression has been reported to promote apoptosis in CC CaSki cells. | [83] |
| CRHR1 | P34998 | Associated with immuno-escape from CC cells by decreasing NKG2D ligand. | [82] |
| ESR2 | Q92731 | Related to the progression of CC. Increases along with the transcription factor BORIS as the degree of the lesion increases (LSIL, HSIL, SC). | [84] |
| FNTA | P49354 | Inhibition of farnesyl transferase protein induces apoptosis in SiHa CC cells. | [85] |
| HCRTR2 | O43614 | Related to migration and invasion of CC. | [86] |
| HSD11B1 | P28845 | Identified in primary cancerous tissue from CC. | [48] |
| HRH1 | P35367 | Promotes the proliferation of HeLa cisR cells. | [87] |
| IGF1R | P08069 | Induces autophosphorylation and activation of specific tyrosine kinase residues, initiating signaling cascades such as Ras/Raf/MAPK and PI3K, involved in resistance to radiotherapy and cell survival. | [88,89] |
| JAK1 | P23458 | Takes part in the evasion of the immune system by HPV+ CC cells. | [90] |
| JAK2 | O60674 | Necessary for activation of the STAT3 pathway and inhibition of apoptosis. Involved in the proliferation and survival of CC cells. Promotes invasion and metastasis by activating REX1. | [91,92] |
| JAK3 | P52333 | Activates the transcription factor STAT, taking part in chemoresistance, proliferation, anti-apoptosis, angiogenesis, migration, invasion, and the Warburg effect. | [93,94] |
| KCNH2 | Q12809 | Expressed in HeLa cells of CC. It has been related to invasion and metastasis in other cancers. | [95] |
| KDR | P35968 | Endothelial cell germination increased vascular permeability; expression of tissue matrix metalloproteinases (MMPs). Regulates EMT-linked stemming in CC cells via the Akt/GSK3β/β-Catenin and Snail pathway. More related to progress from CIN I to III. | [96,97,98] |
| MDM2 | Q00987 | Overexpressed in CC cells. Inhibits p53 through ubiquitination and degradation in the proteasome. | [99] |
| NR1H3 | Q13133 | O-GlcNAcylation increases LXR expression in CC, upregulating sCLU transcription related to proliferation and drug resistance. | [84] |
| NR1H4 | Q96RI1 | Decreased in CC. Its overexpression inhibits the proliferation of CC cells by increasing p14ARF, MDM2, and p53. | [100] |
| NR3C1 | P04150 | Expressed in cervical carcinoma tissue. HPV sequences can bind to translocate to the nucleus. | [101] |
| OPRD1 | P41143 | Morphine stimulates the growth of C33A and CaSki CC cells through opioid receptors. | [96] |
| PTPN1 | P18031 | Overexpressed in CC; associated with proliferation, migration, invasion, and EMT. | [102,103] |
| RORC | P51449 | Overexpressed in CC. Related to the polarization of Th22 and Th17 cells, which favor the development of solid tumors. | [104] |
| SRC | P12931 | Related to CC progression. | [105] |
| STS | P08842 | Induces the Wnt/B catenin and EMT pathway through Twist1 and HIF-1alpha. Promotes tumorigenesis and inhibits apoptosis in CC. | [88] |
| TRPV1 | Q8NER1 | Related to tumor immunity through T cells. | [106] |
| VDR | P11473 | Its elevated expression in patients with cervical CC makes the risk of incidence 2 times higher. | [107] |
| Reference Ligands | PubChem ID | Target Protein | UniProt ID | PDB ID | Reference |
|---|---|---|---|---|---|
| NVP-TAE684 | 16038120 | ALK | Q9UM73 | 2XB7 | [111] |
| CBD | 644019 | CNR1 | P21554 | 5TGZ | [112] |
| CBD | 644019 | CNR2 | P34972 | 5ZTY | [113] |
| CP-376395 | 9862166 | CRHR1 | P34998 | 4K5Y | [114] |
| Estradiol | 5757 | ESR2 | Q92731 | 5TOA | [115] |
| PD036027 | 1266 | FNTA | P49354 | 1LD8 | [116] |
| Suvorexant | 24965990 | HCRTR2 | O43614 | 4S0V | [105] |
| Arylsulfonylpiperazine Inhibitor | 735815 | HSD11B1 | P28845 | 3CZR | [89] |
| Doxepin | 667477 | HRH1 | P35367 | 3RZE | [97] |
| Benzimidazole inhibitor | 5798 | IGFR1 | P08069 | 2OJ9 | [117] |
| Upadacitinib | 58557659 | JAK1 | P23458 | 4EHZ | [118] |
| Upadacitinib | 58557659 | JAK2 | O60674 | 3KRR | [119] |
| Baricitinib | 44205240 | JAK3 | P52333 | 1YVJ | [101] |
| Zonisamide | 5734 | KCNH2 | Q12809 | 6SYG | [120] |
| Vatalanib | 151194 | KDR | P35968 | 4AGD | [121] |
| Imidazoline inhibitor | 68156 | MDM2 | Q00987 | 1RV1 | [84] |
| Dexametasone | 5743 | NR1H3 | Q13133 | 5AVI | [100] |
| Tetrahydroazepinoindole | 66694474 | NR1H4 | Q96RI1 | 3L1B | [122] |
| Stigmasterol | 5280794 | NR3C1 | P04150 | 4CSJ | [123] |
| Naltrexone | 5360515 | OPRD1 | P41143 | 6PT2 | [124] |
| 4-phosphonooxybenzyl-[4-phosphonooxy] benzene | 1757 | PTPN1 | P18031 | 1AAX | [99] |
| Digoxin | 2724385 | RORC | P51449 | 3B0W | [125] |
| PP2 | 4878 | SRC | P12931 | 1FMK | [126] |
| Dehydroepiandrosterone sulfatase | 0009025621 | STS | P08842 | 1P49 | [127] |
| Capsaisin | 1548943 | TRPV1 | Q8NER1 | 7LR0 | [128] |
| GW0742 | 9934458 | VDR | P11473 | 3OGT | [129] |
| Oxime | #Aromatic Heavy Atoms | Fraction Csp3 | #Rotatable Bonds | #H-Bond Acceptors | #H-Bond Donors | Log p | Log s | GI Absorption | BBB Permeant | Pgp Substrate | Log Kp (cm/s) | Lipinski | Ghose | Veber | Egan | Muegge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S.O.2 | 17 | 0.48 | 8 | 5 | 1 | 5.31 | −6.57 | High | No | Yes | −4.71 | 1 | 3 | 0 | 1 | 1 |
| S.O.3 | 12 | 0.42 | 6 | 3 | 1 | 5.53 | −6.19 | High | No | Yes | −4.14 | 1 | 1 | 0 | 1 | 1 |
| S.O.6 | 0 | 0.79 | 5 | 3 | 1 | 6.42 | −7.18 | Low | No | No | −3.41 | 1 | 3 | 0 | 1 | 1 |
| S.O.7 | 6 | 0.72 | 8 | 6 | 0 | 6.18 | −7.22 | Low | No | Yes | −4.36 | 2 | 4 | 0 | 1 | 1 |
| S.O.8 | 0 | 0.78 | 5 | 4 | 2 | 5.71 | −7.14 | Low | No | No | −3.3 | 1 | 2 | 0 | 1 | 1 |
| S.O.9 | 6 | 0.56 | 2 | 7 | 2 | 2.18 | −4.00 | High | No | Yes | −6.61 | 0 | 0 | 0 | 0 | 0 |
| S.O.10 | 6 | 0.61 | 4 | 5 | 2 | 5.81 | −6.36 | High | No | Yes | −4.32 | 1 | 1 | 0 | 1 | 1 |
| S.O.11 | 6 | 0.56 | 0 | 4 | 2 | 2.81 | −4.16 | High | Yes | Yes | −5.58 | 0 | 0 | 0 | 0 | 0 |
| S.O.12 | 6 | 0.61 | 0 | 3 | 2 | 3.38 | −3.96 | High | Yes | Yes | −5.63 | 0 | 0 | 0 | 0 | 0 |
| S.O.13 | 6 | 0.62 | 4 | 4 | 2 | 3.38 | −3.96 | High | Yes | Yes | −5.63 | 0 | 0 | 0 | 0 | 0 |
| S.O.14 | 0 | 0.74 | 0 | 2 | 1 | 4.36 | −5.08 | High | Yes | No | −4.13 | 1 | 0 | 0 | 0 | 1 |
| S.O.15 | 6 | 0.72 | 5 | 5 | 2 | 5.34 | −6.52 | High | No | Yes | −4.65 | 1 | 3 | 0 | 1 | 1 |
| S.O.16 | 6 | 0.6 | 1 | 2 | 2 | 5.34 | −6.52 | High | No | Yes | −4.65 | 1 | 3 | 0 | 1 | 1 |
| S.O.17 | 6 | 0.61 | 3 | 4 | 2 | 5.75 | −6.69 | Low | No | No | −4.24 | 1 | 1 | 0 | 1 | 1 |
| S.O.18 | 0 | 0.85 | 0 | 6 | 2 | 4.53 | −5.96 | High | No | Yes | −5.38 | 0 | 2 | 0 | 0 | 1 |
| S.O.19 | 0 | 0.96 | 5 | 4 | 3 | 5.25 | −6.62 | High | No | No | −3.96 | 1 | 2 | 0 | 0 | 1 |
| S.O.20 | 6 | 0.72 | 8 | 3 | 1 | 7.4 | −8.49 | Low | No | No | −3.24 | 2 | 4 | 0 | 1 | 1 |
| S.O.21 | 0 | 0.95 | 0 | 3 | 2 | 3.61 | −4.22 | High | Yes | Yes | −5.36 | 0 | 0 | 0 | 0 | 0 |
| S.O.22 | 6 | 0.56 | 0 | 4 | 2 | 2.81 | −4.16 | High | Yes | Yes | −5.58 | 0 | 0 | 0 | 0 | 0 |
| S.O.23 | 0 | 0.89 | 7 | 6 | 1 | 5.53 | −7.20 | Low | No | Yes | −3.96 | 1 | 4 | 0 | 1 | 1 |
| S.O.24 | 0 | 0.89 | 7 | 4 | 2 | 5.12 | −6.52 | High | No | Yes | −4.45 | 1 | 3 | 0 | 1 | 1 |
| S.O.25 | 12 | 0.48 | 5 | 5 | 0 | 4.76 | −5.73 | High | Yes | Yes | −5.28 | 0 | 0 | 0 | 0 | 1 |
| S.O.26 | 12 | 0.43 | 8 | 4 | 0 | 5.81 | −6.36 | High | No | Yes | −4.32 | 1 | 1 | 0 | 1 | 1 |
| S.O.27 | 6 | 0.71 | 7 | 6 | 1 | 5.78 | −7.00 | Low | No | Yes | −4.5 | 2 | 4 | 0 | 1 | 1 |
| S.O.28 | 0 | 0.75 | 2 | 5 | 2 | 2.6 | −3.36 | High | No | Yes | −6.65 | 0 | 0 | 0 | 0 | 0 |
| S.O.29 | 6 | 0.5 | 0 | 3 | 2 | 3.32 | −3.88 | High | Yes | Yes | −5.69 | 0 | 0 | 0 | 0 | 0 |
| S.O.30 | 6 | 0.65 | 4 | 3 | 1 | 4.51 | −5.13 | High | Yes | No | −4.38 | 0 | 0 | 0 | 0 | 1 |
| S.O.31 | 6 | 0.6 | 1 | 3 | 1 | 4.51 | −5.13 | High | Yes | No | −4.38 | 0 | 0 | 0 | 0 | 1 |
| S.O.32 | 0 | 0.88 | 5 | 5 | 1 | 4.49 | −5.1 | High | No | No | −5.46 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Valdeolivar, C.A.; Carrasco-Carballo, A.; Organista-Nava, J.; Sandoval-Ramírez, J.; Illades-Aguiar, B. Steroidal Oximes and Cervical Cancer: An In Silico Mechanistic Pathway Approach. Sci. Pharm. 2025, 93, 36. https://doi.org/10.3390/scipharm93030036
Sánchez-Valdeolivar CA, Carrasco-Carballo A, Organista-Nava J, Sandoval-Ramírez J, Illades-Aguiar B. Steroidal Oximes and Cervical Cancer: An In Silico Mechanistic Pathway Approach. Scientia Pharmaceutica. 2025; 93(3):36. https://doi.org/10.3390/scipharm93030036
Chicago/Turabian StyleSánchez-Valdeolivar, Carlos Antonio, Alan Carrasco-Carballo, Jorge Organista-Nava, Jesús Sandoval-Ramírez, and Berenice Illades-Aguiar. 2025. "Steroidal Oximes and Cervical Cancer: An In Silico Mechanistic Pathway Approach" Scientia Pharmaceutica 93, no. 3: 36. https://doi.org/10.3390/scipharm93030036
APA StyleSánchez-Valdeolivar, C. A., Carrasco-Carballo, A., Organista-Nava, J., Sandoval-Ramírez, J., & Illades-Aguiar, B. (2025). Steroidal Oximes and Cervical Cancer: An In Silico Mechanistic Pathway Approach. Scientia Pharmaceutica, 93(3), 36. https://doi.org/10.3390/scipharm93030036









