Abstract
This study systematically reviews the non-traditional pharmacological effects of diclofenac, a well-known nonsteroidal anti-inflammatory drug, to explore its potential for drug repositioning beyond its established analgesic and anti-inflammatory applications. A comprehensive literature search was conducted using the PubMed, Scopus and Web of Science databases, covering studies from 1981 to 2025. It was revealed that over 94% of records in Scopus and Web of Science are duplicated in PubMed, so the latter was used for the search in our study. After duplicate removal and independent screening, 89 from 1123 retrieved studies were selected for the search. The analysis revealed a broad spectrum of diclofenac’s non-traditional pharmacological activities, including neuroprotective, antiamyloid, anticancer, antiviral, immunomodulatory, antibacterial, antifungal, anticonvulsant, radioprotective, and antioxidant properties, primarily identified through preclinical In vitro and In vivo studies. These effects are mediated through diverse molecular pathways beyond cyclooxygenase inhibition, such as modulation of neurotransmitter release, apoptosis, and cellular proliferation. Diclofenac showed potential for repositioning in oncology, neurodegenerative disorders, infectious diseases, and immune-mediated conditions. Its hepatotoxicity and cardiovascular risks necessitate strategies like advanced drug formulations, dose optimization, and personalized medicine to enhance safety. Large-scale randomized clinical trials are essential to validate these findings and ensure safe therapeutic expansion.
1. Introduction
The modern pharmaceutical science and industry constantly search for new, more effective, and safer therapeutic solutions to combat various human diseases. However, the traditional de novo drug development pathway, which begins with target identification and synthesis of new chemical compounds, is facing increasing and often insurmountable challenges. This process is highly time-consuming, usually taking 10–15 years from an idea to enter the market. This requires tremendous financial investments, reaching billions of dollars per successful drug, and is characterized by an extremely high failure rate. Only a small part of molecules from preclinical and early clinical trials eventually receives regulatory approval [1,2].
Drug repositioning (also known as repurposing or “drug repurposing”) is one of the most attractive and strategically essential paradigms in modern pharmacology. This approach consists of identifying, substantiating, and developing new therapeutic indications for existing medicinal products previously approved for use for other indications [3]. The key advantage of drug repurposing is the ability to use a large array of existing data on the molecules investigated. This approach significantly reduces the time and cost of developing new drugs, reducing the risk of unforeseen side effects and clinical trial failures. It is estimated that repositioning can be 40–90% cheaper than creating a fundamentally new drug from scratch [4].
Particular attention merits the repositioning of generic drugs, which have been widely used in clinical practice for decades. These drugs possess a well-studied primary mechanism of action and an established safety profile. [5]. Diclofenac, a nonsteroidal anti-inflammatory drug from the phenylacetic acid derivatives group, is a prominent representative of such drugs. Introduced into clinical practice [6] in the 1970s, diclofenac has become one of the most common drugs for treating pain and inflammation of various genesis, particularly in rheumatic diseases, arthritis, postoperative pain, and injuries. Its classical mechanism of action is well studied. It involves non-selective inhibition of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes. This inhibition reduces the synthesis of prostaglandins, which are key mediators of inflammation, pain, and fever. The effectiveness of diclofenac in this role is undeniable. The use of diclofenac, like other NSAIDs, carries a risk of side effects, primarily affecting the gastrointestinal tract and cardiovascular system. These side effects are mainly due to COX-1 inhibition. This inhibition impacts the homeostatic functions of prostaglandins [7].
However, over the past two decades, an increasing body of evidence has accumulated suggesting that the pharmacological action of diclofenac is not limited solely to its effect on COX. Preclinical In vitro and In vivo studies, some clinical observations, and computer modeling indicate that diclofenac has several additional pharmacological properties. Recent studies reported on the potential anticancer activity of diclofenac, which is mediated through its effects on angiogenesis, apoptosis, and cellular proliferation [8]. Its effect on bacterial and viral strains is being studied [9]. Its impact on the processes involved in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease, is also being studied [10]. These potential new effects are likely mediated through mechanisms independent or only partially dependent on COX inhibition, including effects on other signaling pathways. Such multifaceted pharmacological action opens new prospects for expanding the therapeutic horizons of Diclofenac through its repositioning beyond its traditional applications.
The aim of this study was to analyze and systematize scientific information on the non-traditional pharmacological effects of diclofenac, beyond its well-known anti-inflammatory and analgesic properties. The study focused on its potential repositioning for treating conditions not covered by its established clinical indications.
2. Materials and Methods
2.1. Search Strategies
The information resources of the PubMed (a widely recognized resource for biomedical and pharmacological research), Scopus and Web of Science database were used to search for information. A scoping probe in Scopus (94.9%) and Web of Science (94%) showed that >94% of records retrieved there were duplicates of PubMed citations, with no additional primary studies meeting the a priori inclusion criteria. Therefore, the study included data search, analysis, and systematization, and generalization was used from the PubMed database [11]. The study covered the period from 1981 to 2025, when various studies of different non-traditional pharmacological properties of diclofenac started (Figure 1). This broad time frame ensured a comprehensive collection of the relevant literature. The search strategy was likely structured to combine these terms using Boolean operators (AND, OR) to capture studies addressing diclofenac’s diverse pharmacological applications. It includes an analysis of 89 literature sources (Figure 1, for Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6, Section 3.7, Section 3.8, Section 3.9 and Section 3.10 below in Section 3, Results and Discussion). The search combined free-text keywords relating to “diclofenac” and “drug repurposing” OR (“pleiotropic”) AND specific effect-related terms. These included “neuroprotective,” “amyloid”, “antiamyloid”, “Alzheimer disease”, “Parkinson’s disease”, “cancer”, “anticancer,” “antitumor”, “antiviral,” “immunomodulatory,” “antibacterial,” “antimicrobial”, “antifungal,” “anticonvulsant,” “antiseizure”, “epilepsy”, “antiepileptic”, “radiation”, “radioprotective,” “prooxidant”, and “antioxidant”.
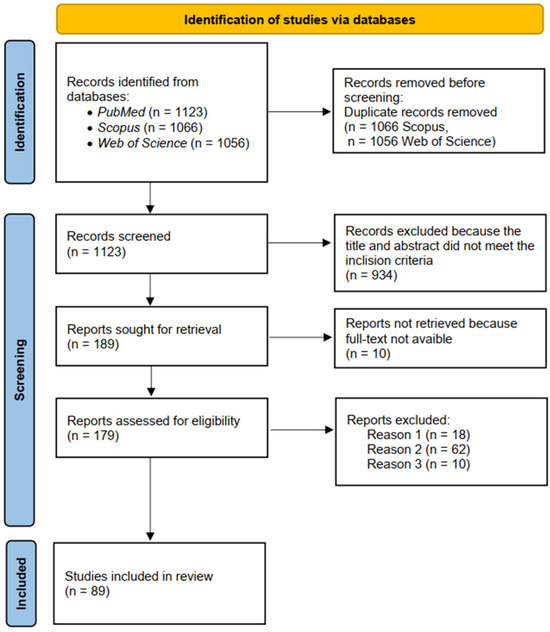
Figure 1.
PRISMA flow diagram of systematic review process.
2.2. Filters Applied
Both preclinical (In vitro and In vivo) and clinical studies were included to provide a holistic view of diclofenac’s non-traditional effects. No specific filters for study type (e.g., randomized controlled trials, observational studies) were noted, indicating a broad approach to capturing relevant literature. We focused our search on studies reported in English.
2.3. Screening Process
The screening process involved the following stages: initial screening, full-text assessment, and resolution of disagreements. The initial screening included reviewing titles and abstracts to identify studies relevant to diclofenac’s non-traditional pharmacological effects. Then evaluating the full texts of potentially relevant studies to confirm eligibility based on inclusion and exclusion criteria was carried out. Duplicate removal was performed and verified manually. Two reviewers (M.D. and M.S.) independently screened records against pre-defined eligibility criteria. Any discrepancies between reviewers were resolved through discussion.
2.4. Inclusion and Exclusion Criteria
Studies were included if they
- were original research articles, reviews, or meta-analyses reporting on diclofenac’s non-traditional pharmacological effects (any formulation or derivative of diclofenac) beyond COX inhibition (e.g., neuroprotection, anticancer activity);
- reported data from In vitro assays, animal experiments, or clinical studies in contexts beyond its traditional anti-inflammatory and analgesic uses;
- provided sufficient methodological detail to allow extraction of dose, model, and outcome.
Exclusion criteria were non-original publications (descriptive, theoretical papers, letters, comments), studies focusing solely on analgesic/anti-inflammatory endpoints, and articles not in English or without access to the full text.
2.5. Data Extraction
Data extraction was conducted to synthesize information from the 89 studies included. Two reviewers extracted data independently to minimize bias, adopting the dual-extraction practice. Detailed tables summarize study methods, doses, models, effects, and references for each non-traditional property. The extracted data included
- Study characteristics: study design and experimental models (e.g., In vitro, In vivo, clinical studies);
- Intervention details: doses of diclofenac used in the studies;
- Outcomes: observed pharmacological effects (e.g., neuroprotective, anticancer) and underlying mechanisms;
- Safety data: reported adverse effects or toxicity concerns;
- References: bibliographic details for each study.
3. Results and Discussion
The analysis of information sources on diverse studies of diclofenac’s pharmacological activities identified 10 main non-traditional directions, primarily investigated at the preclinical stage. These include neuroprotective, anti-amyloidogenic, anticancer, antiviral, immunomodulatory, antibacterial, antifungal, anticonvulsant, radioprotective, and antioxidant properties (Figure 2).
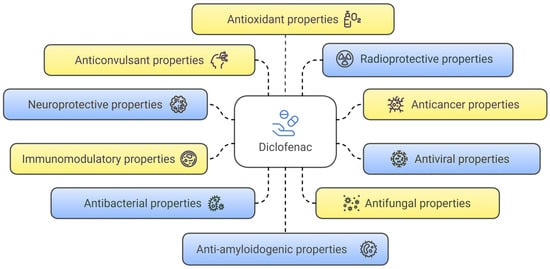
Figure 2.
Non-traditional directions in the research of diclofenac’s pharmacological properties.
3.1. Neuroprotective Properties
It is known that the mechanism of neuroprotective action is identical to anti-inflammatory and analgesic effects. It involves the inhibition of the cyclooxygenase enzyme, which reduces the synthesis of prostaglandins. Reducing the latter’s level can help reduce neuroinflammation, which plays an essential role in the development of neurodegenerative processes [12]. Diclofenac may affect other molecular pathways related to neuroprotection, such as modulation of neurotransmitter release and stabilization of cell membranes [13]. Several scientific studies provided evidence of diclofenac’s potential benefits in treating neurodegenerative diseases. In particular, a study [14] investigated the effect of diclofenac on cognitive function and daily activities in patients with Parkinson’s disease. The study showed that diclofenac can restore behavioral motor impairment and neuronal loss caused by Parkinson’s disease. These effects may be related to its potent anti-inflammatory activity [14]. In a rat model of parkinsonism (chlorpromazine-induced), diclofenac was found to significantly reduce catalepsy, improve motor activity, and alleviate histological damage in the midbrain. It was found that dopamine and DOPAC levels were higher in the diclofenac-treated group than in the control group. The authors also observed improved neuronal preservation, indicating a neuroprotective effect, likely due to anti-inflammatory effects, antioxidant activity, and activation of PPARγ receptors [14]. After two months of treatment, the diclofenac group exhibited a significant improvement in cognitive function according to the MMSE and MoCA tests and activities of daily living (ADCS-ADL). The severity of Parkinson’s disease also significantly decreased, as measured by the UPDRS III and Hoehn & Yahr scales [14].
Another study established a tendency toward slowing the progression of Alzheimer’s disease with the use of diclofenac in combination with misoprostol [10]. In a study by the authors of [15], conducted In vivo on a rat model, diclofenac was shown to inhibit the activity of the hepatic enzyme TDO and increase the levels of the brain enzyme IDO. These enzymes were involved in the metabolism of tryptophan through the kynurenine pathway. This could potentially alter the levels of neuroactive metabolites, in particular kynurenic acid and quinolinic acid, which play a role in the pathogenesis of Alzheimer’s disease. The authors suggested that diclofenac may have a neuroprotective effect by modulating kynurenine metabolism in the brain [15]. In a retrospective clinical study [16], it was determined that the incidence of Alzheimer’s disease was 6–8 times lower among patients taking diclofenac compared to those taking naproxen or etodolac. The authors of this study attributed this effect to the ability of diclofenac to inhibit interleukin-1β (IL-1β) and reduce neuroinflammation, particularly by affecting NLRP3 inflammasomes. A study by American scientists [16] showed the potential of diclofenac in the treatment of Alzheimer’s disease due to its ability to suppress neuroinflammation and reduce microglia activity. It also decreased NLRP3 inflammasome expression and proinflammatory cytokines (IL-1β, IL-6, TNF-α).
In a joint study by German and Swedish researchers [17], the neuroprotective effect of diclofenac was evaluated in a model of penetrating traumatic brain injury In vivo in male rats. The study specifically assessed its impact on apoptosis, neuronal degeneration, and the size of brain damage. The authors found that COX-2 inhibition by diclofenac after penetrating traumatic brain injury was associated with lower levels of apoptosis and minor brain tissue damage. This study indicated the neuroprotective potential of diclofenac in the treatment or prevention of secondary brain damage after trauma [17].
The main results of neuroprotective study of diclofenac are summarized in Table 1.

Table 1.
The main results of neuroprotective study of diclofenac.
The reviewed studies suggest that diclofenac’s neuroprotective properties are multifaceted, encompassing direct anti-inflammatory effects in the brain and modulation of key neurodegenerative pathways. Additionally, it provides beneficial systemic effects, such as inflammation reduction and hematopoietic recovery. Diclofenac demonstrates compelling, albeit preliminary and sometimes controversial, evidence of neuroprotective properties in animal models of Alzheimer’s disease, Parkinson’s disease, and traumatic brain injury (TBI). However, significant gaps in the research remain. There is an urgent need for large, well-controlled randomized placebo-controlled trials to confirm the effect of diclofenac in humans. These trials should preferably focus on individuals with mild cognitive impairment or early stages of Alzheimer’s/Parkinson’s disease. Ideally, the studies should be conducted before disease onset. Patient selection should be based on a combination of clinical, blood, and imaging biomarkers for a homogeneous population, with serial biomarker testing throughout the study. A standard dose with careful monitoring for side effects is recommended [10,12,13,14,15,16]. For TBI, further studies should investigate the specific apoptotic pathways and the effects of diclofenac on neurons and glia. These studies should also assess diclofenac’s potential to restore axonal functionality. Additionally, exploring specific TBI subgroups, such as males, and evaluating the feasibility of local drug administration in clinical settings are warranted [12,13,14,15,16].
3.2. Anti-Amyloidogenic Properties
A group of American scientists investigated the effect of diclofenac and its derivatives as potential transthyretin amyloid fibril formation inhibitors [18]. In 2016, Canadian researchers studied the ability of diclofenac to inhibit the aggregation of islet amyloid polypeptide, the formation of which is associated with neurodegenerative diseases such as Alzheimer’s [19]. In 2023, a group of Canadian investigators [20] examined the ability of disease-associated proteins, including β-amyloid, to aggregate in intranuclear amyloid bodies (A-bodies). The research specifically focused on these aggregation processes under stressful conditions. The authors also investigated the effect of diclofenac on inhibiting this aggregation, particularly in cellular conditions. Scientists determined that diclofenac significantly reduced the ability of β-amyloid (1–42) to form A-body aggregates in a cellular model (hypoxia + acidosis) [20]. The summarized data on the study of amyloid properties of diclofenac are presented in Table 2.

Table 2.
The summarized data on the study of amyloid properties of diclofenac.
Diclofenac represents a multifaceted anti-amyloid agent [18,19,20]. Its direct protein-stabilizing role in TTR amyloidosis contrasts with a potentially indirect, cellular pathway-dependent mechanism for β-amyloid aggregation. The most critical insight is the stark contradiction between diclofenac’s performance in In vitro Thioflavin T assays versus cellular A-body assays for β-amyloid aggregation [20]. This emphasizes that In vitro assays may not fully capture the complex biological environment and indirect mechanisms relevant to disease [20]. Diclofenac directly binds to TTR, but its effect on β-amyloid appears to be indirect, mediated through modulation of cellular pathways such as the COX pathway. This suggests that diclofenac may not act as a “universal amyloid inhibitor” by directly targeting fibril structure across all proteins. Instead, it likely influences upstream cellular processes that affect amyloid formation or sequestration. Diclofenac shows promise for TTR and IAPP amyloidosis [18,19]. However, issues like plasma partitioning selectivity (for TTR) and potential toxicity (for NSAIDs in general) need to be carefully considered for drug development. For Alzheimer’s disease, the cellular A-body model supports an epidemiological link to reduced risk with diclofenac, but In vitro assays of fibril inhibition do not confirm this association. This implies that its protective effects might stem from modulating inflammatory pathways or other cellular processes [18,19].
3.3. Anticancer Properties
Research on diclofenac as a potential anticancer agent encompasses several complementary approaches. In silico methods are extensively employed to elucidate its molecular mechanisms of action [21]. A broad spectrum of In vitro experiments was conducted to demonstrate its anticancer activity in various cancer cell lines. Numerous In vivo studies using animal models provided additional evidence supporting its anticancer potential. The discussion of diclofenac as a potential anticancer agent is presented in works [8,22,23]. Its efficacy was demonstrated in various types of tumors, including fibrosarcoma, colorectal cancer, neuroblastoma, glioblastoma, melanoma, pancreatic cancer, and more.
As shown in Table 3, diclofenac may exert its effects through a variety of mechanisms. The primary mechanisms of diclofenac’s action are diverse. One of the key mechanisms involves the inhibition of cyclooxygenase-2 (COX-2) and the reduction in prostaglandin E2 (PGE2) synthesis. This contributes to decreased angiogenesis and immunosuppression. Additionally, diclofenac suppresses angiogenesis, induces apoptosis, and causes cell cycle arrest. It also destabilizes microtubules and disrupts autophagy. Furthermore, it exerts significant effects on tumor metabolism [8,23,24]. In addition, diclofenac has been found to act on DNA (apurinic/apyrimidinic) lyase, ADAM10, and tyrosine-protein kinase, which are involved in the development of various types of cancer [8,23,24]. Diclofenac also found its place in studies of combinations with other drugs for the treatment of osteosarcoma, breast cancer, ovarian cancer, gastrointestinal cancer, and non-small cell lung cancer [25]. In 2024, a group of researchers [26] conducted a promising study on the production of diclofenac-based prodrugs. They studied their anticancer effect on mouse colon adenocarcinoma, human colorectal carcinoma, and human colon adenocarcinoma.

Table 3.
The results of studies on the antitumor properties of diclofenac on some types of cancer.
The results of studies on the anticancer properties of diclofenac on some types of cancer are presented in Table 3.
Although the evidence of diclofenac’s antitumor activity is promising, particularly at the preclinical level, the vast majority of these studies remain at the preclinical stage. This represents the primary limitation in the current assessment of its full antitumor potential. There is clear evidence of diclofenac’s ability to influence key cancer processes, such as proliferation, apoptosis, angiogenesis, and immune response [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Researchers are making significant efforts to reduce the toxicity of diclofenac and enhance its efficacy through chemical modification [24] and prodrugs development [26]. Overcoming challenges related to its side effects and bioavailability through novel structural solutions and combination therapies is critical for further development.
3.4. Antiviral Properties
Reports on the repositioning of diclofenac as a promising antiviral agent are relatively limited in the literature. The available studies of antiviral properties are represented by laboratory (In vitro, In vivo) or computational (in silico) results. Diclofenac was first studied in 1998 by Y. Gordon and co-authors [43] In vitro and In vivo for its effect on adenovirus replication, but no significant effect was found.
In 2001, Y. Gordon and E. Romanowski studied the effect of diclofenac on the inhibitory activity of cidofovir on adenovirus replication in the Ad5/NZW rabbit model [44]. The authors found that the simultaneous use of diclofenac with the antiviral drug did not reduce its antiviral inhibitory activity against adenovirus replication. Additionally, it did not prevent the formation of subepithelial infiltrates in the In vivo model [44].
A study on rabbit eyes [45] with acute herpetic keratitis (herpes simplex viruse HSV-1) found that topical 0.1% diclofenac sodium did not exacerbate the disease. Lesions were less severe or no more severe than those treated with other anti-inflammatory agents. Crucially, virus shedding was not prolonged.
In 2011, Colombian researchers tested diclofenac for its effect on ECwt (wild-type) rotavirus in ICR mice [46]. The study showed that treatment of mice infected with this virus with diclofenac led to a decrease in cell infection. In 2017, Turkish scientists proposed a synthetic approach to structurally modify diclofenac, resulting in its hydrazone and spirothiazolidinone derivatives [47]. These derivatives demonstrated good antiviral activity against herpes simplex viruses (HSV-1, HSV-2, and HSV-1 TK-), vaccinia virus, and Coxsackie B4 virus.
The main results of antiviral study of diclofenac are summarized in Table 4.

Table 4.
The main results of antiviral study of diclofenac.
Analysis of works [43,44,45,46,47] revealed that diclofenac’s antiviral research journey reflects both challenges and opportunities. Diclofenac is not consistently a direct and potent antiviral agent. However, research highlights its significant role as a safe and effective anti-inflammatory adjunct therapy in various viral infections. Additionally, it serves as an effective immunomodulatory therapy in these contexts. Its ability to reduce inflammation and manage severe immune responses without exacerbating viral replication or interfering with primary antiviral treatments makes it a valuable tool. Diclofenac, in its current form, may not serve as a stand-alone antiviral solution. However, its incorporation into combination treatment regimens [44] shows promise for developing adjunctive therapies. Additionally, further structural optimization [47] could enhance its potential. These therapies aim to address both viral replication and the damaging host’s inflammatory response.
3.5. Immunomodulatory Properties
Diclofenac, in addition to its main anti-inflammatory properties, may exert immunomodulatory effects by affecting various immune system components. These properties are indirect and associated with its effect on inflammatory cascades and metabolic pathways. In particular, the review [48] highlighted In vitro and In vivo studies on diclofenac, demonstrating its ability to inhibit the activation of the NLRP3 inflammasome. Additionally, it showed diclofenac’s immunomodulatory activity through the suppression of PPAR-γ expression. This mechanism may contribute to a reduction in the production of proinflammatory cytokines in COVID-19 disease. The study [49] found that diclofenac and its metabolite, 4-hydroxydiclofenac, modulate the production of proinflammatory cytokines in lymphoblastoid cell lines, indicating immunomodulatory effects. Villalonga et al. [50] found that diclofenac may inhibit the activity of Kv1.3 potassium channels, which play a key role in the activation and proliferation of T-lymphocytes and macrophages. Blocking these channels can reduce the immune response, which is potentially valuable for autoimmune diseases.
Diclofenac also inhibited the differentiation of monocytes into mature dendritic cells under the influence of 27-hydroxycholesterol. This may reduce T-cell activation and modulate the adaptive immune response [51]. In addition, it is considered a potential treatment for autoimmune diseases and in combination with other therapies, such as metronomic chemotherapy, to enhance the antitumor response [25].
Thus, diclofenac has a complex effect on the immune system. On the one hand, it may inhibit certain aspects of the immune response, such as macrophage activation and migration and IL-2 production in T lymphocytes. It may be helpful in the treatment of autoimmune diseases, including rheumatoid arthritis [50]. On the other hand, in different experimental conditions, it can modulate cytokine production, particularly by increasing IL-2 levels [49]. The effect of diclofenac on Kv1.3 channels in leukocytes is an essential mechanism of its immunomodulatory effect [49,50].
The main results of immunomodulatory study of diclofenac are summarized in Table 5.

Table 5.
The main results of immunomodulatory study of diclofenac.
The immunomodulatory effects of diclofenac are often dose-dependent, with higher concentrations (e.g., 15 mM) demonstrating significant effects. In contrast, lower concentrations (e.g., 1.5 mM) do not exhibit these effects [50]. The effects also vary based on the cell type and its activation state [50]. Its ability to inhibit macrophage activation and migration underscores its potential to attenuate overactive immune responses relevant in autoimmune diseases [50]. Additionally, it suppresses T-lymphocyte IL-2 production, further contributing to immune modulation [50]. Furthermore, its interference with dendritic cell differentiation supports this therapeutic potential in autoimmune conditions [50]. Diclofenac role in acute infectious diseases, especially viral ones like SARS-CoV-2, presents a more nuanced picture. While concerns about potential adverse effects were raised, current evidence generally suggests that diclofenac does not worsen outcomes in COVID-19 [48]. Future research should focus on optimizing its use in combination therapies. It is also important to explore its impact on host metabolic responses during infections. These efforts will help fully leverage its therapeutic potential while mitigating potential risks.
3.6. Antibacterial Properties
Diclofenac demonstrates a wide range of antibacterial activity against both Gram-positive and Gram-negative bacteria [9,52]. There are Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Bacillus pumilus, Bacillus subtilis, Listeria monocytogenes, Mycobacterium tuberculosis, Stenotrophomonas maltophilia, Acinetobacter baumannii, Aeromonas trota, Streptococcus suis, Paenibacillus lactis, Pasteurella canis, Salmonella typhimurium, Salmonella virchow and Staphylococcus equorum.
The mechanisms of antibacterial action of diclofenac could be manifested through inhibition of DNA synthesis [9], disruption of membrane activity and inhibition of enzymes [53], effect on biofilm formation [54,55], synergy with antibiotics [9,53,56,57,58].
A study by Salem Milani et al. showed that diclofenac has a pronounced antibacterial activity against E. faecalis, but less than the antibacterial activity of conventional antibiotics (amoxicillin, gentamicin, etc.) [59]. In combination with antibiotics, diclofenac reduced the inflammatory response and improve the patient’s condition, for example, in treating urinary system diseases [56,58]. Diclofenac also inhibited some mechanisms of bacterial resistance [60]. Studies in mice with infections caused by methicillin-resistant S. aureus (MRSA) showed that combined treatment with diclofenac and oxacillin significantly reduces biofilm formation and bacterial load [54].
Studies [53,55,61] explored the potential use of diclofenac as an individual antibacterial agent, as it inhibits the growth of bacteria of different strains, such as B. subtilis, E. coli, P. aeruginosa, and S. typhi.
The main results of antibacterial study of diclofenac are summarized in Table 6.

Table 6.
The main results of antibacterial study of diclofenac.
The existing research strongly suggests that diclofenac holds significant promise as an agent with notable antibacterial and anti-biofilm properties. This potential is particularly evident when diclofenac is used in synergistic combination with conventional antibiotics [9,52,53,54,55,56,57,58,59,60,61]. This could be a valuable strategy for combating multi-drug resistant pathogens and managing biofilm-associated infections. A major concern is that the effective in vitro concentrations of diclofenac can be significantly higher (25,600 µg/mL) than clinically achievable human plasma concentrations (0.5–2.5 µg/mL). However, for its clinical application as an antibacterial agent, rigorous in vivo and clinical trials are essential to determine optimal, safe, and effective dosing strategies. This is especially important considering its known toxicological profile at high concentrations.
3.7. Antifungal Properties
Research on the antifungal activity of diclofenac is represented by a relatively limited number of scientific studies. However, based on the available evidence, diclofenac demonstrates considerable antifungal activity in vitro and in vivo against pathogenic fungi, particularly Aspergillus fumigatus and various Candida species, including C. albicans and C. tropicalis. The antifungal effect is provided by inhibition of growth, hyphal formation, biofilm formation, and the potential for synergy with other antifungal drugs. In particular, a study [62] reported that diclofenac sodium significantly reduced both the growth of A. fumigatus mycelium growth and Ef-1 gene expression in a dose-dependent manner. Studies [63,64,65] showed that diclofenac inhibits the growth and morphogenetic processes of C. albicans, particularly the formation of hyphae and biofilms. A significant inhibitory effects of diclofenac on the adhesion and formation of C. albicans biofilms [63,66,67] and an increase in the permeability of biofilm cell membranes [64,68,69] were established.
Diclofenac in combination with essential oils, such as Mentha piperita, Melaleuca alternifolia, and Pelargonium graveolens, had a synergistic effect, significantly reducing the formation of biofilms of various Candida strains [70].
Another study found that diclofenac increases the sensitivity of C. albicans biofilms to caspofungin, which is promising in the combination therapy of candidiasis [68]. Diclofenac also exhibited synergistic effects with fluconazole and voriconazole against biofilms of resistant strains of C. tropicalis [66,71] and fluconazole against C. albicans strains.
The main results of antifungal study of diclofenac are summarized in Table 7.

Table 7.
The main results of antifungal study of diclofenac.
Therefore, diclofenac exhibits significant antifungal activity, particularly through its ability to inhibit fungal morphogenesis (hyphae/germ tube formation) and reduce biofilm production. A critical observation is the wide range of effective diclofenac concentrations reported across studies and even within a single study depending on the model [62,63,64,65,66,67,68,69,70,71]. While the In vitro results are encouraging, the number of In vivo studies is limited. Some In vivo animal model data showed synergistic activity (e.g., with caspofungin against C. albicans biofilms) [68]. Crucially, diclofenac demonstrates strong synergistic effects when combined with conventional antifungals and essential oils, which is a promising strategy to overcome antifungal resistance and manage biofilm-associated infections by allowing for lower, potentially less toxic, effective concentrations [66,68,71]. The precise molecular mechanisms by which diclofenac exerts its antifungal and synergistic effects, especially in complex combination therapies, require deeper investigation.
3.8. Anticonvulsant and Proconvulsant Properties
Diclofenac is also the subject of some In vitro studies investigating its potential anticonvulsant effect, which is associated with its influence on inflammatory and neural mechanisms. The first studies in this area were conducted in the early 1980s. In particular, an In vivo study in mice conducted in 1981 [72] showed that diclofenac reduces the seizure threshold. The authors found that mice administered with the convulsant pentylenetetrazol exhibited a significant increase in immunoreactive material resembling prostaglandins (PGs) and thromboxane B2 (TXB2) in brain extracts. Diclofenac was shown to inhibit the pentetrazole-induced elevation in immunoreactive PGF2α, PGE2, and TXB2 by more than 90%.
Studies in 2005 and 2018 found that diclofenac may act as a KCNQ2/Q3 potassium channel opener [73,74,75]. In particular, In vivo, the combined administration of retigabine (a KCNQ potassium channel activator) and diclofenac significantly increased the percentage of protection against seizures induced by picrotoxin and maximum electroshock. They prolonged the average latency period of seizures in pilocarpine-induced persistent epilepsy [73]. The efficacy of diclofenac in the maximum electroshock test was blocked by a KV-channel blocker, which confirmed its possible action as a KCNQ2/3 potassium channel opener.
In two studies conducted in 2016 and 2022 [76,77] on rat models of seizures induced by pentylenetetrazole, administration of diclofenac sodium led to a significant reduction in seizure severity according to the Racine seizure scale. This effect was accompanied by a decrease in the levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the hippocampus [76]. There was also a significant decrease in MDA and TNF-α levels and an increase in SOD levels, suggesting a possible role for the antioxidant and anti-inflammatory effects of diclofenac in reducing seizure activity [77].
A study in 2023 [75] reported that the combined administration of diclofenac potassium and diazepam was more effective in reducing the amplitude of spikes than diclofenac potassium alone. This study also noted that the effects of diclofenac potassium on epileptic seizures may vary as either proconvulsant or anticonvulsant. These effects depended on the form of diclofenac, dose, type of experimental model, and study protocol.
The main results of antiseizure study of diclofenac are summarized in Table 8.

Table 8.
The main results of antiseizure study of diclofenac.
Therefore, the initial interpretation of diclofenac lowering the convulsive threshold contrasts with later studies showing broader anticonvulsant effects. This may reflect a developing understanding of the complex interplay between prostaglandins and seizure activity. Additionally, the discovery of mechanisms such as KCNQ channel opening and KMO inhibition directly contributes to the anticonvulsant effects [73]. Diclofenac’s effects are highly dependent on the dose administered and the experimental model used. For instance, its effectiveness in MES and PTZ models appears stronger than in the pilocarpine model when used alone [72,73,74,75,76,77]. While early research presented some proconvulsant indications in specific acute contexts, the bulk of the evidence points to diclofenac having significant anticonvulsant properties. This is particularly supported by more recent studies and findings from combination therapies. Its multifaceted actions, including COX and kynurenine 3-monooxygenase inhibition, make it a promising candidate. Additionally, its KCNQ potassium channel opening and general anti-inflammatory/anti-oxidative effects further enhance its therapeutic potential. Diclofenac appears particularly effective as an adjunctive therapy, enhancing the effects of established anticonvulsants like diazepam and retigabine. Importantly, it also combats drug resistance in epilepsy models. Further investigations, especially in chronic epilepsy models and clinical trials, are warranted to fully establish its therapeutic role in epilepsy management.
3.9. Radioprotective Properties
In vitro and In vivo studies of diclofenac as a potential radioprotective agent began in the 1980s, and their results indicated the possibility of protecting normal tissues from radiation damage. In particular, in 1985, the effect of radiation exposure on the inflammatory process and the effect of diclofenac on it were studied. It was found that it effectively suppressed inflammatory reactions in both normal and irradiated animals. This effect may be due to its inhibitory action on prostaglandin synthetase and stabilization of lysosomal membranes [78]. In 1992 and 1993 [79,80], it was determined that the combination of diclofenac and carboxymethyl glucan (an immunomodulator and hematopoietic stimulant) enhanced the recovery of hematopoiesis in gamma-irradiated mice.
In another study [81], repeated administration of diclofenac to mice before each irradiation resulted in a statistically significant increase in leukopoiesis compared to the control group. However, after a lethal total radiation dose accumulated, a slight decrease in survival rate was observed in diclofenac-treated mice [81].
Studies on cultured human peripheral blood lymphocytes showed that both pre- and post-irradiation with diclofenac sodium reduced the formation of gamma radiation-induced dicentric chromosomes, cytochalasin-blocked micronuclei, and γ-H2AX foci [82].
In vitro and in vivo studies were conducted to investigate the effects of diclofenac on cancer cells’ sensitivity to radiation and chemotherapy [83]. They demonstrated radio- and chemosensitizing effects on some cancer cells [83], particularly on colorectal adenocarcinoma cell lines (LS174T and LoVo). The combined use of diclofenac and radiation significantly increased the production of reactive oxygen species (ROS) in LS174T cells but not in A549 lung cancer cells. In contrast to colorectal cancer cells, the radiosensitizing effect of diclofenac was not observed in lung cancer (A549), breast cancer (MDA-MB-231), and pancreatic cancer (COLO357) cells, where diclofenac did not induce changes in lactate metabolism and stress response. A study in the LS174T colorectal adenocarcinoma mouse model showed that diclofenac also enhances the effectiveness of radiotherapy, leading to a reduction in tumor size, especially when used diclofenac and radiation treatment [83].
Possible mechanisms of diclofenac’s radioprotective properties may be realized by reducing prostaglandin levels [81] and decreasing radiation-induced inflammatory reactions [79]. It may also act by reducing free radicals [83]. Additionally, when combined with radiation, diclofenac can increase ROS production, affect cancer cell metabolism, and modulate the stress response [83].
The main results of radioprotective study of diclofenac are summarized in Table 9.

Table 9.
The main results of radioprotective activity study of diclofenac.
Therefore, diclofenac demonstrates a complex and context-dependent role in radiation exposure (Table 9). As a radioprotector in normal cells and tissues, particularly the hematopoietic system and DNA, its efficacy is supported by strong In vitro and In vivo evidence. However, a critical perspective highlights its capacity as a radiosensitizer in specific cancer cells, particularly colorectal cancer. This effect is achieved by disrupting tumor metabolism and stress response and potentially increasing ROS production. This dual effect suggests diclofenac might be beneficial in cancer therapy, potentially enhancing tumor cell killing while simultaneously offering some protection to surrounding normal tissues [83]. Despite its potential, diclofenac’s known gastrointestinal and other systemic side effects remain a significant concern. This is particularly evident with repeated administration at high radiation doses. These safety issues limit its broad application as a general radioprotector in accidental exposure scenarios [81]. Repurposing diclofenac as a radiation countermeasure agent is a promising avenue, benefiting from its wide availability and well-known pharmacokinetics. However, its precise application must carefully consider the specific context, including the type of cells or tissue (normal vs. cancerous) and the radiation dose. The treatment regimen should also be optimized to enhance beneficial effects while mitigating potential adverse outcomes [82,84].
3.10. Antioxidant and Prooxidant Properties
The literature sources indicated that diclofenac and its derivatives are capable of exhibiting a variety of antioxidant properties. These include the ability to scavenge different types of free radicals (such as DPPH, ABTS, hydroxyl, and peroxyl radicals), protect lipids and DNA from oxidative damage, and modulate the activity of antioxidant enzymes. In particular, a study [85] found that diclofenac sodium in rat liver model (In vivo) can bind the stable free radical DPPH but does not show the ability to bind superoxide anion (O2-) or hydroxyl radicals (-OH). The same study showed that diclofenac sodium prevented lipid peroxidation in the liver of rats subjected to ischemia–reperfusion by reducing the level of phosphatidylcholine hydroperoxide in plasma and suppressing the increase in serum enzymes.
In work [86], the authors developed an In vitro assay (membrane model) to test the ability of diclofenac to scavenge free radicals using phosphatidylcholine liposomes as a membrane model. They found that diclofenac demonstrated significant oxygen and lipid radical scavenging activity.
The authors of the study [87] showed that diclofenac exhibits In vitro dose-dependent antioxidant activity in preventing human erythrocyte hemolysis induced by free radicals generated during the decomposition of 2,2′-azobis(2-amidinopropane) hydrochloride (AAPH). They also suggested that diclofenac may recycle radicals from other antioxidants, such as vitamins E and C.
In a work by Brazilian researchers [88], it was shown that diclofenac sodium led to a decrease in oxidized low-density lipoprotein and lipid hydroperoxides In vivo (male Wistar rats). It also resulted in an increase in total serum antioxidant capacity and superoxide dismutase activity. A subsequent study in 2009 [89] found that diclofenac and its sodium salt protected DNA from oxidation induced by AAPH in a concentration-dependent manner and effectively scavenged ABTS+ and DPPH radicals in the brain, liver, gill and blood of common carp.
Some studies suggested that diclofenac can cause oxidative stress, disrupting the body’s balance between free radicals and antioxidant systems in the brain, liver, gill, and blood of common carp and male Wistar rats [90,91]. It can generate excess free radicals and inhibit the activity of antioxidant enzymes such as superoxide dismutase and catalase. This leads to disruption of cellular redox homeostasis and potential tissue damage (male albino rats) [92,93]. However, when combined with certain drugs, diclofenac may contribute to the reduction in oxidative stress [93].
The main results for study of antioxidant and prooxidant properties of diclofenac are summarized in Table 10.

Table 10.
The main results for study of antioxidant and prooxidant properties of diclofenac.
Therefore, studies on antioxidant activity are conducted using both In vitro models (in test tubes, on chemical or cellular systems) and In vivo models (on living organisms, primarily animals, as well as on human cells). The In vitro models are chemical or cellular systems that, while allowing for the evaluation of certain aspects of antioxidant activity, cannot fully replicate the complexity of a living organism. In vitro studies, such as interactions with DNA and serum albumin, are considered promising for further biological studies and potential applications [89]. In vivo studies are conducted on whole organisms, such as rats or human cells (neutrophils, erythrocytes), allowing for the assessment of more complex biological interactions. Even the most complex In vivo animal models do not fully reflect human physiology and pathophysiology. This makes translating results to clinical studies a challenging task and requires caution when extrapolating conclusions. For example, studies using human neutrophils [94] and other cells are more relevant for local drug application in humans.
3.11. Diclofenac’s Safety and Repositioning
The discussion over diclofenac’s safety is emblematic of the broader challenges inherent in balancing therapeutic efficacy with adverse risk in pharmacotherapy. On the one hand, diclofenac’s potent anti-inflammatory and analgesic properties have made it a mainstay in the treatment of various painful conditions. On the other hand, its association with significant hepatotoxic and cardiovascular adverse events cannot be ignored. Accumulating evidence from clinical trials and epidemiological studies has revealed that diclofenac has a compromised safety profile due to two major categories of adverse effects: hepatotoxicity and cardiovascular risks. Clinical evidence suggests that the risk of both hepatotoxicity and cardiovascular events increases in a dose- and duration-dependent manner [96,97,98]. Advancements in pharmaceutical formulation led to the development of alternative diclofenac preparations that aim to reduce systemic exposure and, by extension, toxicity. The main strategies and pathways to mitigate hepatotoxicity and cardiovascular risks of diclofenac are presented in Figure 3.
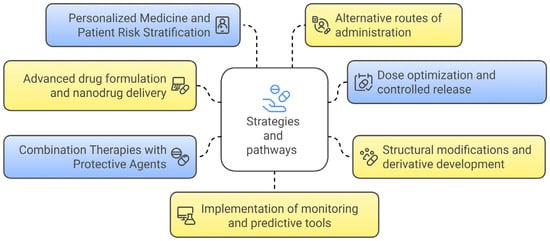
Figure 3.
Strategies and pathways to mitigate hepatotoxicity and cardiovascular risks of diclofenac.
In particular, one promising approach to counter diclofenac’s toxicity involves the development of nanodrug delivery systems that alter its pharmacokinetics and biodistribution. Polymeric micelle formulations reduced cardiac exposure by preferentially modulating the drug’s tissue distribution. This effectively normalizes CYP-mediated metabolism, thereby diminishing systemic cardiovascular injury [99]. Liposomal and nanosuspension carriers were explored to reduce peak plasma concentrations. These carriers ensured that sufficient local concentrations were achieved with minimal systemic toxicity [100].
Careful dose management is another key pathway for limiting both hepatotoxic and cardiovascular events. Research suggested that administering the lowest effective dose of diclofenac, ideally below threshold levels that triggered significant COX-2 inhibition, could reduce the risk of thrombotic events and liver injury. This approach also preserved analgesic efficacy [8,23]. Controlled-release formulations were designed to minimize peak plasma levels, thereby reducing off-target exposures and mitigating adverse effects associated with rapid spikes in drug concentration [23].
Chemical modification of diclofenac was employed as a molecular-level strategy to reduce toxicity. For instance, the synthesis of diclofenac methyl ester and metal salt derivatives, such as diclofenac zinc, copper, or choline salts, offered enhanced pharmacokinetic properties with potentially lower hepatic and cardiovascular risks [23]. Advances in computer-aided drug design allowed for the selective modification of molecular structures to attenuate toxic metabolite formation while retaining the desired anti-inflammatory actions [23].
Co-administration of hepatoprotective agents represents another viable strategy. Agents such as silymarin demonstrated significant efficacy in ameliorating diclofenac-induced liver injury, as evidenced by reductions in elevated liver enzymes and improvements in histopathological liver architecture [23,101]. Adjunctive therapies using omega-3 fatty acids, vitamin B12, or curcuminoid complexes explored to reduce diclofenac-induced oxidative stress and inflammatory damage. These approaches have the potential to reduce the risk of adverse outcomes in both the liver and cardiovascular systems [23].
The adoption of individualized treatment strategies based on patient-specific risk factors, including pharmacogenomic profiling, offers a path to safer diclofenac therapy. Identifying individuals with genetic variants in CYP enzymes (e.g., CYP2C9 metabolizer status) could assist clinicians in predicting hepatic metabolism rates and subsequent risk of hepatotoxicity, thereby allowing for tailored dosing regimens or alternative treatment selections [102]. This approach also enables stratification based on cardiovascular risk factors, ensuring that high-risk patients are either excluded from diclofenac therapy or managed with enhanced monitoring protocols [23].
Employing non-oral routes, such as topical formulations, is another effective method to reduce systemic exposure. Topical diclofenac administration has proven effective in treating localized conditions, such as osteoarthritis. This approach has been shown to exhibit a substantially lower risk of systemic toxicity, including both cardiovascular and hepatic adverse effects [102,103]. This strategy minimizes drug plasma levels, thereby reducing the burden on the liver and cardiovascular system.
Leveraging modern data analytics and machine learning can further enhance patient safety by enabling early detection and prediction of adverse drug events. Recent studies employed machine learning models to analyze electronic health record data. These studies identified key drug interactions and risk factors associated with diclofenac-induced liver injury. The findings provided clinicians with actionable insights to prevent toxicity [104]. Such predictive approaches can be integrated into clinical decision support systems to facilitate real-time monitoring and risk mitigation during diclofenac therapy.
Therefore, each of these strategies has been supported by research demonstrating the potential to reduce systemic exposure and adverse effects while maintaining the drug’s therapeutic efficacy. By carefully addressing these risk factors, it is feasible to repurpose diclofenac for the treatment of other complex diseases, thereby expanding its clinical utility beyond its current indications.
The safety concerns associated with diclofenac, particularly hepatotoxicity and cardiovascular risks, are well established and supported by a robust body of evidence ranging from research studies and clinical trials. However, the convergence of advanced drug delivery systems, structural modifications, combination protective therapies, and personalized dosing regimens fosters a promising environment for repurposing diclofenac. By overcoming its hepatotoxic and cardiovascular risks, diclofenac could be repositioned to leverage its off-target pharmacological effects. These effects include modulation of voltage-gated potassium channels and inhibition of acid-sensing ion channels. Such mechanisms may offer benefits in treating neurological disorders, certain cancers, and autoimmune conditions [23,105]. The repositioning of diclofenac for oncological applications has been driven by its emerging role in inducing cell cycle arrest and promoting apoptosis in malignant cells. However, ensuring a favorable safety profile remains paramount [101]. Such repurposing strategies necessitate robust benefit–risk evaluations and the incorporation of risk mitigation techniques as described above [23].
Despite these promising strategies, several challenges remain in the safe repurposing of diclofenac. Comprehensive clinical validation through rigorously designed trials is required to confirm the safety and efficacy of novel diclofenac formulations and combination therapies. These trials should demonstrate that such approaches could reduce hepatotoxic and cardiovascular risks. At the same time, they should maintain therapeutic efficacy in new disease contexts [8,106]. The heterogeneity in patient populations and the influence of comorbid conditions underscore the need for individualized dosing and enhanced monitoring, which could complicate large-scale clinical implementation [102,103]. Structural modifications of diclofenac show promise but require extensive preclinical and clinical evaluation. These evaluations are necessary to ensure that new derivatives do not introduce unforeseen toxicities. They must also confirm that the pharmacodynamic properties making diclofenac effective are preserved [23]. Furthermore, the implementation of advanced predictive analytics, such as machine learning-based risk stratification, holds significant promise. However, integrating these tools into everyday clinical practice remains challenging due to limitations in data interoperability and the need for constant model validation against evolving clinical data sets [104].
Future research should focus on multi-center collaborative studies to better identify the risk factors associated with diclofenac use. These studies should also refine approaches for mitigating these risks while exploring diclofenac’s repositioning potential. Additionally, regulatory hurdles and the need for post-marketing surveillance are critical considerations. The approval of modified diclofenac formulations or combination therapy regimens will require robust evidence demonstrating both efficacy and significantly improved safety outcomes compared to conventional formulations [106,107].
4. Conclusions
As a result of the conducted analysis of the scientific literature, it was established that diclofenac possesses not only well-known anti-inflammatory and analgesic properties. It also demonstrates a range of non-traditional pharmacological effects. These effects may hold potential for therapeutic application within the framework of drug repositioning.
Particular interest lies in the potential use of diclofenac in the treatment of neurodegenerative diseases (such as Parkinson’s and Alzheimer’s diseases), epilepsy, various types of cancer, bacterial and fungal infections, as well as protection against radiation-induced damage. Its reported antiviral, antioxidant, and immunomodulatory effects also merit attention. Equally important is the identification of synergistic effects of diclofenac or the use in combination with conventional therapeutic agents for the treatment of the aforementioned diseases.
Thus, repositioning diclofenac is a promising strategy for the pharmaceutical industry and may lead to the creation of novel therapeutic approaches based on a well-established generic drug. However, a major barrier to diclofenac repositioning lies in the fact that existing evidence is the data supported mainly by preclinical In vitro and In vivo studies and a limited number of clinical trials.
Therefore, further research on the non-traditional pharmacological effects of diclofenac (Figure 4) should primarily focus on conducting in-depth preclinical studies to verify the molecular mechanisms of action. In addition, exploring the combination of diclofenac with other therapeutic agents—such as antibiotics, antiviral, or anticancer drugs—as part of combination therapies, as well as optimizing its dosage regimens, appears to be a promising direction. It is also necessary to evaluate the toxicological profile and develop strategies to reduce side effects under new therapeutic conditions of diclofenac use to ensure patient safety. Ultimately, to expand the clinical application of diclofenac and enable the development of effective new treatments for various diseases, a wide range of randomized clinical trials targeting novel indications is required.
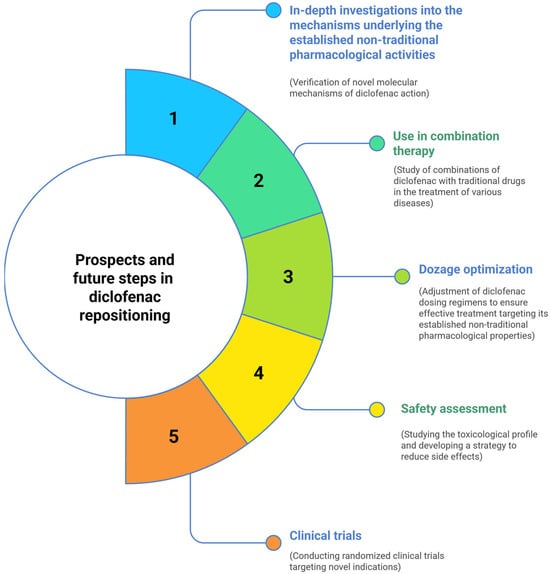
Figure 4.
Directions for further research in the repositioning of diclofenac as a multifunctional pharmacological agent.
Author Contributions
Conceptualization, M.S.; writing—original draft preparation and editing, M.S. and M.D.; visualization, M.S.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernald, K.D.S.; Förster, P.C.; Claassen, E.; van de Burgwal, L.H.M. The Pharmaceutical Productivity Gap—Incremental Decline in R&D Efficiency despite Transient Improvements. Drug Discov. Today 2024, 29, 104160. [Google Scholar] [CrossRef] [PubMed]
- Langevin, M.; Bianciotto, M.; Vuilleumier, R. Balancing Exploration and Exploitation in de Novo Drug Design. Digit. Discov. 2024, 3, 2572–2588. [Google Scholar] [CrossRef]
- Nair, R. Drug Repurposing: Finding New Therapeutic Uses for Existing Pharmaceuticals. Univers. Res. Rep. 2024, 11, 37–43. [Google Scholar] [CrossRef]
- Khatami, F.; Hairima, H.; Fitria, R. Drug Repurposing and Computational Drug Discovery: Strategies and Advances. Crystallogr. Rev. 2024, 30, 226–228. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S.; Bhasin, B. Drug Repurposing: An Emerging Tool for Drug Reuse, Recycling and Discovery. Curr. Drug Res. Rev. 2021, 13, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Sallmann, A.R. The History of Diclofenac. Am. J. Med. 1986, 80, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Diclofenac: An Update on Its Mechanism of Action and Safety Profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, K.; Dastidar, S.G.; Park, J.H.; Dutta, N.K. The Anti-Inflammatory Non-Antibiotic Helper Compound Diclofenac: An Antibacterial Drug Target. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.; Mander, A.; Ugoni, A.; Vajda, F.; Christophidis, N. A Double-Blind, Placebo-Controlled Trial of Diclofenac/Misoprostol in Alzheimer’s Disease. Neurology 1999, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. PubMed. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 1 May 2025).
- Tzeng, S.-F.; Hsiao, H.-Y.; Mak, O.-T. Prostaglandins and Cyclooxygenases in Glial Cells during Brain Inflammation. Curr. Drug Target-Inflamm. Allergy 2005, 4, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Najam, R.; Khan, S.S.; Mirza, T.; Sikandar, B. Neuroprotective Effect of Diclofenac on Chlorpromazine Induced Catalepsy in Rats. Metab. Brain Dis. 2019, 34, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Wambiya, E.; Bano, S. Diclofenac Sodium Inhibits Hepatic Tryptophan 2,3-Dioxygenase but Augments Brain Indoleamine 2,3-Dioxygenase Activities in Rats. J. Basic Appl. Sci. 2016, 12, 140–145. [Google Scholar] [CrossRef]
- Stopschinski, B.E.; Weideman, R.A.; McMahan, D.; Jacob, D.A.; Little, B.B.; Chiang, H.-S.; Saez Calveras, N.; Stuve, O. Microglia as a Cellular Target of Diclofenac Therapy in Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231156674. [Google Scholar] [CrossRef] [PubMed]
- Jadid, K.D.; Davidsson, J.; Lidin, E.; Hånell, A.; Angéria, M.; Mathiesen, T.; Risling, M.; Günther, M. COX-2 Inhibition by Diclofenac Is Associated with Decreased Apoptosis and Lesion Area after Experimental Focal Penetrating Traumatic Brain Injury in Rats. Front. Neurol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Oza, V.B.; Smith, C.; Raman, P.; Koepf, E.K.; Lashuel, H.A.; Petrassi, H.M.; Chiang, K.P.; Powers, E.T.; Sachettinni, J.; Kelly, J.W. Synthesis, Structure, and Activity of Diclofenac Analogues as Transthyretin Amyloid Fibril Formation Inhibitors. J. Med. Chem. 2001, 45, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.S.; Benoit-Biancamano, M.-O. Inhibition of Islet Amyloid Polypeptide Aggregation and Associated Cytotoxicity by Nonsteroidal Anti-Inflammatory Drugs. Can. J. Physiol. Pharmacol. 2016, 94, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Chandhok, S.; Pereira, L.; Momchilova, E.A.; Marijan, D.; Zapf, R.; Lacroix, E.; Kaur, A.; Keymanesh, S.; Krieger, C.; Audas, T.E. Stress-Mediated Aggregation of Disease-Associated Proteins in Amyloid Bodies. Sci. Rep. 2023, 13, 14471. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Yadav, S.; Goel, Y.; Temre, M.K.; Singh, V.K.; Singh, S.M. Molecular Docking of Anti-Inflammatory Drug Diclofenac with Metabolic Targets: Potential Applications in Cancer Therapeutics. J. Theor. Biol. 2019, 465, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.H.; Palmeira, A.; Sousa, S.M.; Pinto, C. Repurposing Some of the Well-Known Non-Steroid Anti-Inflammatory Drugs (NSAIDs) for Cancer Treatment. Curr. Top. Med. Chem. 2023, 23, 1171–1195. [Google Scholar] [CrossRef] [PubMed]
- Satar, H.A.; Yousif, E.; Ahmed, A. DICLOFENAC: A Review on Its Synthesis, Mechanism of Action, Pharmacokinetics, Prospect and Environmental Impact. Al-Mustaqbal J. Pharm. Med. Sci. 2025, 2, 134–157. [Google Scholar] [CrossRef]
- Alshargabi, A. Diclofenac Derivatives as Promising Anticancer and Anti-Inflammatory Drug: Synthesis, Formulations, and Pharmacokinetics. J. Drug Deliv. Sci. Technol. 2024, 95, 105544. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Melhuis, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Diclofenac as an Anti-Cancer Agent. Ecancermedicalscience 2016, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Selg, C.; Gordić, V.; Krajnović, T.; Buzharevski, A.; Laube, M.; Kazimir, A.; Lönnecke, P.; Wolniewicz, M.; Sárosi, M.B.; Schädlich, J.; et al. Re-Design and Evaluation of Diclofenac-Based Carborane-Substituted Prodrugs and Their Anti-Cancer Potential. Sci. Rep. 2024, 14, 30488. [Google Scholar] [CrossRef] [PubMed]
- Leidgens, V.; Seliger, C.; Jachnik, B.; Welz, T.; Leukel, P.; Vollmann-Zwerenz, A.; Bogdahn, U.; Kreutz, M.; Grauer, O.; Hau, P. Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms. PLoS ONE 2015, 10, e0140613. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Kesanakurti, D.; Kirti, P.B.; Babu, P.P. Nonsteroidal Anti-Inflammatory Drugs Diclofenac and Celecoxib Attenuates Wnt/β-Catenin/Tcf Signaling Pathway in Human Glioblastoma Cells. Neurochem. Res. 2013, 38, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Chirasani, S.R.; Leukel, P.; Gottfried, E.; Hochrein, J.; Stadler, K.; Neumann, B.; Oefner, P.J.; Gronwald, W.; Bogdahn, U.; Hau, P.; et al. Diclofenac Inhibits Lactate Formation and Efficiently Counteracts Local Immune Suppression in a Murine Glioma Model. Int. J. Cancer 2012, 132, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sanyal, S.N. Diclofenac, a Selective COX-2 Inhibitor, Inhibits DMH-Induced Colon Tumorigenesis through Suppression of MCP-1, MIP-1α and VEGF. Mol. Carcinog. 2011, 50, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Cecere, F.; Iuliano, A.; Albano, F.; Zappelli, C.; Castellano, I.; Grimaldi, P.; Masullo, M.; De Vendittis, E.; Ruocco, M.R. Diclofenac-Induced Apoptosis in the Neuroblastoma Cell Line SH-SY5Y: Possible Involvement of the Mitochondrial Superoxide Dismutase. J. Biomed. Biotechnol. 2010, 2010, 801726. [Google Scholar] [CrossRef] [PubMed]
- Poku, R.A.; Jones, K.J.; Van Baren, M.; Alan, J.K.; Amissah, F. Diclofenac Enhances Docosahexaenoic Acid-Induced Apoptosis in Vitro in Lung Cancer Cells. Cancers 2020, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Erfani, M.; Ahmadi, R.; Karimi Ghezeli, Z. Diclofenac Induces Intrinsic Apoptotic Pathway in in Cervical Cancer Cells. In Proceedings of the 8th International Conference on Agricultural, Environment, Biology and Medical Sciences (AEBMS-2017), Dubai, United Arab Emirates, 21–22 December 2017. [Google Scholar] [CrossRef]
- Erfani, M.; Ghorbani, M.; Ahmadi, R. The Effect of Diclofenac on Caspase-8 and Caspase-9 Activity in Cervical Cancer Cells (HeLa) in Cell Culture. Qom Univ. Med. Sci. J. 2018, 12, Pe2–Pe9. [Google Scholar] [CrossRef]
- Forget, P.; Machiels, J.-P.; Coulie, P.G.; Berlière, M.; Poncelet, A.; Tombal, B.; Stainier, A.; Legrand, C.; Canon, J.-L.; Kremer, Y.; et al. Neutrophil: Lymphocyte Ratio and Intraoperative Use of Ketorolac or Diclofenac Are Prognostic Factors in Different Cohorts of Patients Undergoing Breast, Lung, and Kidney Cancer Surgery. Ann. Surg. Oncol. 2013, 20, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Li, Y.; Zhou, Y.; Wang, Z.; Zhang, D.; Liu, J.; Zhang, X. Diclofenac Impairs the Proliferation and Glucose Metabolism of Triple-Negative Breast Cancer Cells by Targeting the C-Myc Pathway. Exp. Ther. Med. 2021, 21, 10016. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Lang, S.A.; Renner, K.; Bosserhoff, A.; Gronwald, W.; Rehli, M.; Einhell, S.; Gedig, I.; Singer, K.; Seilbeck, A.; et al. New Aspects of an Old Drug—Diclofenac Targets MYC and Glucose Metabolism in Tumor Cells. PLoS ONE 2013, 8, e66987. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, Z.; Wu, J.; Liu, X.; Wang, R.; Xu, J.; Zhu, X. Diclofenac Enhances the Response of BRAF Inhibitor to Melanoma through ROS/P38/P53 Signaling. Clin. Exp. Pharmacol. Physiol. 2025, 52, e70022. [Google Scholar] [CrossRef] [PubMed]
- Arisan, E.D.; Akar, R.O.; Rencuzogullari, O.; Obakan Yerlikaya, P.; Coker Gurkan, A.; Akın, B.; Dener, E.; Kayhan, E.; Palavan Unsal, N. The Molecular Targets of Diclofenac Differs from Ibuprofen to Induce Apoptosis and Epithelial Mesenchymal Transition due to Alternation on Oxidative Stress Management P53 Independently in PC3 Prostate Cancer Cells. Prostate Int. 2019, 7, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Anai, S.; Onishi, S.; Miyake, M.; Tanaka, N.; Hirayama, A.; Fujimoto, K.; Hirao, Y. Inhibition of COX-2 Expression by Topical Diclofenac Enhanced Radiation Sensitivity via Enhancement of TRAIL in Human Prostate Adenocarcinoma Xenograft Model. BMC Urol. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Valle, B.L.; D’Souza, T.; Becker, K.G.; Wood, W.H.; Zhang, Y.; Wersto, R.P.; Morin, P.J. Non-Steroidal Anti-Inflammatory Drugs Decrease E2F1 Expression and Inhibit Cell Growth in Ovarian Cancer Cells. PLoS ONE 2013, 8, e61836. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.J.A.; Frew, D.; Tatoud, R.; Wolf, C.R.; Palmer, C.N.A. Diclofenac Antagonizes Peroxisome Proliferator-Activated Receptor-γ Signaling. Mol. Pharmacol. 2002, 61, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J. The Effects of Topical Nonsteroidal Anti-Inflammatory Drugs on Adenoviral Replication. Arch. Ophthalmol. 1998, 116, 900. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Gordon, Y.J. Effects of Diclofenac or Ketorolac on the Inhibitory Activity of Cidofovir in the Ad5/NZW Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2001, 42, 158–162. [Google Scholar][Green Version]
- Trousdale, M.D.; Barlow, W.E.; McGuigan, L.J.B. Assessment of Diclofenac on Herpes Keratitis in Rabbit Eyes. Arch. Ophthalmol. 1989, 107, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.A.; Paula Pardo, V.R.; Rafael Guerrero, O.A. Inhibition of Rotavirus ECwt Infection in ICR Suckling Mice by N-Acetylcysteine, Peroxisome Proliferator-Activated Receptor Gamma Agonists and Cyclooxygenase-2 Inhibitors. Memórias Do Inst. Oswaldo Cruz 2013, 108, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Kocabalkanlı, A.; Cihan-Üstündağ, G.; Naesens, L.; Mataracı-Kara, E.; Nassozi, M.; Çapan, G. Diclofenac-Based Hydrazones and Spirothiazolidinones: Synthesis, Characterization, and Antimicrobial Properties. Arch. Der Pharm. 2017, 350, 1700010. [Google Scholar] [CrossRef] [PubMed]
- Moshawih, S.; Jarrar, Q.; Alim Bahrin, A.; Fern Lim, A.; Ming, L.; Poh Goh, H. Evaluating NSAIDs in SARS-CoV-2: Immunomodulatory Mechanisms and Future Therapeutic Strategies. Heliyon 2024, 10, e25734. [Google Scholar] [CrossRef] [PubMed]
- Klopčič, I.; Markovič, T.; Mlinarič-Raščan, I.; Sollner Dolenc, M. Endocrine Disrupting Activities and Immunomodulatory Effects in Lymphoblastoid Cell Lines of Diclofenac, 4-Hydroxydiclofenac and Paracetamol. Toxicol. Lett. 2018, 294, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, N.; David, M.; Bielańska, J.; González, T.; Parra, D.; Soler, C.; Comes, N.; Valenzuela, C.; Felipe, A. Immunomodulatory Effects of Diclofenac in Leukocytes through the Targeting of Kv1.3 Voltage-Dependent Potassium Channels. Biochem. Pharmacol. 2010, 80, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, B.-Y.; Park, Y.C.; Kim, K. Diclofenac Inhibits 27-Hydroxycholesterol-Induced Differentiation of Monocytic Cells into Mature Dendritic Cells. Immune Netw. 2017, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Martins Silva, P. Non-Antibiotic Compounds: The Activity of the NSAID Diclofenac on Bacteria—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 340–351. [Google Scholar] [CrossRef]
- Padma, R.; Yalavarthy, P.D. Screening of Diclofenac for Antibacterial Activity against Pathogenic Microorganisms. Int. J. Adv. Inpharmacy Biol. Chem. 2015, 4, 554–558. [Google Scholar]
- Xi, H.; Luo, Z.; Liu, M.; Chen, Q.; Zhu, Q.; Yuan, L.; Sheng, Y.; Zhao, R. Diclofenac Sodium Effectively Inhibits the Biofilm Formation of Staphylococcus epidermidis. Arch. Microbiol. 2024, 206, 289. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.; Abu-El-Azayem, M.; Kotb, M.; Arnaout, H.; Samir, N.; Latif, A.; Soliman, M.; Mostafa, M. Antibiofilm and Antibacterial Activity of Diclofenac against Clinical Enterococcal Isolates. Int. J. Clin. Exp. Med. 2021, 14, 1507–1515. [Google Scholar]
- Annadurai, S.; Guha-Thakurta, A.; Sa, B.; Ray, S.G.D.R.; Chakrabarty, A.N. Experimental studies on synergism between aminoglycosides and the antimicrobial antiinflammatory agent diclofenac sodium. J. Chemother. 2002, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Eman, F.A.; Rehab, M.A.E.-B.; Abo, B.F.A.; Nancy, G.F.; Neveen, A.A.; Gamal, F.M.G. Evaluation of Antibacterial Activity of Some Non-Steroidal Anti-Inflammatory Drugs against Escherichia coli Causing Urinary Tract Infection. Afr. J. Microbiol. Res. 2016, 10, 1408–1416. [Google Scholar] [CrossRef]
- Kbyeh, F.R.; Abedsalih, A.N. Study the Antibacterial Mechanism of Diclofenac and Its Activity Alone or Combined with Ciprofloxacin in Treating Urinary Tract Infection. Wasit J. Pure Sci. 2023, 2, 292–310. [Google Scholar] [CrossRef]
- Salem-Milani, A.; Balaei-Gajan, E.; Rahimi, S.; Moosavi, Z.; Abdollahi, A.; Zakeri-Milani, P.; Bolourian, M. Antibacterial Effect of Diclofenac Sodium on Enterococcus faecalis. J. Dent. 2013, 10, 16–22. [Google Scholar]
- Dutta, N.K.; Annadurai, S.; Mazumdar, K.; Dastidar, S.G.; Kristiansen, J.E.; Molnar, J.; Martins, M.; Amaral, L. Potential Management of Resistant Microbial Infections with a Novel Non-Antibiotic: The Anti-Inflammatory Drug Diclofenac Sodium. Int. J. Antimicrob. Agents 2007, 30, 242–249. [Google Scholar] [CrossRef] [PubMed]
- El-Soudany, I.; Abdelwahab, I.A.; Yakout, M.A. Antibacterial and Antibiofilm Activities of Diclofenac against Levofloxacin-Resistant Stenotrophomonas maltophilia Isolates; Emphasizing Repurposing of Diclofenac. Iran. J. Microbiol. 2024, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Nargesi, S.; Rezaie, S. Investigation an Antifungal Activity of Diclofenac Sodium against Hyphae Formation in Aspergillus fumigatus with Attention to the Expression of Ef-1 Gene. Iran. J. Public Health 2018, 47, 770. [Google Scholar] [PubMed]
- Rashki Ghalehnoo, Z.; Rashki, A.; Najimi, M.; Dominguez, A. The Role of Diclofenac Sodium in the Dimorphic Transition in Candida albicans. Microb. Pathog. 2010, 48, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Carrieri, A.; Tardugno, R.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils. Antibiotics 2023, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Yousri, F.; Taha, N.; El-Waly, O.A.; Ramadan, A.E.-K.; Ismail, E.; Hamada, R.; Khalaf, M.; Refaee, M.; Ali, S.; et al. Effect of Some Non Steroidal Anti-Inflammatory Drugs on Growth, Adherence and Mature Biofilms of Candida Spp. Am. J. Microbiol. Res. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Brasil, J.A.; de Oliveira, J.S.; Pereira, V.S.; Pereira-Neto, W.d.A.; Sidrim, J.J.C.; Rocha, M.F.G. Diclofenac Exhibits Synergism with Azoles against Planktonic Cells and Biofilms of Candida tropicalis. Biofouling 2020, 36, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.; Radu-Popescu, M.; Pelinescu, D.; Vassu, T. Treatment with Some Anti-Inflammatory Drugs Reduces Germ Tube Formation in Candida albicans Strains. Braz. J. Microbiol. 2014, 45, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Bink, A.; Kucharíková, S.; Neirinck, B.; Vleugels, J.; Van Dijck, P.; Cammue, B.P.A.; Thevissen, K. The Nonsteroidal Antiinflammatory Drug Diclofenac Potentiates the in Vivo Activity of Caspofungin against Candida albicans Biofilms. J. Infect. Dis. 2012, 206, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Yu, C.; Li, T.; Liu, J.; Sun, S. Potential Antifungal Targets against a Candida Biofilm Based on an Enzyme in the Arachidonic Acid Cascade—A Review. Front. Microbiol. 2016, 7, 01925. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Altini, E.; Sblano, S.; Salvagno, L.; Maggi, F.; de Michele, G.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Synergistic Activity of New Diclofenac and Essential Oils Combinations against Different Candida Spp. Antibiotics 2021, 10, 688. Antibiotics 2021, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Yücesoy, M.; Oktem, I.; Gülay, Z. In-Vitro Synergistic Effect of Fluconazole with Nonsteroidal Anti-Inflammatory Agents against Candida albicans Strains. J. Chemother. 2000, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, H.B.; Hertting, G. Lowering of the Convulsive Threshold by Non-Steroidal Anti-Inflammatory Drugs. Eur. J. Pharmacol. 1981, 69, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.I.; Kamel, E.S.M.; Abbas, N.A.T.; Kaoud, A. Diclofenac Influence on the Anticonvulsant Effect of Retigabine: The Potential Role of KCNQ Channels. Egypt. J. Basic Clin. Pharmacol. 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Degani, N.; Nachman, R.; Uziyel, Y.; Gibor, G.; Shabat, D.; Attali, B. Meclofenamic Acid and Diclofenac, Novel Templates of KCNQ2/Q3 Potassium Channel Openers, Depress Cortical Neuron Activity and Exhibit Anticonvulsant Properties. Mol. Pharmacol. 2004, 67, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Türel, C.A.; Çelik, H.; Çetinkaya, A.; Türel, İ. Electrophysiologic and Anti-Inflammatorial Effects of Cyclooxygenase Inhibition in Epileptiform Activity. Physiol. Rep. 2023, 11, e15800. [Google Scholar] [CrossRef]
- Vieira, V.; Glassmann, D.; Marafon, P.; Pereira, P.; Gomez, R.; Coitinho, A.S. Effect of Diclofenac Sodium on Seizures and Inflammatory Profile Induced by Kindling Seizure Model. Epilepsy Res. 2016, 127, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Erdogan, M.A.; Gurgul, S.; Erbas, O. Effects of Diclofenac Sodium on Seizure Activity in Rats with Pentylenetetrazole-Induced Convulsions. Neurochem. Res. 2022, 48, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazaly, M.; Kenawy, S.; Khayyal, M.T.; Roushdy, H.; Saleh, S. Effect of Exposure to Radiation on the Inflammatory Process and Its Influence by Diclofenac. Br. J. Pharmacol. 1985, 85, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pospísil, M.; Hofer, M.; Pipalová, I.; Viklická, S.; Netíková, J.; Sandula, J. Enhancement of Hematopoietic Recovery in Gamma-Irradiated Mice by the Joint Use of Diclofenac, an Inhibitor of Prostaglandin Production, and Glucan, a Macrophage Activator. Exp. Hematol. 1992, 20, 891–895. [Google Scholar] [PubMed]
- Hofer, M.; Pospíšil, M.; Viklická, Š.; Vacek, A.; Pipalovà, I.; Bartoničková, A. Hematopoietic Recovery in Repeatedly Irradiated Mice Can Be Enhanced by a Repeatedly Administered Combination of Diclofenac and Glucan. J. Leukoc. Biol. 1993, 53, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospísil, M.; Pipalová, I.; Holá, J. Modulation of Haemopoietic Radiation Response of Mice by Diclofenac in Fractionated Treatment. Physiol. Res. 1996, 45, 213–220. [Google Scholar] [PubMed]
- Alok, A.; Agrawala, P.K. Repurposing Sodium Diclofenac as a Radiation Countermeasure Agent: A Cytogenetic Study in Human Peripheral Blood Lymphocytes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2020, 856–857, 503220. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Dezfouli, A.B.; Khosravi, M.; Alkotub, B.; Bauer, L.; Birgani, M.J.T.; Multhoff, G. The Radiation- and Chemo-Sensitizing Capacity of Diclofenac Can Be Predicted by a Decreased Lactate Metabolism and Stress Response. Radiat. Oncol. 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Adhikari, J.S.; Chaudhury, N.K. Radioprotective Role of Clinical Drug Diclofenac Sodium. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2013, 755, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Egashira, T.; Yamanaka, Y. Effect of Diclofenac, a Non-Steroidal Anti-Inflammatory Drug, on Lipid Peroxidation Caused by Ischemia-Reperfusion in Rat Liver. Jpn. J. Pharmacol. 1994, 64, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Saibene, L.; Macciocchi, A. Antioxidant Profile of Nimesulide, Indomethacin and Diclofenac in Phosphatidylcholine Liposomes (PCL) as Membrane Model. Int. J. Tissue React. 1993, 15, 225–234. [Google Scholar] [PubMed]
- Tang, Y.-Z.; Liu, Z.-Q. Evaluation of the Free-Radical-Scavenging Activity of Diclofenac Acid on the Free-Radical-Induced Haemolysis of Human Erythrocytes. J. Pharm. Pharmacol. 2006, 58, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Curcelli, E.C.; Muller, S.S.; Luiz, J. Beneficial Effects of Diclofenac Therapy on Serum Lipids, Oxidized Low-Density Lipoprotein and Antioxidant Defenses in Rats. Life Sci. 2008, 82, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Z. Diclofenac Acid: A Free-Radical-Scavenger to Protect DNA against Radical-Induced Oxidation. Drug Dev. Res. 2009, 70, 520–524. [Google Scholar] [CrossRef]
- Islas-Flores, H.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Colín-Cruz, A.; Neri-Cruz, N.; García-Medina, S. Diclofenac-Induced Oxidative Stress in Brain, Liver, Gill and Blood of Common Carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2013, 92, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Dada, S.A.; Akintoye, O.; Ezekpo, O.; Dada, O.S.; Sanya, J. Diclofenac-Induced Alterations in Renal Antioxidants and Cytokines in Male Wistar Rats. Int. J. Med. Sci. Clin. Res. Stud. 2023, 3, 2221–2226. [Google Scholar] [CrossRef]
- Tella, T.; Adegbegi, A.; Emeninwa, C.; Odola, A.; Ayangbenro, A.; Adaramoye, O. Evaluation of the Antioxidative Potential of Diisopropyldithiocarbamates Sodium Salt on Diclofenac-Induced Toxicity in Male Albino Rats. Toxicol. Rep. 2022, 9, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Majumder, R.; Banerjee, A.; Bandyopadhyay, D.; Chattopadhyay, A. Melatonin Protects against Diclofenac Induced Oxidative Stress Mediated Myocardial Toxicity in Rats: A Mechanistic Insight. Food Chem. Toxicol. 2024, 190, 114813. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Lattuada, N.; Greco, V.; Sibilia, V.; Falchi, M.; Bianchi, T.; Dal Sasso, M. Diclofenac-Choline Antioxidant Activity Investigated by means of Luminol Amplified Chemiluminescence of Human Neutrophil Bursts and Electron Paramagnetic Resonance Spectroscopy. Drug Res. 2015, 65, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rojo, C.; Álvarez-Figueroa, M.J.; Soto, M.; Cañete, A.; Pessoa-Mahana, D.; López-Alarcón, C. Scavenging Activity of Diclofenac: Interaction with Abts Radical Cation and Peroxyl Radicals. J. Chil. Chem. Soc. 2009, 54, 58–62. [Google Scholar] [CrossRef][Green Version]
- Moore, N. Coronary Risks Associated with Diclofenac and Other NSAIDs: An Update. Drug Saf. 2020, 43, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Arfè, A.; Scotti, L.; Varas-Lorenzo, C.; Nicotra, F.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H.; et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ (Clin. Res. Ed.) 2016, 354, i4857. [Google Scholar] [CrossRef] [PubMed]
- García Rodríguez, L.A.; Tacconelli, S.; Patrignani, P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J. Am. Coll. Cardiol. 2008, 52, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawati, H.; Vakili, M.R.; Lavasanifar, A.; Ahmed, S.; Jamali, F. Reduced Heart Exposure of Diclofenac by Its Polymeric Micellar Formulation Normalizes CYP-Mediated Metabolism of Arachidonic Acid Imbalance in An Adjuvant Arthritis Rat Model: Implications in Reduced Cardiovascular Side Effects of Diclofenac by Nanodrug Delivery. Mol. Pharm. 2020, 17, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Iftode, C.; Olaru, I.; Chioran, D.; Coricovac, D.; Geamantan, A.; Borza, C.; Ursoniu, S.; Ardelean, S. Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) as Repurposed Anticancer Drugs in Skin Cancer. Farmacia 2024, 72, 28–43. [Google Scholar] [CrossRef]
- Alharbi, F.K. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Vet. J. 2025, 15, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, I.M.; Roman, Y.M. Individualized Management of Osteoarthritis: The Role of Pharmacogenomics to Optimize Pain Therapy. Future Pharmacol. 2025, 5, 30. [Google Scholar] [CrossRef]
- Hnepa, Y.Y.; Chopey, I.V.; Chubirko, K.I.; Bratasyuk, A.M. Short- and Long-Term Effects of Nsaids on the Gastrointestinal Mucosa: Complex Analysis of Benefits and Complications Prevention. Wiadomości Lek. 2021, 74, 1011–1018. [Google Scholar] [CrossRef]
- Datta, A.; Flynn, N.R.; Barnette, D.A.; Woeltje, K.F.; Miller, G.P.; Swamidass, S.J. Machine learning liver-injuring drug interactions with non-steroidal anti-inflammatory drugs (NSAIDs) from a retrospective electronic health record (EHR) cohort. PLoS Comput. Biol. 2021, 17, e1009053. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Gkika, D.A.; Siadimas, I.; Christodoulopoulos, I.; Efthymiopoulos, P.; Kyzas, G.Z. The multifaceted effects of Non-Steroidal and Non-Opioid Anti-Inflammatory and analgesic drugs on platelets: Current knowledge, limitations, and future perspectives. Pharmaceuticals 2024, 17, 627. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Sørensen, H.T.; Pedersen, L. Diclofenac use and cardiovascular risks: Series of nationwide cohort studies. BMJ 2018, 362, k3426. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.E. Anti-Inflammatory and antipyretic analgesics and drugs used in gout. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2018; pp. 141–153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).