1. Introduction
Lung cancer remains the primary cause of cancer-related deaths around the world. This type of cancer develops in lung tissues and can occur in several forms including small cell lung carcinoma [
1]. Small-cell lung cancer (SCLC) is one of the most aggressive forms of lung cancer, characterized by rapid growth and early metastasis. It is often diagnosed at an advanced stage, leading to a high recurrence rate and poor survival outcomes [
2]. Historically, platinum-based chemotherapy (carboplatin or cisplatin) combined with etoposide has been the first-line therapy for patients with advanced-stage SCLC. However, prognosis remains poor, with a median overall survival (OS) of 8–13 months and a high relapse rate following initial treatment [
3], particularly in extensive-stage SCLC (ES-SCLC).
In recent years, immunotherapy has revolutionized the management of SCLC. Two pivotal phase III clinical trials, IMpower133 and CASPIAN, have led to a paradigm shift in treatment, demonstrating that the addition of immune checkpoint inhibitors, such as atezolizumab (IMpower133) and durvalumab (CASPIAN), to chemotherapy significantly improves both OS and progression-free survival (PFS) [
4,
5]. The IMpower133 trial reported a median OS of 12.3 months in patients treated with atezolizumab plus chemotherapy, compared to 10.3 months with chemotherapy alone
2. Similarly, the CASPIAN confirmed the efficacy of immunotherapy, showing positive results with durvalumab in combination with chemotherapy [
6].
Given these advancements, immunotherapy combined with chemotherapy is now a key component of standard treatment for advanced-stage SCLC. This report presents a notable case of long-term disease control in a patient with SCLC, treated with chemotherapy and atezolizumab, followed by maintenance immunotherapy.
2. Case Presentation
The A 76-year-old man, a former heavy smoker (80 pack-years), with a medical history of COPD, hypertensive heart disease, hyperuricemia, and benign prostatic hyperplasia, was admitted to the Pulmonology Unit at San Severo Hospital in January 2023 due hyperpyrexia, cough, and dyspnea. Blood tests revealed elevated inflammatory markers (ESR 58 mm/h, CRP 145 mg/L) and leukocytosis (white blood cells 14,500/mm
3). Chest X-ray (
Figure 1A) revealed a suspected consolidation in the lower left lung lobe, prompting treatment with meropenem, levofloxacin, and methylprednisolone. After clinical improvement, the patient was discharged with instructions to continue levofloxacin and prednisone at home.
A follow-up chest X-Ray (
Figure 1B) performed 15 days later detected an oval-shaped mass with well-defined margins in the lower left lung lobe, raising suspicion of pulmonary neoplasm.
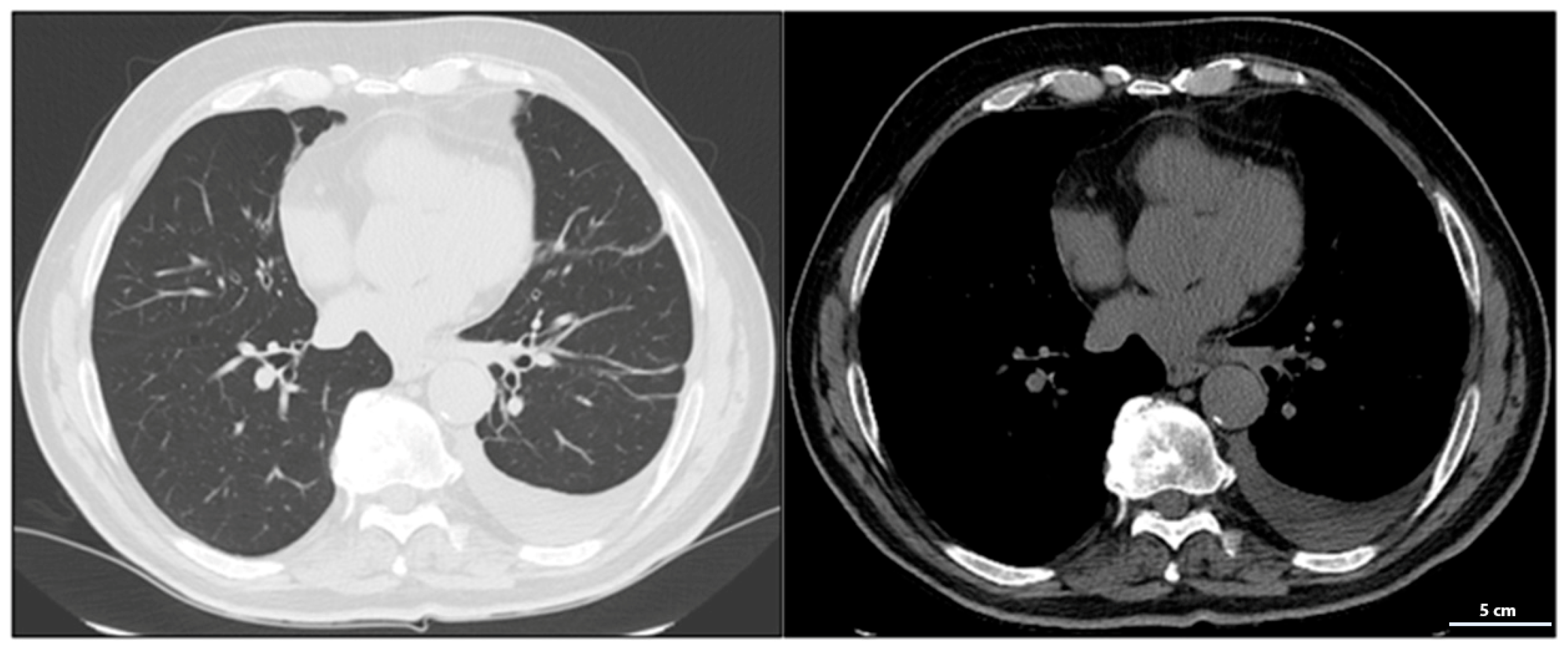
A chest CT scan performed in February 2023 confirmed the presence of a 35 mm solid nodular mass with irregular margins in the apical segment of the lower left lung lobe (
Figure 2).
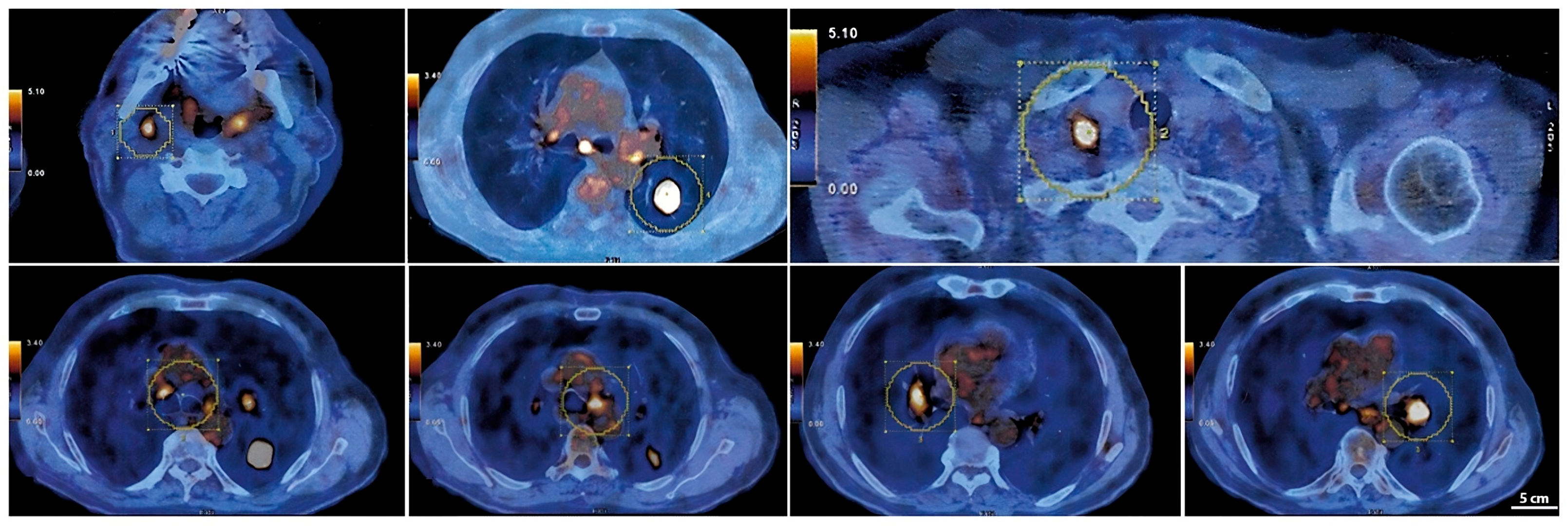
A PET-CT scan with 18F-FDG performed in March 2023 (
Figure 3) revealed intense radiotracer uptake in the nodular lesion of the apical segment of the lower left lung lobe (SUV max 12.5). The scan also showed hypermetabolic lymph nodes in the Barety space (SUV max 4), aorto-pulmonary window (SUV max 5.1), subcarinal region (SUV max 6.4), and bilateral hilar regions (SUV max up to 6 on the left). Additional hypermetabolic lymph nodes were detected in the right supraclavicular region (SUV max 10.2) and in the ipsilateral lateral cervical region (SUV max 5.9), consistent with advanced-stage disease (T2aN3M0) [
7].
To confirm the diagnosis, the patient underwent EBUS-TBNA. The sample was adequate [
8]; however, the cytology report described “lymphatic tissue with rare macrophages containing anthracotic pigment and fragments of respiratory columnar epithelium, occasionally with squamous metaplasia”, with no evidence of atypical cells. Therefore, a wedge resection of the apical segment of the lower left lung lobe was performed. The final histopathological examination confirmed the diagnosis of small-cell lung cancer (SCLC), with a Ki67 proliferation index of 70%. A representative histopathological image showing the Ki67 immunostaining and proliferation index has been provided in the
Supplementary Materials (Figures S1 and S2).
3. Treatment and Clinical Response
After the diagnosis of advanced-stage SCLC, the patient initiated combination therapy with carboplatin, etoposide, and atezolizumab. The chemotherapy regimen consisted of four cycles administered every 21 days. Each cycle included carboplatin and etoposide (100 mg/m
2 intravenously on days 1 to 3), administered concurrently with atezolizumab. Atezolizumab was delivered intravenously at a fixed dose of 1200 mg on day 1 of each cycle. Following the induction phase, the patient continued with maintenance immunotherapy, receiving atezolizumab at the same dose (1200 mg) every three weeks until disease progression or the onset of unacceptable toxicity. Carboplatin was selected for its more favorable toxicity profile in elderly patients compared to cisplatin, particularly considering the patient’s comorbidities, including COPD and hypertensive heart disease [
9]. The patient completed four cycles of this combination therapy. Atezolizumab, an anti-PD-L1 immune checkpoint inhibitor, was administered every three weeks according to standard extensive-stage SCLC treatment protocols. The patient’s baseline performance status remained stable throughout therapy, a crucial factor given that SCLC patients, particularly those with extensive-stage disease, often experience rapid health declines due to both the disease progression and treatment toxicity [
4].
Remarkably, the patient tolerated the treatment regimen exceptionally well, without significant hematologic, gastrointestinal, or pulmonary toxicities (common adverse effects of both chemotherapy and immunotherapy). Immune-related adverse events (irAEs), including pneumonitis, colitis, or endocrinopathies, were closely monitored; however, none occurred in this patient, likely facilitating uninterrupted therapy and sustained treatment efficacy [
10].
After completing four cycles of chemotherapy and atezolizumab, the patient transitioned to maintenance therapy with atezolizumab alone. This transition aligns with clinical trial findings, such as those from IMpower133, which demonstrated the efficacy of maintenance atezolizumab in extending both OS and PFS in extensive-stage SCLC patients [
4]. The patient continued atezolizumab maintenance without interruption, and follow-up clinical assessments showed stable disease with no new symptoms indicative of progression.
Serial imaging, including CT and PET-CT scans, confirmed ongoing disease stability, with a marked reduction in mediastinal and hilar lymph node size and no evidence of new pulmonary lesions or metastases. The patient’s prolonged disease stability was particularly remarkable considering the typically rapid progression of extensive-stage SCLC [
11]. The sustained maintenance of good quality of life, without significant treatment-related toxicities, further highlights the role of atezolizumab in managing extensive-stage SCLC [
12].
Neutrophil-to-lymphocyte ratio (NLR) values were monitored over time and reported in
Table 1, showing a consistent association with disease stability (
Table 1).
4. Radiological Monitoring and Disease Stability
During treatment, the patient underwent serial total-body CT scans to assess the response to therapy (
Table 1). The first follow-up CT, performed in June 2023, detected a left postero-basal pleural effusion, likely related to the prior wedge resection surgery (
Figure 4). To manage this complication, dexamethasone 8 mg daily was administered for one week. Although the dexamethasone dose was higher than the generally recommended threshold for minimizing immunosuppression (equivalent to <10 mg prednisone/day), it was prescribed for a short duration to achieve rapid symptom control, balancing the therapeutic benefit against potential impact on atezolizumab efficacy. As is well-known, corticosteroid use in such cases must be limited due to concurrent immunotherapy with atezolizumab. While corticosteroids effectively reduce inflammation and pleural effusion, their immunosuppressive effects can attenuate the efficacy of immune checkpoints inhibitors. High doses or prolonged corticosteroid use may compromise atezolizumab’s antitumor activity by dampening the immune response against cancer cells [
13]. Therefore, a short-term corticosteroid regimen was chosen to control the pleural effusion while minimizing the potential interference with atezolizumab’s immune-mediated effects.
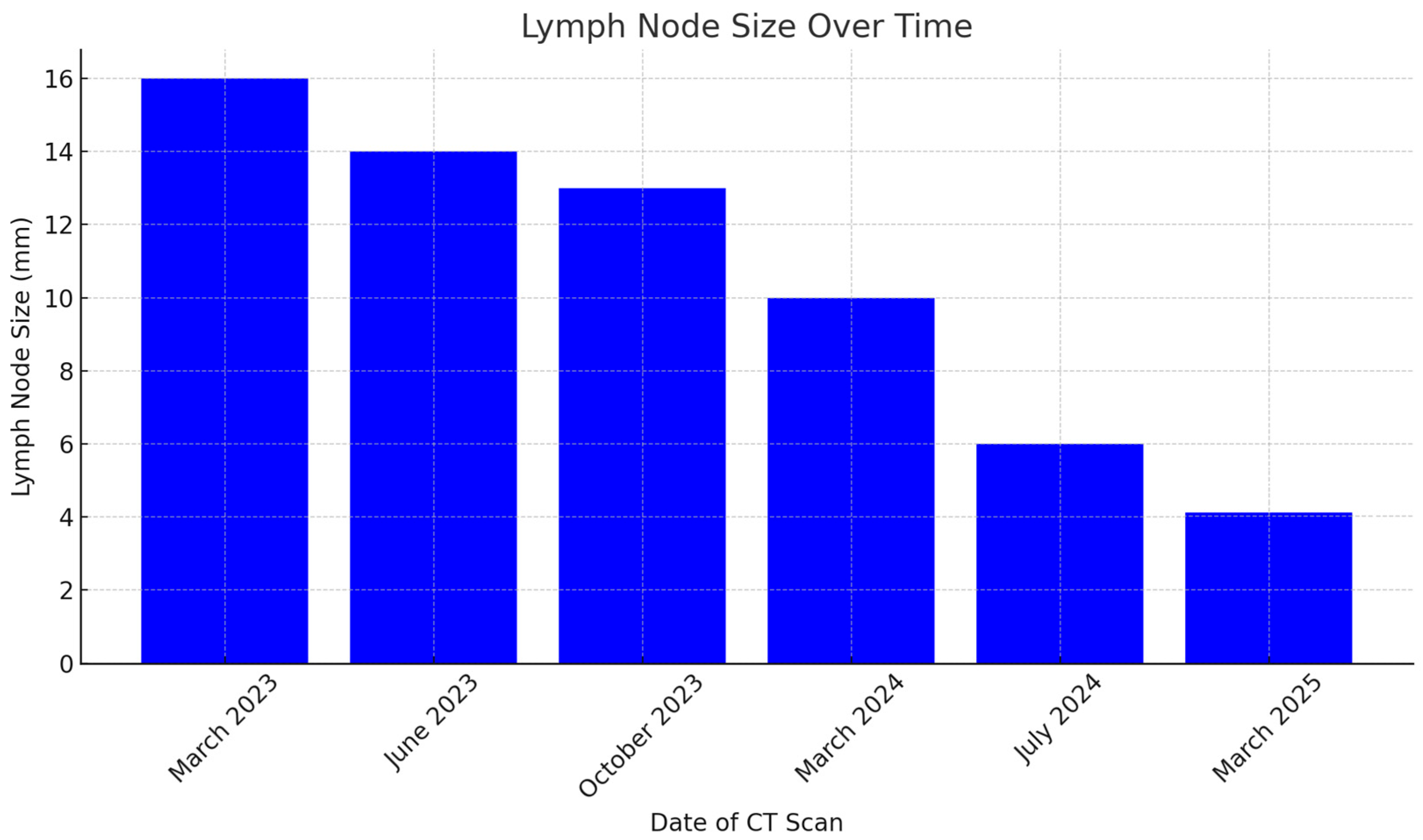
The October 2023 CT scan confirmed the absence of disease progression, with further stabilization of the mediastinal and lateral cervical lymph nodes, now measuring <1 cm and no longer clinically significant, indicating good disease control.
By March 2024, CT imaging showed further improvement, with a reduction in mediastinal lymph node size to 7 mm in the aorto-pulmonary window and 11 mm at the right hilum. No new pulmonary or nodal lesions were observed, confirming continued disease control without signs of progression.
At the radiological follow-up in July 2024, imaging demonstrated sustained disease stability, with no new pleural effusions and stable lymph nodes. These findings confirmed a prolonged response to atezolizumab, representing a rare case of long-term disease control in advanced-stage small-cell lung cancer (SCLC), with some lymph nodes completely resolving and others reduced to clinically insignificant sizes. At the most recent radiological follow-up performed on 10 March 2025, total-body CT imaging confirmed continued disease stability. No evidence of recurrence was observed in the thoracic region, and there were no new lesions detected either in the lungs or at extra-pulmonary sites. This further supports the sustained response to maintenance immunotherapy with atezolizumab.
This case is remarkable for the exceptionally prolonged disease stability observed in a patient with extensive-stage SCLC treated with chemo-immunotherapy. Unlike the typical clinical course of SCLC, characterized by rapid progression, this patient exhibited an unusually sustained response to atezolizumab.
Serial CT and PET-CT scans over a 24-months period confirmed the absence of new lesions and sustained tumor control. Lymph node involvement, initially extensive, showed significant regression, with some nodes completely resolving. This case suggests that, in select patients, chemo-immunotherapy may induce durable responses beyond standard expectations, warranting further investigation into predictive biomarkers (
Figure 5 and
Figure S3).
5. Discussion
Advanced-stage small-cell lung cancer (SCLC) remains a significant therapeutic challenge due to its aggressive nature and generally poor prognosis. Despite initial sensitivity to chemotherapy, most patients experience early disease progression following standard treatment. This case demonstrates the efficacy of the therapeutic approach combining chemotherapy with immune checkpoint inhibition using atezolizumab; the results are encouraging towards disease’s stability and tolerability, as suggested in other studies adopting a similar approach [
14].
Phase III clinical trials, such as IMpower133, have shown that adding atezolizumab to standard chemoterapy significantly improves both overall survival (OS) and progression-free survival (PFS) in extensive-stage SCLC [
4,
12]. Atezolizumab functions by blocking the interaction between PD-L1 on tumor cells and PD-1 receptors on T cells, thereby preventing immune evasion enhancing antitumor immunity [
15]. In the IMpower133 trial, patients treated with atezolizumab achieved a median overall survival of 12.3 months, compared to 10.3 months with chemotherapy alone, with a 12-month survival rate of 51.7% versus 38.2% for chemotherapy alone [
4].
In this patient, atezolizumab administration led to prolonged disease stability, as evidenced by follow-up imaging showing significant reduction in mediastinal lymphadenopathy and resolution of previously involved nodal stations. This outcome underscores the pivotal role of immunotherapy in maintaining clinical and radiological response in extensive-stage SCLC.
While chemo-immunotherapy with atezolizumab yielded significant benefits in this case, alternative therapeutic strategies for extensive-stage SCLC should be considered, including: (1) alternative chemotherapy regimens, such as topotecan, commonly used in relapsed SCLC; (2) other immune checkpoint inhibitors, like nivolumab or pembrolizumab, which have shown promising results in clinical trials (e.g., CheckMate 032 trial) [
16]; (3) emerging therapies, such as bispecific antibodies, antibody-drug conjugates, and targeted radiotherapy; (4) combination strategies involving radiotherapy, particularly for cases with limited progression, where local disease control may enhance overall survival [
17].
A key aspect of this case is the treatment’s tolerability. Despite advanced age and significant comorbidities such as COPD and hypertensive heart disease, the patient tolerated atezolizumab well, without requiring treatment interruptions due to severe adverse effects. This demonstrates that immunotherapy can be safely administered even in frail populations [
16]. Notably, immune-related adverse events (irAEs), such as autoimmune pneumonitis or colitis, did not occur in this patient, enabling uninterrupted maintenance therapy with atezolizumab.
Moreover, atezolizumab’s immunomodulatory effects provided not only local disease control but also systemic protection against tumor dissemination. This is particularly relevant given SCLC’s propensy for rapid metastatic spread. These findings reinforce immunotherapy’s role in extending overall survival and maintaining long-term disease control.
The neutrophil-to-lymphocyte ratio (NLR) is a widely studied systemic inflammatory marker with prognostic significance in various solid tumors, including small-cell lung cancer (SCLC). A high NLR is generally associated with poor outcomes, while a low NLR correlates with longer overall survival (OS) and progression-free survival (PFS). Specifically, an NLR < 4 has been linked to improved prognosis, whereas values ≥ 4 predict more aggressive disease and reduced survival times. Mirili et al. demonstrated that patients with SCLC and NLR < 4 had significantly longer OS and PFS compared to those with higher values, and also found strong correlations between NLR and PET-CT metabolic parameters such as MTV and TLG, further supporting its prognostic role [
18]. Moreover, recent findings by Xiong et al. show that post-treatment NLR may predict early response to immunotherapy with anti-PD-1/PD-L1 agents, suggesting a role for NLR not only as a static prognostic factor but also as a dynamic biomarker of treatment efficacy in SCLC [
19]. These observations highlight the potential of NLR as a prognostic and predictive biomarker in SCLC. Reflecting this trend, in our patient, the NLR remained consistently below 4.0 throughout the entire treatment period. Notably, the most recent measurements—1.37 in March 2025 and 1.45 in May 2025—continued to indicate a favorable systemic inflammatory status. This sustained low NLR paralleled the long-term radiological disease stability, supporting the hypothesis that effective immunotherapy not only controlled tumor progression but also modulated systemic inflammation.
This case highlights the efficacy and tolerability of atezolizumab in the management of advanced-stage SCLC. The combined chemo-immunotherapy approach, followed by maintenance with atezolizumab, resulted in sustained disease stability, supporting its role as a key therapeutic strategy for improving survival and quality of life in this aggressive malignancy. This patient’s experience aligns with findings from major clinical trials, such as IMpower133, further strengthening the rationale for integrating immunotherapy as a standard of care in extensive-stage SCLC.
6. Conclusions
The treatment landscape for advanced-stage small-cell lung cancer (SCLC) has evolved significantly with the introduction of immunotherapy. The addition of atezolizumab to standard chemotherapy has led to substantial improvements in both survival outcomes and disease management for this highly aggressive malignancy. In this case report, the patient demonstrated prolonged disease stability, with some lymph nodes completely resolving and others significantly decreasing in size, without the emergence of new lesions or debilitating side effects.
An important limitation of this case is the lack of immunohistochemical analysis of the tumor microenvironment (TME), particularly regarding the presence of immune-suppressive cell populations such as regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), which are known to negatively influence the efficacy of immune checkpoint inhibitors. Future studies should incorporate a broader panel of immunohistochemical markers to better characterize the immune landscape of SCLC, enabling a more comprehensive understanding of factors that predict response to immunotherapy.
These findings, supported by robust clinical evidence such as the IMpower133 trial, reinforce the crucial role of immunotherapy with atezolizumab in extending overall survival and enhancing the quality of life in SCLC patients. Notably, the excellent tolerability of treatment in this elderly patient with multiple comorbidities highlights the importance of considering immunotherapy not only for disease control but also for its favorable long-term safety profile.
Despite advanced age and pre-existing conditions, including COPD and hypertensive heart disease, the patient tolerated therapy exceptionally well, with: (1) absence of severe hematologic, gastrointestinal, or pulmonary toxicities; (2) absence of immune-related adverse events, such as pneumonitis, colitis, or endocrinopathies; (3) effective management of post-surgical pleural effusion; the only notable complication, a transient pleural effusion following wedge resection, was successfully managed with short-term corticosteroids without compromising the efficacy of immunotherapy.
This case raises important questions regarding potential patient-specific factors, such as immune system profile or tumor microenvironment, that may contribute to enhanced tolerability of atezolizumab.
Ultimately, this case exemplifies how a multimodal therapeutic strategy, integrating chemotherapy and immunotherapy, can lead to more effective management of advanced-stage SCLC. These findings pave the way for further advancements in optimizing treatment approaches for this challenging disease.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/scipharm93030029/s1, Figure S1: Immunohistochemical staining of MIB-1 (Ki-67) at 10× magnification, showing a proliferation index greater than 70%. Image acquired with a Nikon Eclipse microscope. Figure S2: Immunohistochemical staining of MIB-1 (Ki-67) at 20× magnification, showing a proliferation index greater than 70%. Image acquired with a Nikon Eclipse microscope. Figure S3: Sequential Chest CT Scans. (A) March 2023, (B) June 2023, (C) October 2023, (D) March 2024, (E) July 2024, (F) March 2025.
Author Contributions
Conceptualization, S.N., R.L. and S.R.; investigation, M.L., M.T. and M.S.; resources, L.S., P.C., E.L. and F.C.; writing—original draft preparation, S.N., R.L., M.L., M.T., M.S., G.L., L.S., P.C., F.C., E.L., A.G., R.B. and S.R.; writing—review and editing, S.N., R.L., M.L., M.T., M.S., G.L., L.S., P.C., F.C., E.L., A.G., R.B. and S.R.; visualization, S.R.; supervision, S.N., R.L. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Kazarnowicz, A.; Karaseva, N.; Sanchez, A.; De Boer, R.; Andric, Z.; Reck, M.; Atagi, S.; Lee, J.S.; Garassino, M.; et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): A randomized phase I/III trial. Ann. Oncol. 2020, 31, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liang, H.; Zhong, W.; Zhao, J.; Chen, M.; Zhu, Z.; Xu, Y.; Wang, M. Prognostic impact of maximum standardized uptake value on (18) F-FDG PET/CT imaging of the primary lung lesion on survival in advanced non-small cell lung cancer: A retrospective study. Thorac. Cancer 2021, 12, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Fontana, M.; Marinelli, F.; Piro, R.; Casalini, E.; Ruggiero, P.; Bisceglia, I.; Pezzarossi, A.; Simeone, M.S.; Tacconi, M.; et al. The Predictive Accuracy of Systematic Versus Selective Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Assessing Mediastinal Staging in Non-Small Cell Lung Cancer. Ann. Res. Oncol. 2024, 4, 122–126. [Google Scholar] [CrossRef]
- Delfanti, S.; Tancredi, R.; Serra, F.; Monaco, T.; Gandini, C.; Ferrari, A.; Tinelli, C.; Brugnatelli, S. Prevention of Oxaliplatin-Induced Peripheral Neuropathy in Colorectal Cancer: The Planet Trial Experience. Ann. Res. Oncol. 2021, 1, 43–55. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Saida, Y.; Watanabe, S.; Kikuchi, T. Extensive-Stage Small-Cell Lung Cancer: Current Landscape and Future Prospects. Onco Targets Ther. 2023, 16, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Dziadziuszko, R.; Sugawara, S.; Kao, S.; Hochmair, M.; Huemer, F.; de Castro, G., Jr.; Havel, L.; Caro, R.B.; Losonczy, G.J.L.C. Five-year survival in patients with extensive-stage small cell lung cancer treated with atezolizumab in the Phase III IMpower133 study and the Phase III IMbrella A extension study. Lung Cancer 2024, 196, 107924. [Google Scholar] [CrossRef] [PubMed]
- Politi, K.; Herbst, R.S. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015, 21, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pacheco, G.; Chandler, G.S.; Maiya, V.; Socinski, M.A.; Sonpavde, G.; Puente, J.; Essioux, L.; Carter, C.; Cardona, J.V.; Mohindra, R.; et al. Correlation of safety and efficacy of atezolizumab therapy across indications. J. Immunother. Cancer 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial (vol 17, pg 883, 2016). Lancet Oncol. 2019, 20, E70. [Google Scholar]
- De Ruysscher, D.; Pijls-Johannesma, M.; Vansteenkiste, J.; Kester, A.; Rutten, I.; Lambin, P. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann. Oncol. 2006, 17, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Guney, I.B.; Paydas, S.; Seydaoglu, G.; Kapukaya, T.K.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, A.E.; Karaalioglu, B.; et al. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET-CT metabolic parameters in small cell lung cancer (SCLC). Int. J. Clin. Oncol. 2019, 24, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, Z.; Xin, L.; Qin, B.; Zhao, X.; Zhang, J.; Shi, W.; Yang, B.; Zhang, G.; Hu, Y. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol. Immunother. 2021, 70, 713–720. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).