1. Introduction
The problem of somatic nerve regeneration is still one of the most urgent and understudied topics in biology and medicine due to the lack of effective treatment techniques. Every year, more than 2 million operations are performed worldwide to repair post-traumatic defects in the peripheral nervous system, and there are currently increasing rates of damage to somatic nerves due to an increase in road accidents and domestic and industrial injuries. Thus, damage to peripheral nerves is a priority in terms of disability treatment. Despite the widespread use of various approaches and treatment methods, the degree of disability remains quite high, and the time required to restore the functions of nerve conductors is long. The current approaches to the therapy of damaged somatic nerves fail to maintain over a long period of time the survival of nerve cells and neurite growth, which are the key factors of nerve regeneration [
1]. Considering the problem’s significance, over recent years there has been growing interest from researchers in the search for potential therapeutic agents—the substances enhancing neuroprotection and stimulating regenerative processes [
2]. One of the promising trends in the field is the use of physiologically active compounds of natural origin, particularly, IGF-1. The scientific literature increasingly frequently demonstrates data on the effect IGF-1 has on the regulation of neuron proliferation and differentiation, which is likely to be associated with the launching of the intracellular signaling pathways necessary to restore the functioning of injured nerve conductors [
3,
4,
5].
Since the membrane of nerve cells is a multi-component system, where structural organization and the performing functions are interrelated, the protein composition change to a large extent determines the development of the pathological processes that impact on the physicochemical condition of a lipid layer and the functional activity of a nerve fiber. According to reports in the literature, the activation of various signal cascades, particularly, MAPK/ERK, is accompanied by protein synthesis intensification due to the expression of the genes participating in cell differentiation and survival [
6].
Based on the above statements, the present work aimed to study the effect of IGF-1 on the change in the quantitative and qualitative composition of the protein fraction and DNA content in somatic nerve injury and regeneration. To achieve the goal, we completed the following tasks:
- -
To study the content of total protein and certain protein fractions in the proximal and distal ends of the rat sciatic nerve after its transection and intramuscular administration of IGF-1.
- -
To study the effect of the preparation on the change in quantitative DNA content and the functional activity of nerve conductors, as well as state the role of IGF-1 in regulating protein synthesis in damaged nerve conductors.
2. Materials and Methods
This study focused on the sciatic nerves of Wistar rats. The rats had an average weight of 250 ± 50 r. Intact (undamaged) nerves in the first experimental group served as a reference. In the second experimental group, the sciatic nerve of the animals was transected at the mid-thigh level. The nerve trunk was approached by dissecting the skin and subcutaneous fat of the hindlimb using blunt branches. The fasciae were dissected in layers, and the hip region muscles were dissected along the posterior limb surface. The nerve was completely transected in the lower third of the thigh at the site of the sural nerve bifurcation into separate ones—the common fibular and tibial nerves. In the third experimental group, after the sciatic nerve transection, IGF-1 (recombinant human insulin-like growth factor type 1) (E. coli) (SIGMA-ALDRICH, Saint Louis, MO, USA)) was administered intramuscularly daily to the animals at 50 and 100 ng/kg concentrations at a volume of 100 µL. The animals of the fourth experimental group were administered saline solution intramuscularly daily instead of IGF-1.
The proximal and distal ends of the nerves were extracted on days 7, 14 and 21 and placed in Ringer’s solution, consisting of 136 mM sodium chloride, 2.7 mM potassium chloride, 1.8 mM calcium chloride, 2.4 mM sodium bicarbonate and 5.55 mM glucose with a continuous oxygen flow. Bioelectrical activity was recorded for a proximal nerve segment with extracellular recording under the following stimulation parameters: amplitude 1.5 V, duration 0.3 ms and stimulation frequency 100 impulses/s using a oscilloscope (GW Instek GDS-71042, Taipei, Taiwan) and laboratory electrical stimulator ESL-2 (Tekknow, Tomsk, Russia) [
7].
Sciatic nerves were crushed using a homogenizer (POTTERS Sartorius, Gottingen, Germany) in a tube with a glass pestle in 0.8 M sucrose (nerve to sucrose solution ratio, 1:2) at 4 °C. The protein concentration in the obtained samples was measured using the Lowry method using bovine serum albumin as a standard [
8]. The optical density was determined on a spectrophotometer (Shimadzu, Kyoto, Japan) at a wavelength of 650 nm.
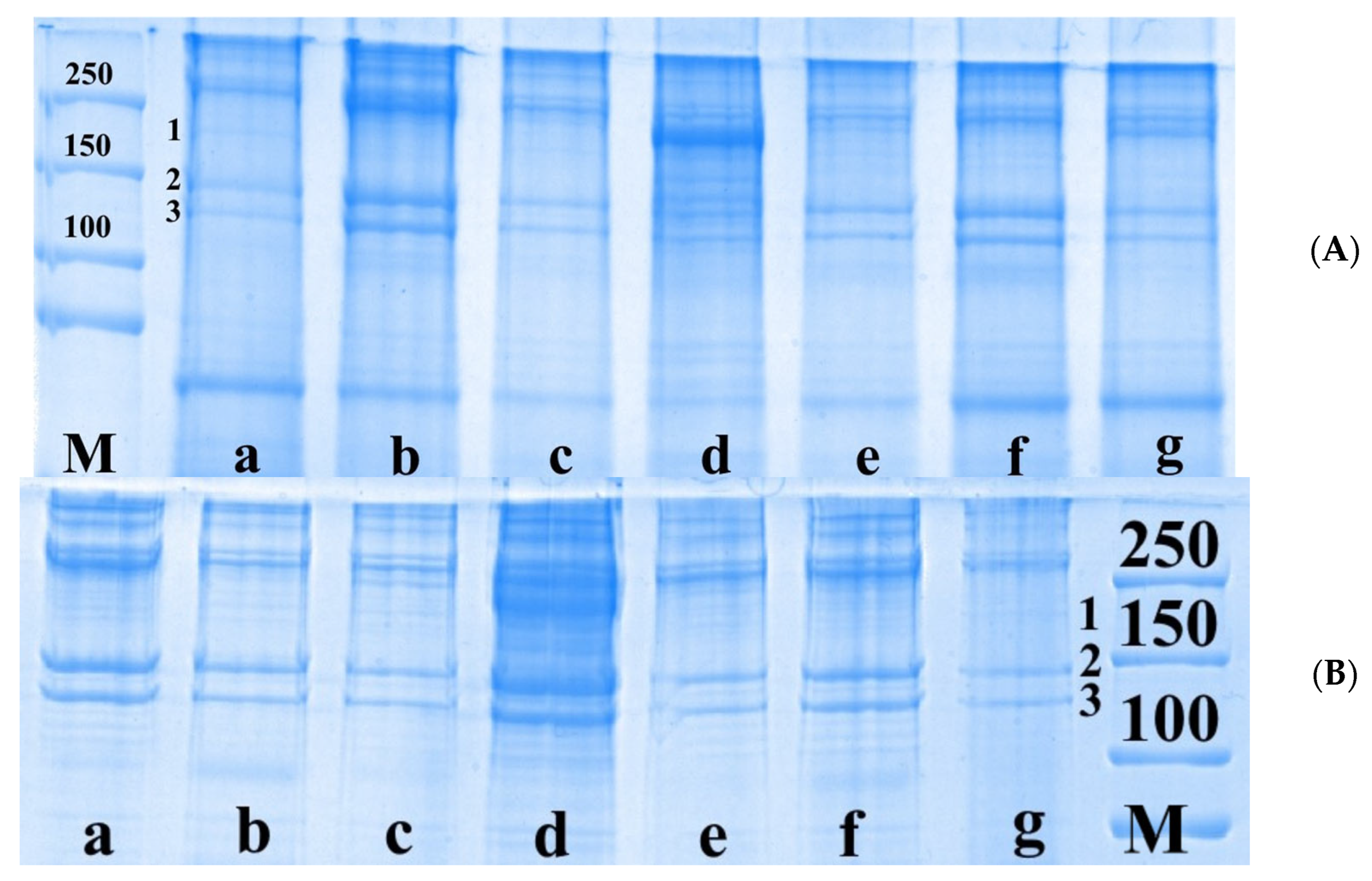
The protein composition of the somatic nerves was studied using electrophoresis in PAG in the presence of sodium dodecyl sulfate according to the Laemmli method [
9]. To assess the protein molecular weight, we used a standard protein solution Precision Plus Protein Dual Color Standards (10–250 kDa) (Bio-Rad, Hercules, CA, USA). The obtained protein gels were imaged, recorded and quantitatively analyzed using the gel imaging system GelDoc XR+ (Bio-Rad, Hercules, CA, USA). The findings were processed using the ImageLab program.
DNA was isolated using the Boodram method [
10], with the concentration being measured using a spectrophotometer NanoPhotometer NP80 (Implen, Munich, Germany) at a wavelength of 260 nm.
The physicochemical condition change in a lipid layer of the nerve fiber was assessed by RS-spectroscopy using a Raman spectrometer inVia Basis (Renishaw GmbH, Pliezhausen, Germany) relative to the intensity of the I
1122/I
1076 and I
2940/I
2853 peaks in the RS spectrum [
11,
12]. The Raman spectrometer is equipped with a short-focus high-aperture monochromator with a focal length of less than 250 mm. A laser with a wavelength of 532 nm and a radiation power of 10 mW was used to excite the spectrum. The signal was detected using a CCD detector (1024 × 256 pixels). The spectra were recorded in the range of 100–3200 cm
−1 with a spectral resolution of at least 2 cm
−1. At least 5 scans were performed for each sample to ensure the statistical reliability and reproducibility of the results.
The statistical analysis was performed using two-way ANOVA followed by Tukey’s multiple comparison test for pairwise group comparisons, with a significance threshold of 5%.
3. Results
According to the literature, neurotrophic factors produced by Schwann cells (SCs) are known to have a stimulating effect on developing neurons, since they are able to launch signaling pathways participating in the synthesis of structural and axonal proteins necessary to recover the functioning of an injured nerve conductor [
13]. Based on the above, the first experimental stage was devoted to the study of the change in total protein content and certain protein fractions in the proximal and distal ends of the rat sciatic nerve following its transection and the intramuscular administration of IGF-1. It should be noted that in a series of experiments with saline solution administered intramuscularly, no reliable changes in the protein composition, DNA content, as well as the functional activity of the sciatic nerve were found compared with an intact nerve conductor; therefore, these data are not presented in the article.
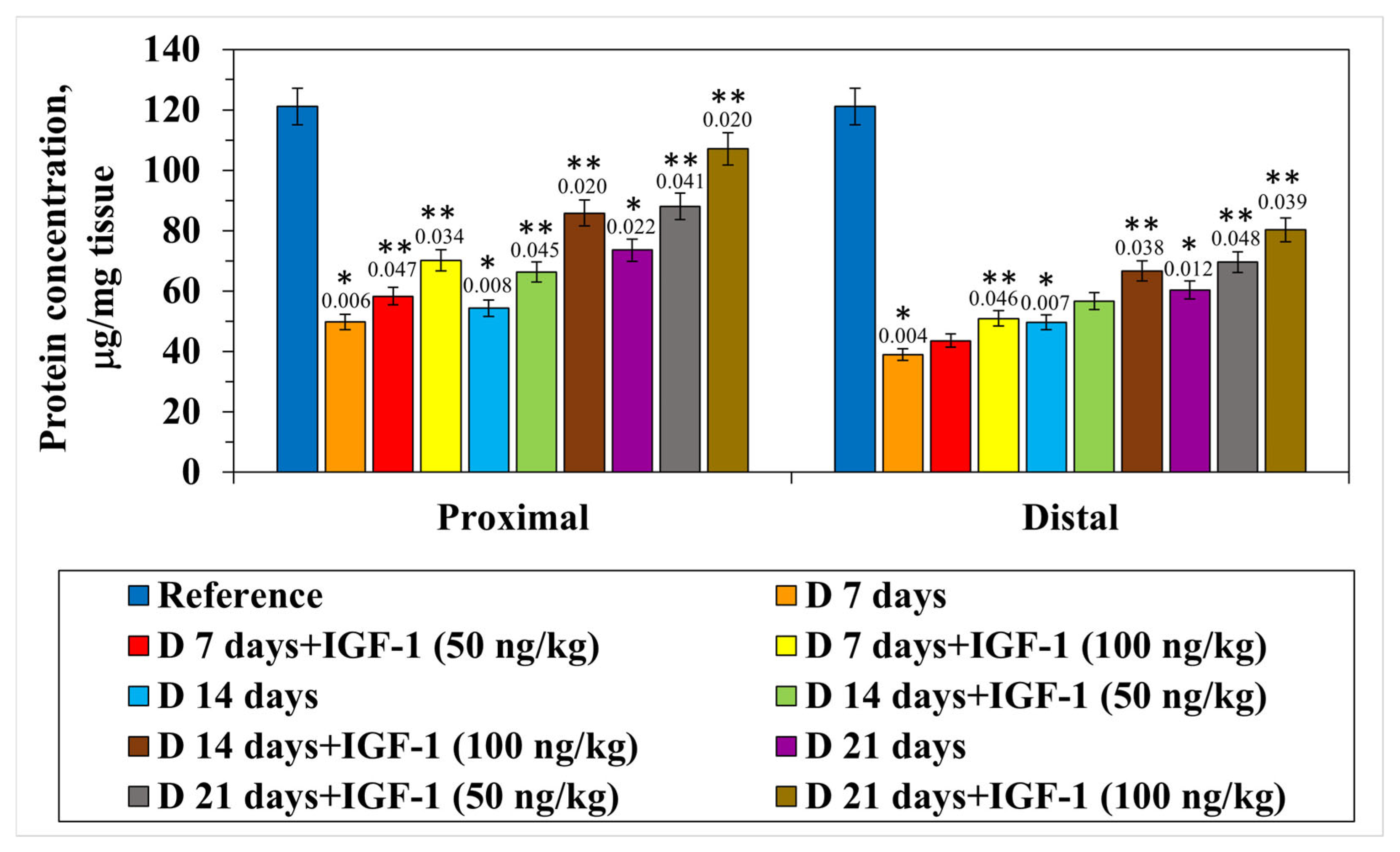
The experiments carried out revealed the transection to be accompanied by the quantitative decrease in the total protein fraction in the proximal nerve end by 60.0% relative to the control on day 7 of the postoperative period. On day 14 of this set of experiments, it was found that the indicator decreased by 55.1% compared with the intact nerve, and as the postoperative period extended to 21 days, the protein content decreased by more than 1.6 times relative to the control. The distal segment of the nerve conductor was observed to have a significant decrease in the protein content by day 7 of the experiment on average by 3.0 times compared with the control. It should be noted that by day 21, the value of the indicator under study was still lower by 50.2% than the control one. With the intramuscular administration of IGF-1 at a concentration of 100 ng/kg, by day 7 of the experiment, in the proximal nerve segment there was an increase in the total protein content by 16.9% compared with the nerve transection. In the same set of experiments, 14 days after injury, the quantitative protein content increased by 26.0%, and by day 21, by 27.8% relative to the set of experiments with the nerve transection. In the distal segment of the nerve conductor, in the set of experiments with the preparation administration, a less pronounced growth in the protein fraction content was observed. On day 7 of the experiment, against the background of IGF-1 administered at a 100 ng/kg concentration, a reliable increase in the indicator by 9.9% compared with the nerve transection was found. The experiment duration extending up to 14 and 21 days was accompanied by a protein fraction content increase in the distal nerve segment of 14.1% and 16.5%, respectively, relative to the nerve transection, when the preparation was administered at its maximal concentration (
Figure 1).
Thus, the sciatic transection was accompanied by a significant decrease in the quantitative content of the protein fraction in both nerve segments; however, the most pronounced changes were found to occur in the distal nerve conductor due to the loss of central regulation and the enhancement of degenerative processes resulting from the absence of regulatory mechanisms [
14].
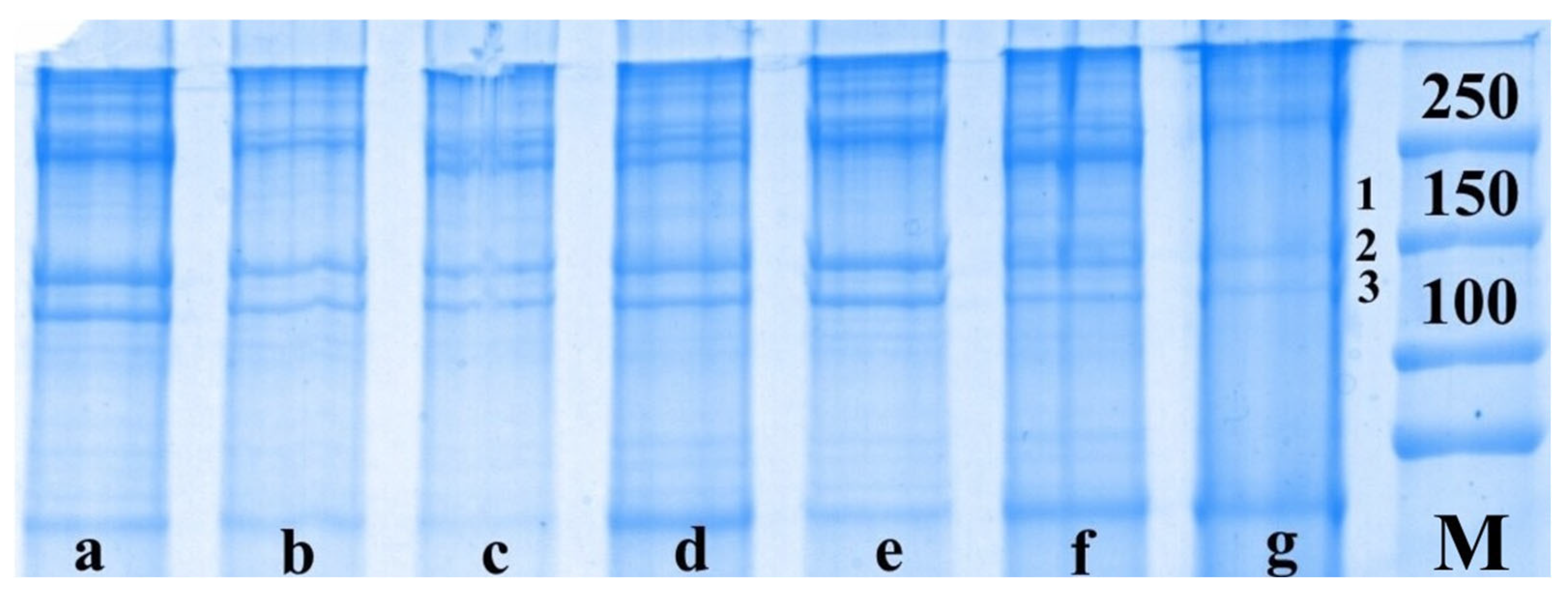
The next set of experiments using the electrophoretic method of separating proteins on PAG involved the study of the qualitative and quantitative composition of certain protein fractions of somatic nerves after injury and IGF-1 administration. In the intact nerve, we identified the proteins of neurofilament-H (NF-H, 190–250 kDa), neurofilament-M (NF-M, 140–160 kDa) and tubulin (110 kDa) (
Figure 2).
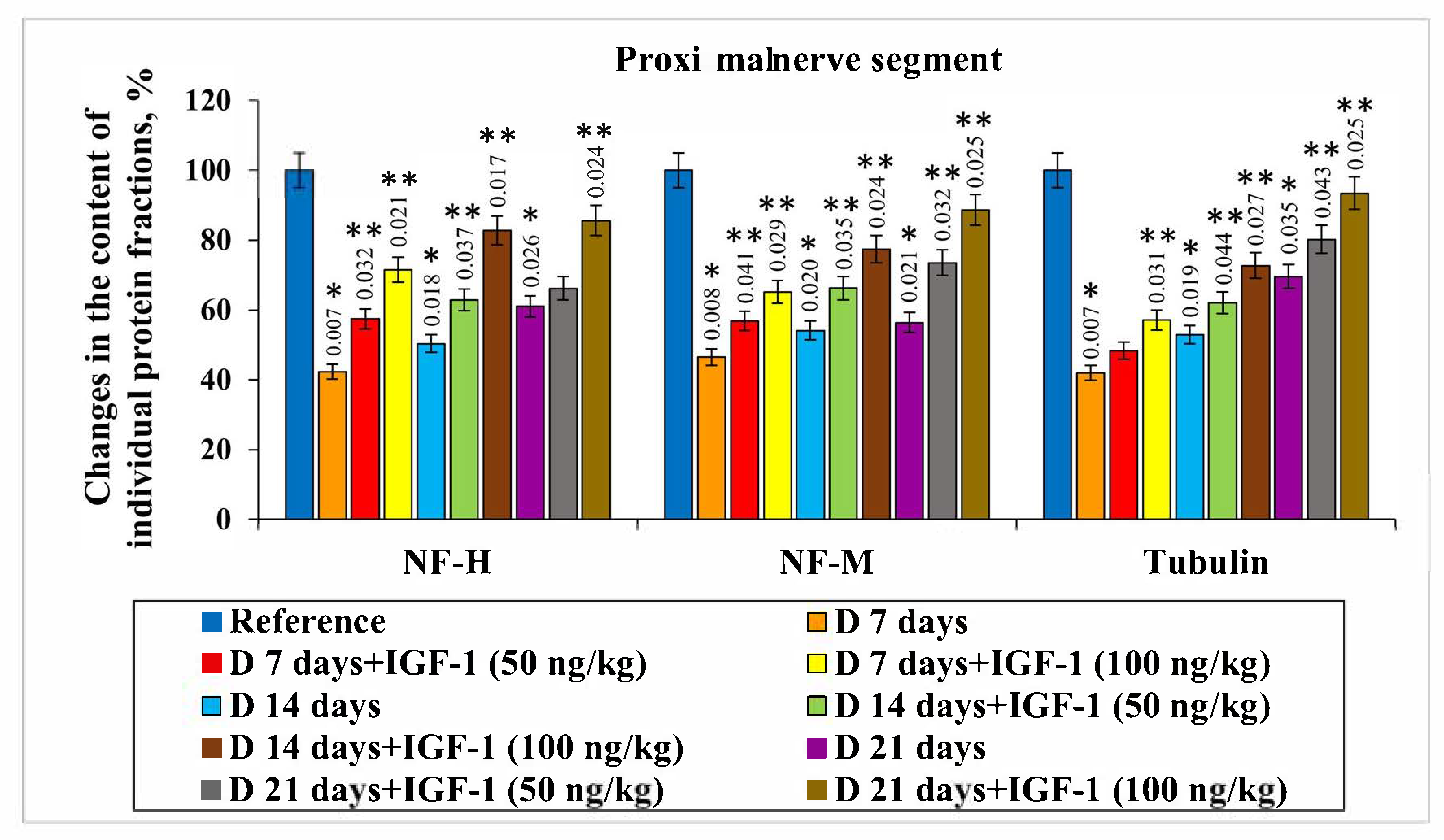
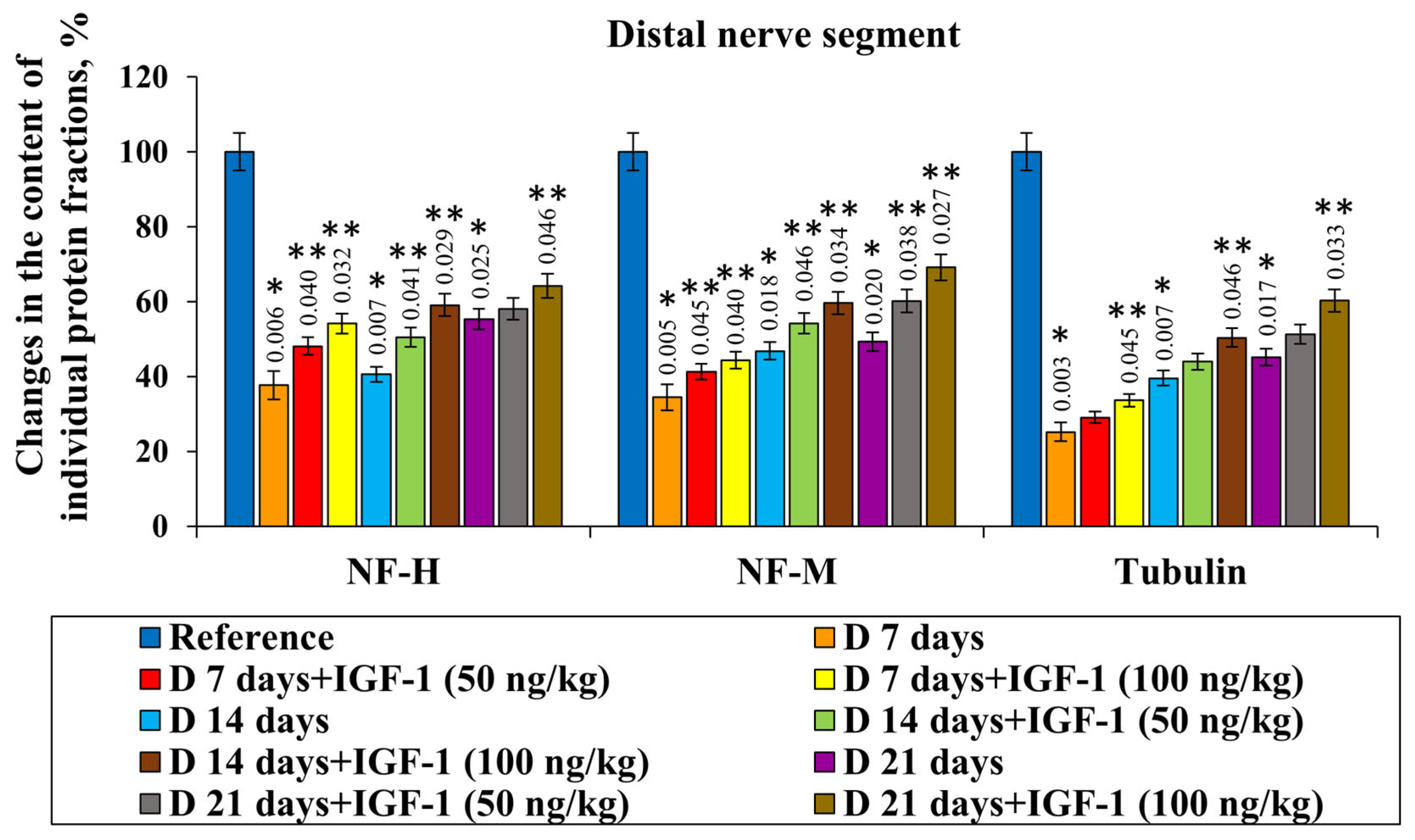
The content of NF-H, NF-M and tubulin fractions in the proximal nerve segment on day 7 after the transection was shown to decrease by 57.7%, 53.5% and 58.0%, respectively, compared with the control group. When extending the damaging effect duration up to 21 days, the level of NF-H, NF-M and tubulin reduced by 40.0%, 43.6% and 30.4%, respectively, compared with the intact nerve. In the distal nerve segment on day 7 after damage, the content of the protein fractions under study was found to decrease by more than 2.0 times relative to the control. On day 14 after damage, we found a decrease in NF-H, NF-M and tubulin levels by 59.4%, 53.2% and 60.4%, and on day 21, by 44.7%, 50.6% and 54.7%, respectively, compared with the control values (
Figure 3).
According to the literature, IGF-1 is an important factor in the growth and development processes in both the central and peripheral nervous systems. IGF-1 promotes the survival and proliferation of nerve cells, has an effect on their maturation, increases the growth of dendrites and axons and stimulates the processes of their myelination [
15]. Accordingly, the next set of experiments was devoted to the study of the effect IGF-1 at 50 and 100 ng/kg concentrations has on the change in certain protein fractions in the damaged somatic nerve (
Figure 4).
The NF-H and NF-M contents on day 7 after damage in the set of experiments with IGF-1 administered at a 50 ng/kg concentration in the proximal nerve segment was shown to increase by 15.2% and 10.3%, respectively, compared with the nerve transection. There were no reliable changes in tubulin levels observed in this set of experiments. IGF-1 administered at a 100 ng/kg concentration resulted in the NF-H, NF-M and tubulin levels increasing by 29.2%, 18.6% and 15.1%, respectively, relative to injury, on day 7 after damage. By day 14 of the experiment, the following changes occurred: the NF-H and NF-M contents increased by 12.5% and 12.1%, respectively, compared with the injury, against the background of using IGF-1 at the rate of 50 ng/kg. Additionally, the preparation concentration growth up to 100 ng/kg was accompanied by a NF-H and NF-M content increase of 32.4% and 23.3%, respectively, relative to the set of experiments with injury. The most pronounced changes were found to occur 21 days after the nerve transection in the set of experiments using IGF-1 at a 100 ng/kg concentration. For this, the NF-H, NF-M and tubulin contents increased by 24.6%, 32.2% and 23.8% compared with the injury (
Figure 3).
The study of the content of certain protein fractions in a distal segment of the damaged somatic nerves in the set of experiments using IGF-1 at a 100 ng/kg concentration showed the followings increases in the NF-H, NF-M and tubulin levels: on day 14 of the observation, the increase in the corresponding protein fractions was 18.4%, 12.9% and 10.8% relative to the nerve transection. Then, 21 days after injury, when administering the preparation at its maximal concentration, the NF-H content barely changed, while the NF-M and tubulin levels increased by 19.9% and 15.1%, respectively, relative to a set of experiments with the transection (
Figure 3).
Since an increase in certain protein fractions was observed both in the proximal and distal segments of the nerve conductor against IGF-1 administration, we carried out a set of experiments to study DNA concentration changes in damaged somatic nerves. According to data from the literature, neurotrophic factors launch several signaling pathways, one of which is MAPK/ERK. MAPK/ERK cascade activation is accompanied by the expression of the genes participating in cell differentiation and survival, along with the transcription factors associated with the axonal growth of nerve fibers and controlling protein synthesis in a cell [
16].
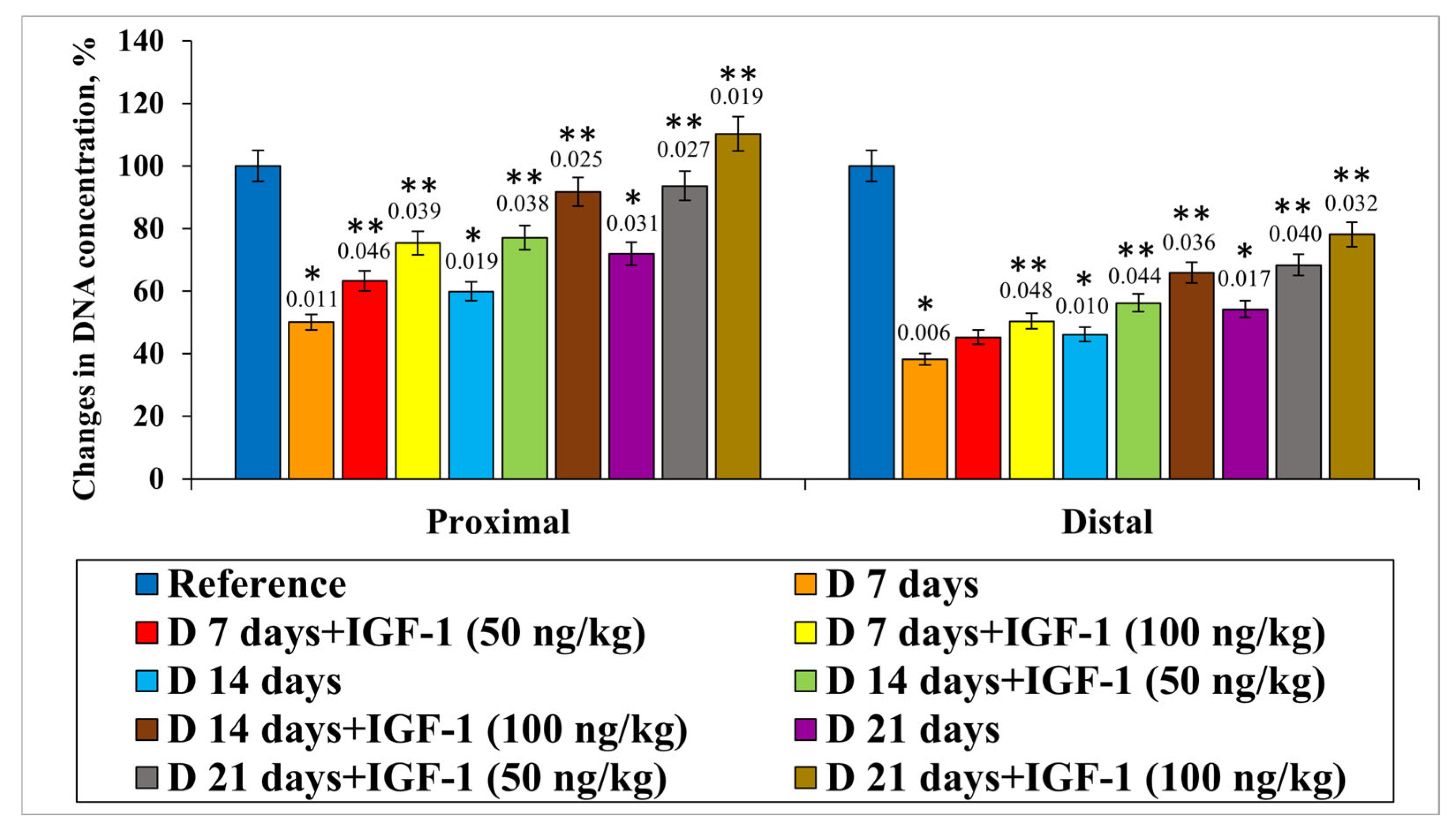
In the course of the present study, we revealed that DNA content decreased in the proximal nerve segment by 50.0%, 40.1% and 28.1% on days 7, 14 and 21 of the experiment, respectively, compared with the control animals. IGF-1 administered at a 50 ng/kg concentration on days 7, 14 and 21 of the experiment resulted in DNA level increases of 13.2%, 17.2% and 21.7%, respectively, relative to injury. If IGF-1 acted at a concentration of 100 ng/kg, the DNA content ratio compared with the nerve transection without IGF-1 introduction significantly increased by 25.3%, 31.9% and 38.4%, respectively, on days 7, 14 and 21 of the experiment (
Figure 5).
In the distal nerve segment when the transection had been performed, we observed a decrease in DNA content compared with the controls in all sets of experiments. The preferential increase in DNA content ratio was found when IGF-1 was used at a 100 ng/kg concentration on days 14 and 21 after damage by 19.8% and 23.9%, respectively, relative to the set of experiments with injury (
Figure 5).
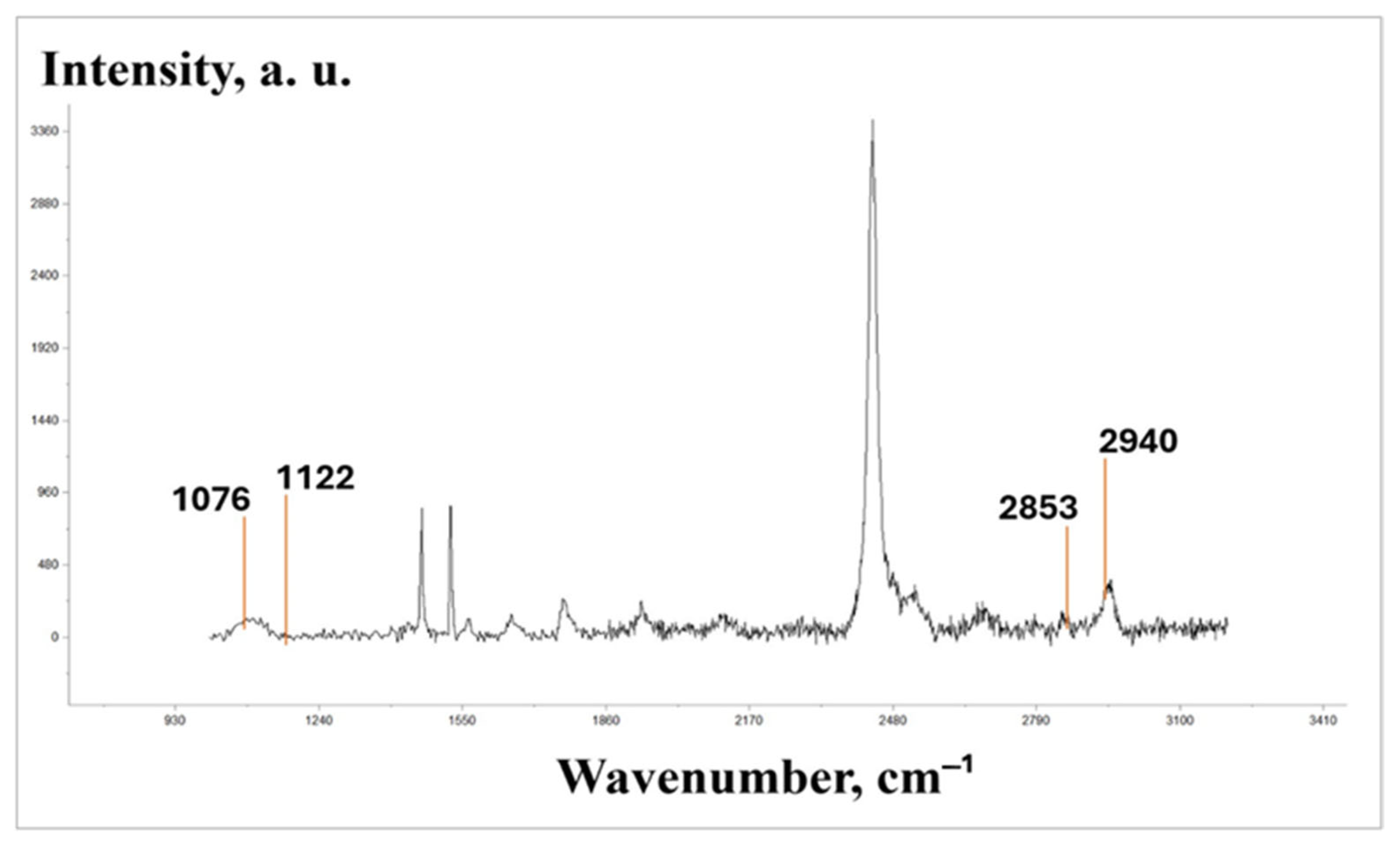
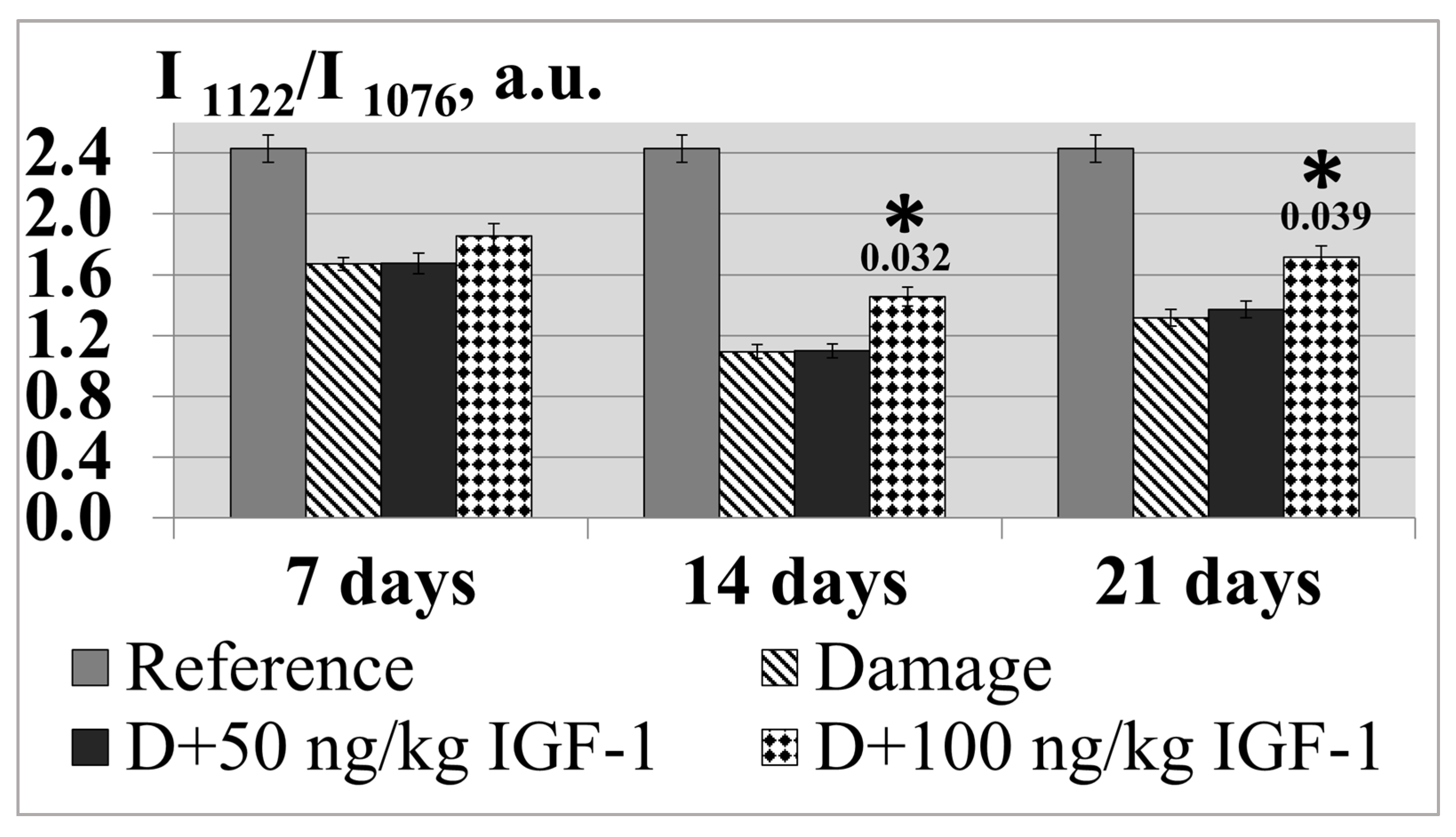
The physicochemical condition of the protein–lipid fraction of somatic nerves plays an essential role in regulating ion permeability, which is known to determine nervous and muscular irritability. Since damage caused the complete loss of nerve conduction in the distal nerve conductor, the next experimental stage using RS-spectroscopy included an assessment of the physicochemical condition of the nerve conductor membrane in its proximal segment. The ratio of the peak intensity (I
1122/I
1076) of an RS spectrum (
Figure 6) indicates the orderliness of fatty acid chains forming a hydrophobic layer in the membrane. In the literature, there are reports demonstrating that the decrease in this indicator is accompanied by a reduction in membrane viscosity [
11].
The experimental findings showed the minimal value of the indicator to be found on day 14 of the experiment: the ordering degree of the nerve fiber membrane decreased by 55.2% relative to the control. In the set of experiments with IGF-1 administered at a 50 ng/kg concentration, the I
1122/I
1076 ratio barely changed compared with the transection during the whole postoperative period. The preparation dose increase up to 100 ng/kg was accompanied by the indicator growth relative to the set of experiments with damage of 11.2, 33.5 and 30.0% by days 7, 14 and 21 of the experiment (
Figure 7).
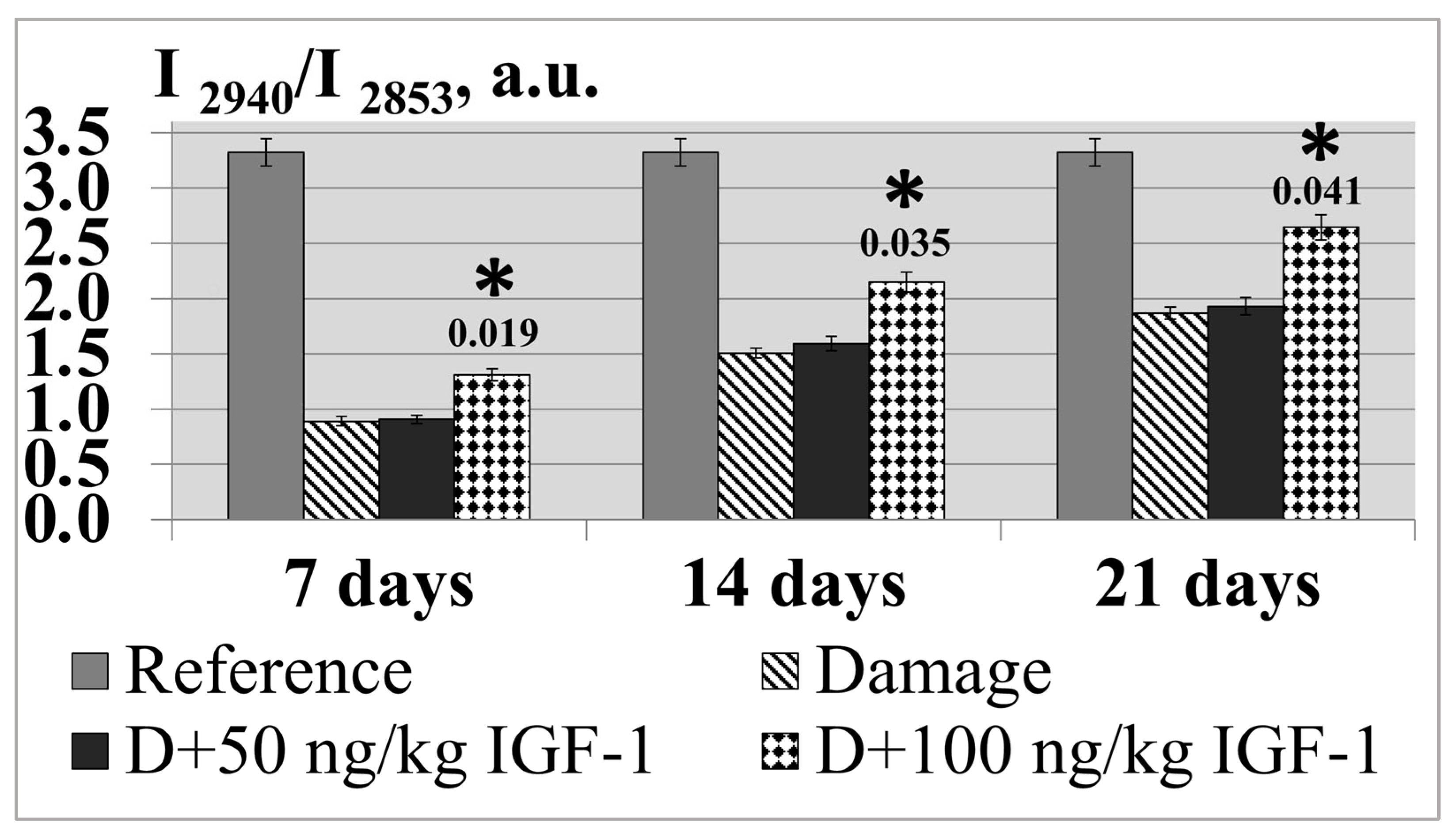
The physicochemical condition of a lipid layer can be judged by the intensity ratio of the I
2940/
I2853 bands, which characterize the relation of a protein component to a lipid one (
Figure 6) [
12]. The experiment showed that the sciatic transection resulted in the I
2940/
I2853 ratio decreasing by 3.7 times in relation to the control by day 7 of the experiment as the result of developing Wallerian degeneration processes, which are accompanied by proteolytic breakdown and lipid fraction destruction [
14]. The increase in the postoperative period up to 21 days resulted in indicator growth; however, it was still lower than the control values by 1.8 times on average. The set of experiments with IGF-1 administered at a 100 ng/kg concentration was observed to have a reliable increase in the indicator; the latter increased by 50.0% on average compared with the damage during the whole observation period (
Figure 8).
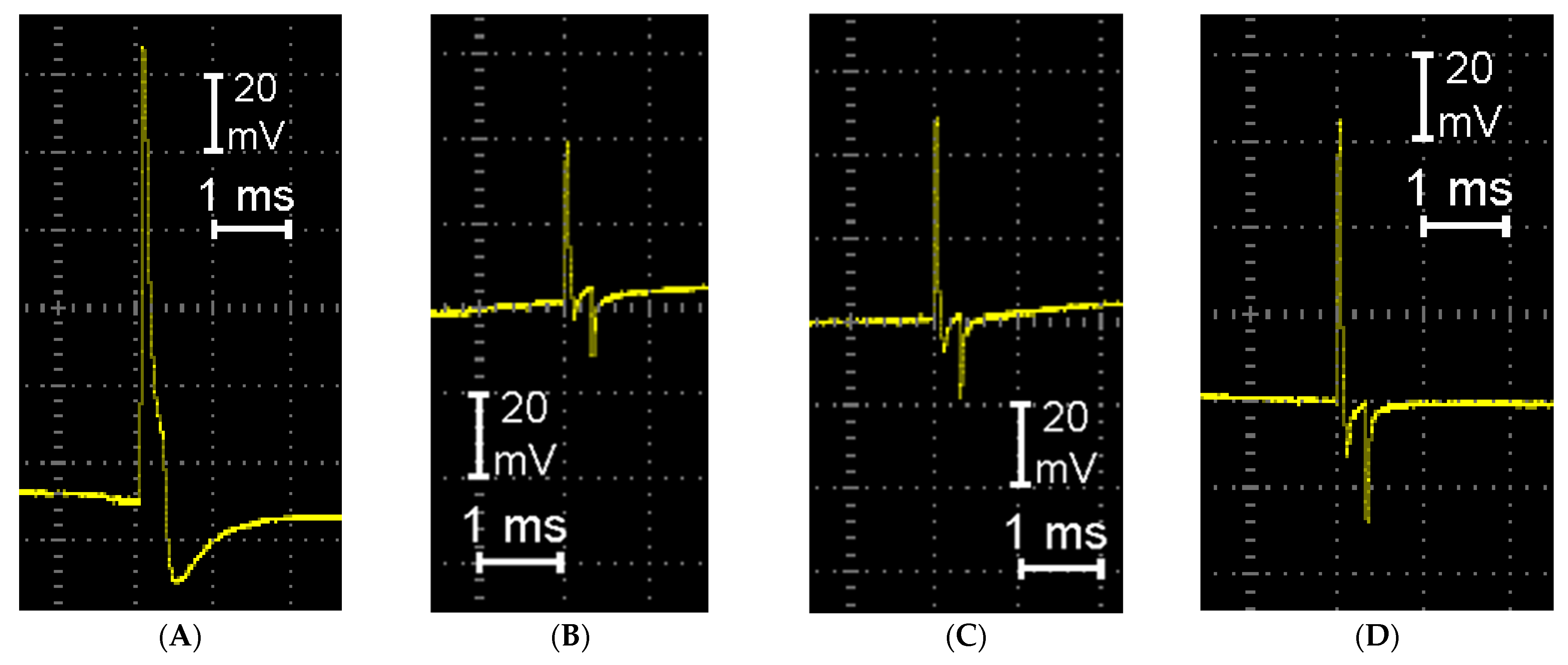
The data obtained on the study of the protein composition and RS-spectroscopy of somatic nerves correlate with the recovery of their functional activity in the course of the intramuscular administration of IGF-1. The nerve conductibility of AP was stated to significantly decrease in its proximal segment and be completely lost in the distal nerve conductor resulting from the nerve transection. With the damaging effect duration extending up to 21 days, the recovery of the AP conductibility with a small amplitude was found in the proximal nerve segment only; this is likely to be explained by central innervation preservation and the partial recovery of neuromuscular transmission. In the set of experiments with injury, the AP amplitude reduced by 3.0 times compared with the control by day 21 of the experiment, while the intramuscular administration of IGF-1 at a 100 ng/kg concentration was accompanied by conductibility recovery in the proximal nerve on average by 1.6 times compared with the damage cases (
Figure 9,
Table 1).
4. Discussion
Axonal growth is an important aspect of neuronal development, especially for the peripheral projecting motor and sensory neurons. IGF-I signaling via IGF-IR plays a key role in this process. IGF expression correlates with repair processes after peripheral nerve injury. According to the literature, in the sciatic nerve, IGF-I is found in axons and SCs and accumulates in damaged axons within 2 h after nerve destruction. It has also been shown that the amount of IGF-I in a lesion site reaches a maximum 2 weeks after the transection of a rat sciatic nerve. The presence of IGF-I also promotes an increase in the length of regenerating axons. IGF-I enhances the differentiation of oligodendrocyte progenitor cells and, consequently, myelination. There is substantial evidence that IGFs play a role in oligodendrocyte differentiation and survival and myelin synthesis, as well as in SCs survival and motility [
17].
It is now recognized that the activation of a transcriptional repair program in SCs is a critical determinant of the success of axon regeneration. According to data from the literature, transient p-ERK activation could have positive effects, for instance through enhanced myelin clearance in the early phase of the injury response. At early time points, the activation of the MAPK/ERK signal pathway produces an increased inflammatory response and faster myelin clearance. It has been shown that the transient and strong activation of the Raf/MEK/ERK pathway was able to induce the recruitment of inflammatory cells (macrophages, mast cells, neutrophils, and T cells). SCs and macrophages participate in myelin phagocytosis following injury and may contribute to the increased myelin clearance observed after MAPK/ERK activation. Following the initial phase of Wallerian degeneration, axons begin to regenerate, re-establishing their close relationship with SCs, which provide critical guidance and growth factors for axons. SCs then redifferentiate and remyelinate axons. Ultimately, axons will reinnervate their targets, leading to the restoration of sensorimotor function, although the degree of recovery is highly dependent on factors such as the type and duration of injury. IGF-1, binding to the corresponding receptor, activates the GTPase of proteins from the Ras family. Ras proteins bind and activate MEKK kinase, which, in turn, activates MEK kinase, which is represented by two components MEK1 and MEK2. MEK1/2 activates ERK1/2. The phosphorylation of ERK1/2 occurs near the cell membrane, after which the enzyme diffuses into the cytoplasm, where it phosphorylates signaling proteins, including the p90 kinase of ribosomal protein S6, and then into the nucleus, where it regulates transcription. ERK1/2 induces the transcription of early c-Fos and c-Myc genes, the products of which are transcription factors, and ensures the transcription of late genes responsible for cell proliferation, survival and motility [
18,
19].
In the present study, we showed that the intramuscular administration of IGF-1 was accompanied by a protein synthesis increase that was likely due to the activation of signaling pathways participating in expressing the genes responsible for the synthesis regulation of certain protein fractions. According to data from the literature, neurofilaments serving as the structural elements of the nerve tissue cytoskeleton participate in providing slow axonal transport, and tubulin, which is a part of cytoplasmatic microtubules, forms an intracellular skeleton [
20]. Additionally, an essential role in regulating the synthesis of cytoskeleton proteins and axon growth is assigned to MAPK/ERK [
21]. IGF-1, when binding to the corresponding receptor on the nerve fiber surface, is known to launch a MAPK/ERK cascade, in which a great number of small G-proteins participate. Small G-proteins transform into an actin cytoskeleton. Moreover, MAP-kinases and MAP family proteins associated with microtubules and stabilizing the tubulin cytoskeleton play a key role in these processes. Actin cytoskeleton rearrangement is associated with two families of small G-proteins, particularly, G-proteins Rho and those of the Ras family. The activation of G-proteins Rho results in cell morphology changes due to the phosphorylation of the protein regulators of actin polymerization, particularly, Apr 2/3 and filamin A, which are responsible for the branching of actin fibrils, as well as the regulatory proteins of tubulin cytoskeleton dynamics. Moreover, the G-protein Rit1, belonging to the family of Ras proteins, initiated axon branching and growth processes through the activation of ERK1/2 referring to the mitogen-activated protein kinases of this signaling pathway [
22].
The data obtained on the study of protein composition and quantitative DNA content are in agreement with the recovered functional activity of damaged somatic nerves associated with IGF-1 administration. We focused on an electrophysiological analysis (the amplitude and velocity of the APs) as the most sensitive and direct marker of early peripheral nerve regeneration under the influence of IGF 1. It is worth noting that the AP reflects the state of myelination, axonal integrity and synaptic transmission. These parameters are of great importance in assessing the regeneration of nerve fibers. In contrast to some other methods, recording the AP enables us to focus on specific nerve segments, which is significant when studying the local effects of IGF-1. In addition, by the 21st day of observation, motor activity does not recover, since a longer period is required to improve sensory function. Nevertheless, measuring the AP, which is the most important indicator of restoring the functional activity of a nerve, enables us to register regeneration processes long before motor function improvement and predict the course and degree of restoration of the functions of the damaged nerve conductor, since it is during this period that the later course of the pathological process and the restoration completeness of normal nerve functioning will depend on the degree of regeneration. The most pronounced recovery of the AP was found on the 21st day of the observation, in the series of experiments using the preparation at a 100 ng/kg concentration.
Moreover, the impaired composition of proteins and lipids along with structural reorganization resulting from the activation of phospholipases were accompanied by the changes in the physicochemical condition of the nerve conductor membrane. We recorded an RS spectrum of myelin to identify the conformational and chemical changes in native, degraded and regenerated myelin. The intensity ratio of I
1122/I
1076, which is related to the ratio of the
trans and
cis conformation populations of C–C bonds, was employed to examine the intramolecular chain ordering in lipid hydrocarbon chains [
11]. The 2940 cm
−1 band results, in part, from underlying infrared active methylene asymmetric stretching modes that become Raman active when the intramolecular chain disorder is increased. The 2853 cm
−1 band is assigned to the antisymmetric C-H stretching modes of the phospholipid acyl chains [
12]. According to RS-spectroscopy, the IGF-1 administered to the experimental animals resulted in the recovery of the orderliness of the fatty acid chains forming a hydrophobic layer in the membrane, suggesting a membrane protective effect of IGF-1 in damaged somatic nerves, along with the stabilization of the protein component composition of the nerve conductors. Thus, IGF-1 contributes to the recovery of nerve fiber microviscosity, the maintenance of which at a certain optimal level is of great importance for a cell and the whole body in case of changes in their functioning conditions.
This study has high translation potential. Small animal models are most frequently used in trauma research, with the aim of improving and developing a basic understanding of the complex post-traumatic regenerative mechanisms. Rat models have become a powerful tool for research on many aspects of trauma. The rat genome was the third complete mammalian genome deciphered. The comparison to the human genome revealed that all human genes known to be associated with disease have corresponding orthologues in the rat genome. The overall orthologous genomic regions in rats and humans correspond to 46%, whereas the “disease orthologous regions” correspond to 76%. Since rats have high translational potential, in particular, in injuries of different origins [
23], the data obtained can be used to develop highly effective drugs resulting from the targeted action on certain parts of the receptor system involved in the regeneration of somatic nerves.