Alpinia zerumbet Extract Mitigates PCB 126-Induced Neurotoxicity and Locomotor Impairment in Adult Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Alpinia Zerumbet Leaf Extract
2.2. Chemical Composition Analyses
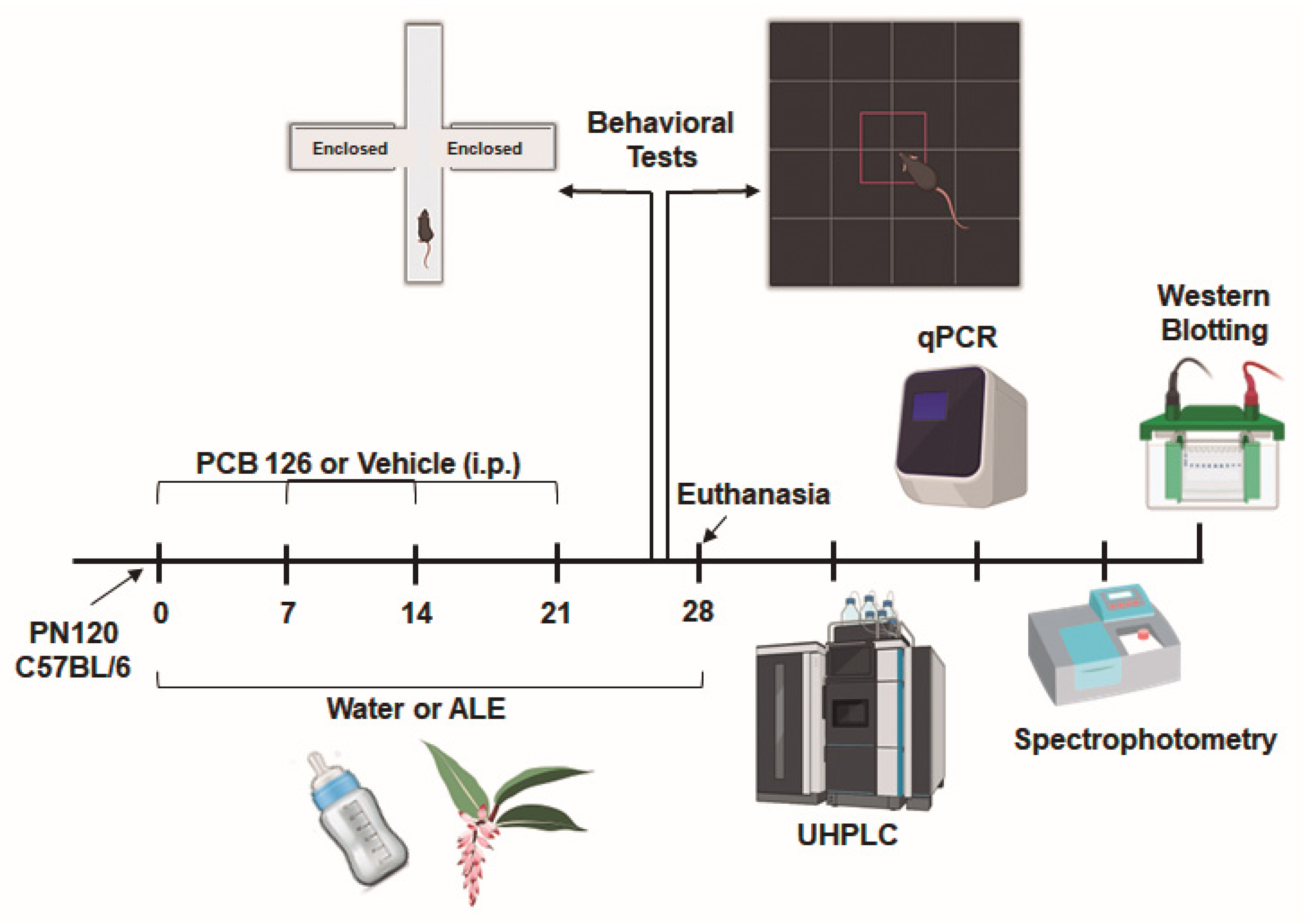
2.3. Experimental Model
2.4. Behavioral Tests
2.4.1. Elevated Plus Maze Test
2.4.2. Open Field Test
2.5. Oxidative Damage Determination
2.6. Superoxide Dismutase (SOD) Activity
2.7. Quantitative Real-Time PCR
2.8. Western Blotting
2.9. Ultra-High Performance Liquid Chromatography (UHPLC)
2.9.1. Tissue Preparation
2.9.2. Neurotransmitter Quantification
2.10. Statistical Analysis
3. Results
3.1. ALE Treatment Prevents PCB 126-Induced Locomotor Deficits Without Affecting Anxiety in Mice
3.2. ALE Treatment Attenuates Oxidative Stress Without Affecting Neuroinflammation in PCB 126-Exposed Mice
3.3. ALE Treatment Modulates Apoptotic Pathway Changes in PCB 126-Exposed Mice
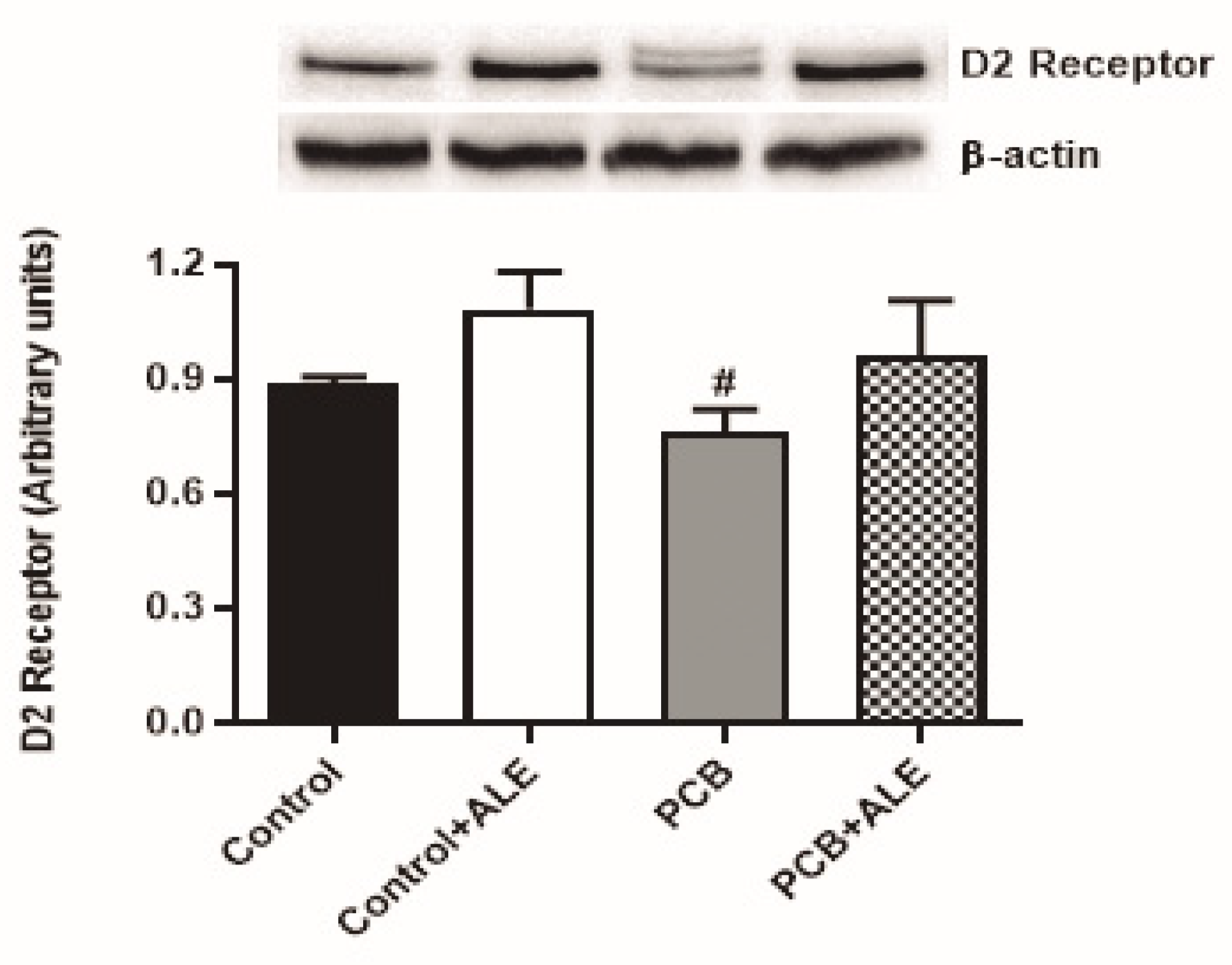
3.4. ALE Treatment Modulates Dopaminergic Pathways in PCB 126-Exposed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AhR | Aryl Hydrocarbon Receptor |
| ALE | Hydroalcoholic Extract of Fresh Leaves of Alpinia zerumbet |
| CNS | Central Nervous System |
| DA | Dopamine |
| DOPAC | 3,4-Dihydroxyphenylacetic Acid |
| EPM | Elevated Plus Maze |
| L-DOPA | Levodopa |
| OF | Open Field |
| PCB 126 | 3,3′,4,4′,5-Pentachlorobiphenyl |
| PCBs | Polychlorinated Biphenyls |
| POPs | Persistent Organic Pollutants |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| TBARS | Thiobarbituric Acid Reactive Substances |
References
- Melymuk, L.; Blumenthal, J.; Sáňka, O.; Shu-Yin, A.; Singla, V.; Šebková, K.; Pullen Fedinick, K.; Diamond, M.L. Persistent Problem: Global Challenges to Managing PCBs. Environ. Sci. Technol. 2022, 56, 9029–9040. [Google Scholar] [CrossRef] [PubMed]
- Coteur, G.; Danis, B.; Fowler, S.W.; Teyssié, J.-L.; Dubois, P.; Warnau, M. Effects of PCBs on Reactive Oxygen Species (ROS) Production by the Immune Cells of Paracentrotus Lividus (Echinodermata). Mar. Pollut. Bull. 2001, 42, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Penteado, J.C.P.; Vaz, J.M. O legado das bifenilas policloradas (PCBs). Quím. Nova 2001, 24, 390–398. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.; Morisseau, C.; Robertson, L.W.; Hammock, B.; Lehmler, H.-J. 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) Decreases Hepatic and Systemic Ratios of Epoxide to Diol Metabolites of Unsaturated Fatty Acids in Male Rats. Toxicol. Sci. 2016, 152, 309–322. [Google Scholar] [CrossRef]
- Shivanna, B.; Chu, C.; Moorthy, B. The Aryl Hydrocarbon Receptor (AHR): A Novel Therapeutic Target for Pulmonary Diseases? Int. J. Mol. Sci. 2022, 23, 1516. [Google Scholar] [CrossRef]
- Schrenk, D.; Chopra, M. Dioxins and Polychlorinated Biphenyls in Foods. In Chemical Contaminants and Residues in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–89. ISBN 978-0-08-100674-0. [Google Scholar]
- Buha Djordjevic, A.; Antonijevic, E.; Curcic, M.; Milovanovic, V.; Antonijevic, B. Endocrine-Disrupting Mechanisms of Polychlorinated Biphenyls. Curr. Opin. Toxicol. 2020, 19, 42–49. [Google Scholar] [CrossRef]
- Klocke, C.; Sethi, S.; Lein, P.J. The Developmental Neurotoxicity of Legacy vs. Contemporary Polychlorinated Biphenyls (PCBs): Similarities and Differences. Environ. Sci. Pollut. Res. 2020, 27, 8885–8896. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.T.; Petriello, M.C.; Newsome, B.J.; Hennig, B. Polychlorinated Biphenyls and Links to Cardiovascular Disease. Environ. Sci. Pollut. Res. 2016, 23, 2160–2172. [Google Scholar] [CrossRef]
- Pessah, I.N.; Lein, P.J.; Seegal, R.F.; Sagiv, S.K. Neurotoxicity of Polychlorinated Biphenyls and Related Organohalogens. Acta Neuropathol. 2019, 138, 363–387. [Google Scholar] [CrossRef]
- Seelbach, M.; Chen, L.; Powell, A.; Choi, Y.J.; Zhang, B.; Hennig, B.; Toborek, M. Polychlorinated Biphenyls Disrupt Blood–Brain Barrier Integrity and Promote Brain Metastasis Formation. Environ. Health Perspect. 2010, 118, 479–484. [Google Scholar] [CrossRef]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Series Introduction: Neurodegeneration: What Is It and Where Are We? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Neuroprotective Effects of Riluzole and Curcumin in Human Astrocytes and Spinal Cord White Matter Hypoxia. Neurosci. Lett. 2020, 738, 135351. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for Amyotrophic Lateral Sclerosis (ALS)/Motor Neuron Disease (MND). Cochrane Database Syst. Rev. 2012, 2012, CD001447. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Thompson, B.L.; Wahlang, B.; Jordan, C.T.; Zach Hilt, J.; Hennig, B.; Dziubla, T. The Environmental Pollutant, Polychlorinated Biphenyls, and Cardiovascular Disease: A Potential Target for Antioxidant Nanotherapeutics. Drug Deliv. Transl. Res. 2018, 8, 740–759. [Google Scholar] [CrossRef]
- de Bem, G.F.; Okinga, A.; Ognibene, D.T.; da Costa, C.A.; Santos, I.B.; Soares, R.A.; Silva, D.L.B.; da Rocha, A.P.M.; Isnardo Fernandes, J.; Fraga, M.C.; et al. Anxiolytic and Antioxidant Effects of Euterpe oleracea Mart. (Açaí) Seed Extract in Adult Rat Offspring Submitted to Periodic Maternal Separation. Appl. Physiol. Nutr. Metab. 2020, 45, 1277–1286. [Google Scholar] [CrossRef]
- Newsome, B.J.; Petriello, M.C.; Han, S.G.; Murphy, M.O.; Eske, K.E.; Sunkara, M.; Morris, A.J.; Hennig, B. Green Tea Diet Decreases PCB 126-Induced Oxidative Stress in Mice by up-Regulating Antioxidant Enzymes. J. Nutr. Biochem. 2014, 25, 126–135. [Google Scholar] [CrossRef]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Ganesh, L.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated Biphenyls Induced Oxidative Stress Mediated Neurodegeneration in Hippocampus and Behavioral Changes of Adult Rats: Anxiolytic-like Effects of Quercetin. Toxicol. Lett. 2013, 222, 45–54. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Phytochemicals of Alpinia Zerumbet: A Review. Molecules 2024, 29, 2845. [Google Scholar] [CrossRef]
- Batista, T.S.C.; Barros, G.S.; Damasceno, F.C.; Cândido, E.A.F.; Batista, M.V.A. Chemical Characterization and Effects of Volatile Oil of Alpinia zerumbet on the Quality of Collagen Deposition and Caveolin-1 Expression in a Muscular Fibrosis Murine Model. Braz. J. Biol. 2024, 84, e253616. [Google Scholar] [CrossRef]
- Faculty of Applied Sciences, UCSI University; Chan, E.W.C.; Wong, S.K.; Chan, H.T. Alpinia Zerumbet, a Ginger Plant with a Multitude of Medicinal Properties: An Update on Its Research Findings. J. Chin. Pharm. Sci. 2017, 26, 775. [Google Scholar] [CrossRef]
- Lahlou, S.; Interaminense, L.F.L.; Leal-Cardoso, J.H.; Duarte, G.P. Antihypertensive Effects of the Essential Oil of Alpinia zerumbet and Its Main Constituent, Terpinen-4-ol, in DOCA-salt Hypertensive Conscious Rats. Fundam. Clin. Pharmacol. 2003, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.A.; Roman-Campos, D.; Carvalho, M.S.; Miranda, F.M.F.; Carneiro, D.C.; Cavalcante, P.H.; Cândido, E.A.F.; Filho, L.X.; Cruz, J.S.; Gondim, A.N.S. Cardiodepressive Effect Elicited by the Essential Oil of Alpinia Speciosa Is Related to L-Type Ca2+ Current Blockade. Phytomedicine 2011, 18, 539–543. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Kikuchi, H.; Koyano, T.; Kowithayakorn, T.; Sakai, T.; Ishibashi, M. Death Receptor 5 Promoter-Enhancing Compounds Isolated from Catimbium Speciosum and Their Enhancement Effect on TRAIL-Induced Apoptosis. Bioorganic Med. Chem. 2009, 17, 6748–6754. [Google Scholar] [CrossRef]
- You, H.; He, M.; Pan, D.; Fang, G.; Chen, Y.; Zhang, X.; Shen, X.; Zhang, N. Kavalactones Isolated from Alpinia zerumbet (Pers.) Burtt. et Smith with Protective Effects against Human Umbilical Vein Endothelial Cell Damage Induced by High Glucose. Nat. Prod. Res. 2022, 36, 5740–5746. [Google Scholar] [CrossRef]
- Xiong, T.; Zeng, J.; Chen, L.; Wang, L.; Gao, J.; Huang, L.; Xu, J.; Wang, Y.; He, X. Anti-Inflammatory Terpenoids from the Rhizomes of Shell Ginger. J. Agric. Food Chem. 2024, 72, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wu, A.; Wang, X.; Guo, Z.; Huang, F.; Cheng, X.; Shen, X.; Tao, L. Anti-Hypertensive and Composition as Well as Pharmacokinetics and Tissues Distribution of Active Ingredients from Alpinia Zerumbet. Fitoterapia 2024, 172, 105753. [Google Scholar] [CrossRef]
- Mpalantinos, M.A.; Soares De Moura, R.; Parente, J.P.; Kuster, R.M. Biologically Active Flavonoids and Kava Pyrones from the Aqueous Extract ofAlpinia Zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.-Y.; Peng, F.; Duan, W.-T.; Wu, C.-H.; Li, H.-T.; Zhang, X.-F.; Shi, Y.-S. Neolignans and Diarylheptanoids with Anti-Inflammatory Activity from the Rhizomes of Alpinia zerumbet. J. Agric. Food Chem. 2021, 69, 9229–9237. [Google Scholar] [CrossRef]
- Da Silva, M.A.; De Carvalho, L.C.R.M.; Victório, C.P.; Ognibene, D.T.; Resende, A.C.; De Souza, M.A.V. Chemical Composition and Vasodilator Activity of Different Alpinia zerumbet Leaf Extracts, a Potential Source of Bioactive Flavonoids. Med. Chem. Res. 2021, 30, 2103–2113. [Google Scholar] [CrossRef]
- Menezes, M.P.; Santos, G.P.; Nunes, D.V.Q.; Silva, D.L.B.; Victório, C.P.; Fernandes-Santos, C.; de Bem, G.F.; Costa, C.A.; Resende, A.C.; Ognibene, D.T. Alpinia zerumbet Leaf Extract Reverses Hypertension and Improves Adverse Remodeling in the Left Ventricle and Aorta in Spontaneously Hypertensive Rats. Braz. J. Med. Biol. Res. 2025, 58, e14210. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Matsuura, M.; Satou, T.; Hayashi, S.; Koike, K. Effects of the Essential Oil from Leaves of Alpinia zerumbet on Behavioral Alterations in Mice. Nat. Prod. Commun. 2009, 4, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Murakami, S.; Matsuura, M.; Hayashi, S.; Koike, K. Anxiolytic Effect and Tissue Distribution of Inhaled Alpinia zerumbet Essential Oil in Mice. Nat. Prod. Commun. 2010, 5, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua, F.; Mocelin, R.; Grimm, C.; Da Silva Junior, N.S.; Buzetto, T.L.B.; Conterato, G.M.M.; Roman, W.A.; Piato, A.L. Involvement of the Catecholaminergic System on the Antidepressant-like Effects of Alpinia zerumbet in Mice. Pharm. Biol. 2016, 54, 151–156. [Google Scholar] [CrossRef]
- De Araújo, F.Y.R.; Chaves Filho, A.J.M.; Nunes, A.M.; De Oliveira, G.V.; Gomes, P.X.L.; Vasconcelos, G.S.; Carletti, J.; De Moraes, M.O.; De Moraes, M.E.; Vasconcelos, S.M.M.; et al. Involvement of Anti-Inflammatory, Antioxidant, and BDNF up-Regulating Properties in the Antipsychotic-like Effect of the Essential Oil of Alpinia zerumbet in Mice: A Comparative Study with Olanzapine. Metab. Brain Dis. 2021, 36, 2283–2297. [Google Scholar] [CrossRef]
- Ferreira, F.D.S.; Silva, P.H.F.D.; Mattos, J.L.A.D.; Resende, A.D.C.; Ognibene, D.T.; Costa, C.A.D.; Montes, G.C.; Fontes-Dantas, F.L.; Bem, G.F.D. Alpinia zerumbet Anxiolytic and Antidepressant Effects: A Literature Review. Ann. Res. Rev. Biol. 2024, 39, 36–51. [Google Scholar] [CrossRef]
- Leece, B.; Denomme, M.A.; Towner, R.; Li, S.M.A.; Safe, S. Polychlorinated Biphenyls: Correlation between in Vivo and in Vitro Quantitative Structure-activity Relationships (QSARs). J. Toxicol. Environ. Health 1985, 16, 379–388. [Google Scholar] [CrossRef]
- Venkataraman, P.; Muthuvel, R.; Krishnamoorthy, G.; Arunkumar, A.; Sridhar, M.; Srinivasan, N.; Balasubramanian, K.; Aruldhas, M.M.; Arunakaran, J. PCB (Aroclor 1254) Enhances Oxidative Damage in Rat Brain Regions: Protective Role of Ascorbic Acid. NeuroToxicology 2007, 28, 490–498. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Emiliano, A.F.; De Carvalho, L.C.R.M.; Souza, M.A.V.; Guedes, D.C.; Tano, T.; Resende, A.C. Antihypertensive and Endothelium-Dependent Vasodilator Effects of Alpinia zerumbet, a Medicinal Plant. J. Cardiovasc. Pharmacol. 2005, 46, 288–294. [Google Scholar] [CrossRef]
- Fraga, M.C.; De Moura, E.G.; Da Silva Lima, N.; Lisboa, P.C.; De Oliveira, E.; Silva, J.O.; Claudio-Neto, S.; Filgueiras, C.C.; Abreu-Villaça, Y.; Manhães, A.C. Anxiety-like, Novelty-Seeking and Memory/Learning Behavioral Traits in Male Wistar Rats Submitted to Early Weaning. Physiol. Behav. 2014, 124, 100–106. [Google Scholar] [CrossRef][Green Version]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 421–431. ISBN 978-0-12-182087-9. [Google Scholar]
- Bannister, J.V.; Calabrese, L. Assays for Superoxide Dismutase. In Methods of Biochemical Analysis; Glick, D., Ed.; Wiley: Hoboken, NJ, USA, 1987; Volume 32, pp. 279–312. ISBN 978-0-471-82195-3. [Google Scholar]
- Rezende, B.; Marques, K.L.; De Carvalho, F.E.A.; Gonçalves, V.M.D.S.; De Oliveira, B.C.C.A.; Nascimento, G.G.; Dos Santos, Y.B.; Antunes, F.; Barradas, P.C.; Fontes-Dantas, F.L.; et al. Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia. Sci. Pharm. 2024, 92, 53. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Ribeiro-Carvalho, A.; Nunes, F.; Araújo, U.C.; Bruno, V.; Marcourakis, T.; Filgueiras, C.C.; Manhães, A.C.; Abreu-Villaça, Y. Lifetime Caffeine and Adolescent Nicotine Exposure in Mice: Effects on Anxiety-like Behavior and Reward. J. Dev. Orig. Health Dis. 2023, 14, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Minkos, A. Polychlorinated Biphenyls (PCB) and Polychlorinated Dibenzo-Para-Dioxins and Dibenzofurans (PCDD/F) in Ambient Air and Deposition in the German Background. Environ. Pollut. 2023, 316, 120511. [Google Scholar] [CrossRef]
- Mohr, S.; Costabeber, I.H. Aspectos Toxicológicos e Ocorrência Dos Bifenilos Policlorados Em Alimentos. Cienc. Rural. 2012, 42, 559–566. [Google Scholar] [CrossRef]
- Megson, D.; Brown, T.; Jones, G.R.; Robson, M.; Johnson, G.W.; Tiktak, G.P.; Sandau, C.D.; Reiner, E.J. Polychlorinated Biphenyl (PCB) Concentrations and Profiles in Marine Mammals from the North Atlantic Ocean. Chemosphere 2022, 288, 132639. [Google Scholar] [CrossRef]
- Steenland, K.; Hein, M.J.; Cassinelli, R.T.; Prince, M.M.; Nilsen, N.B.; Whelan, E.A.; Waters, M.A.; Ruder, A.M.; Schnorr, T.M. Polychlorinated Biphenyls and Neurodegenerative Disease Mortality in an Occupational Cohort. Epidemiology 2006, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, P.R.; Sanz, P.; Eppe, G.; Azevedo, A.F.; Bertozzi, C.P.; Martínez, M.A.; Secchi, E.R.; Barbosa, L.A.; Cremer, M.; Alonso, M.B.; et al. High Accumulation of PCDD, PCDF, and PCB Congeners in Marine Mammals from Brazil: A Serious PCB Problem. Sci. Total Environ. 2013, 463–464, 309–318. [Google Scholar] [CrossRef]
- Kowalski, C.H.; Da Silva, G.A.; Godoy, H.T.; Poppi, R.J.; Augusto, F. Application of Kohonen Neural Network for Evaluation of the Contamination of Brazilian Breast Milk with Polychlorinated Biphenyls. Talanta 2013, 116, 315–321. [Google Scholar] [CrossRef][Green Version]
- Kowalski, C.H.; Costa, J.G.; Godoy, H.T.; Augusto, F. Determination of Polychlorinated Biphenyls in Brazilian Breast Milk Samples Using Solid-Phase Microextraction and Gas Chromatography-Electron Capture Detection. J. Braz. Chem. Soc. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Cocco, R.; Schwanz, T.G.; Mohr, S.; Ceolin, J.; Braga, A.C.M.; Zanatta, N.; Costabeber, I.H. Bifenilos Policlorados Em Arroz e Feijão Do Estado Do Rio Grande Do Sul. Cienc. Rural 2015, 45, 1522–1527. [Google Scholar] [CrossRef][Green Version]
- Costabeber, I.; Sifuentes Dos Santos, J.; Odorissi Xavier, A.A.; Weber, J.; Leal Leães, F.; Bogusz, S.; Emanuelli, T. Levels of Polychlorinated Biphenyls (PCBs) in Meat and Meat Products from the State of Rio Grande Do Sul, Brazil. Food Chem. Toxicol. 2006, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Frenoy, P.; Fiolet, T.; Besson, C.; Mancini, F.R. Dietary Exposure to Polychlorinated Biphenyls (PCB) and Risk of Non-Hodgkin’s Lymphoma: Evidence from the French E3N Prospective Cohort. Environ. Res. 2021, 197, 111005. [Google Scholar] [CrossRef] [PubMed]
- Bandow, N.; Conrad, A.; Kolossa-Gehring, M.; Murawski, A.; Sawal, G. Polychlorinated Biphenyls (PCB) and Organochlorine Pesticides (OCP) in Blood Plasma—Results of the German Environmental Survey for Children and Adolescents 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2020, 224, 113426. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Liu, B.; Bao, W.; Thorne, P.S. Identification of PCB Congeners and Their Thresholds Associated with Diabetes Using Decision Tree Analysis. Sci. Rep. 2023, 13, 18322. [Google Scholar] [CrossRef]
- Frederiksen, M.; Andersen, H.V.; Haug, L.S.; Thomsen, C.; Broadwell, S.L.; Egsmose, E.L.; Kolarik, B.; Gunnarsen, L.; Knudsen, L.E. PCB in Serum and Hand Wipes from Exposed Residents Living in Contaminated High-Rise Apartment Buildings and a Reference Group. Int. J. Hyg. Environ. Health 2020, 224, 113430. [Google Scholar] [CrossRef]
- Ermler, S.; Kortenkamp, A. Systematic Review of Associations of Polychlorinated Biphenyl (PCB) Exposure with Declining Semen Quality in Support of the Derivation of Reference Doses for Mixture Risk Assessments. Environ. Health 2022, 21, 94. [Google Scholar] [CrossRef]
- Choksi, N. Effects of Polychlorinated Biphenyls (PCBs) on Brain Tyrosine Hydroxylase Activity and Dopamine Synthesis in Rats. Fundam. Appl. Toxicol. 1997, 39, 76–80. [Google Scholar] [CrossRef]
- Seegal, R.F. The Neurochemical Effects of PCB Exposure Are Age-Dependent. In Use of Mechanistic Information in Risk Assessment; Bolt, H.M., Hellman, B., Dencker, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 128–137. ISBN 978-3-642-78642-6. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Petriello, M.C.; Newsome, B.; Hennig, B. Influence of Nutrition in PCB-Induced Vascular Inflammation. Environ. Sci. Pollut. Res. 2014, 21, 6410–6418. [Google Scholar] [CrossRef]
- Lai, I.; Chai, Y.; Simmons, D.; Luthe, G.; Coleman, M.C.; Spitz, D.; Haschek, W.M.; Ludewig, G.; Robertson, L.W. Acute Toxicity of 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) in Male Sprague–Dawley Rats: Effects on Hepatic Oxidative Stress, Glutathione and Metals Status. Environ. Int. 2010, 36, 918–923. [Google Scholar] [CrossRef]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.Y.R.; De Oliveira, G.V.; Gomes, P.X.L.; Soares, M.A.; Silva, M.I.G.; Carvalho, A.F.; De Moraes, M.O.; De Moraes, M.E.A.; Vasconcelos, S.M.M.; Viana, G.S.B.; et al. Inhibition of Ketamine-Induced Hyperlocomotion in Mice by the Essential Oil of Alpinia zerumbet: Possible Involvement of an Antioxidant Effect. J. Pharm. Pharmacol. 2011, 63, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Roman Junior, W.A.; Piato, A.L.; Marafiga Conterato, G.M.; Wildner, S.M.; Marcon, M.; Moreira, S.; Santo, G.D.; Mocelin, R.; Emanuelli, T.; Santos, C.A.D.M. Psychopharmacological and Antioxidant Effects of Hydroethanolic Extract of Alpinia zerumbet Leaves in Mice. Pharmacogn. J. 2013, 5, 113–118. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.-S. Astrogliosis in CNS Pathologies: Is There A Role for Microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Rodrigues, M.C.; Monteiro-Neto, J.R.; Teglas, T.; Toborek, M.; Soares Quinete, N.; Hauser-Davis, R.A.; Adesse, D. Early-Life Exposure to PCBs and PFAS Exerts Negative Effects on the Developing Central Nervous System. J. Hazard. Mater. 2025, 485, 136832. [Google Scholar] [CrossRef]
- Walker, K.A.; Rhodes, S.T.; Liberman, D.A.; Gore, A.C.; Bell, M.R. Microglial Responses to Inflammatory Challenge in Adult Rats Altered by Developmental Exposure to Polychlorinated Biphenyls in a Sex-Specific Manner. NeuroToxicology 2024, 104, 95–115. [Google Scholar] [CrossRef]
- Quitete, F.T.; Teixeira, A.V.S.; Peixoto, T.C.; Martins, B.C.; Atella, G.C.; Resende, A.D.C.; Mucci, D.D.B.; Martins, F.; Daleprane, J.B. Long-Term Exposure to Polychlorinated Biphenyl 126 Induces Liver Fibrosis and Upregulates miR-155 and miR-34a in C57BL/6 Mice. PLoS ONE 2024, 19, e0308334. [Google Scholar] [CrossRef]
- Li, T.; Tian, D.; Lu, M.; Wang, B.; Li, J.; Xu, B.; Chen, H.; Wu, S. Gut Microbiota Dysbiosis Induced by Polychlorinated Biphenyl 126 Contributes to Increased Brain Proinflammatory Cytokines: Landscapes from the Gut-Brain Axis and Fecal Microbiota Transplantation. Ecotoxicol. Environ. Saf. 2022, 241, 113726. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Suganya, S.; Ahmad Bhat, F.; Krishnamoorthy, G.; Arunakaran, J. Effect of Quercetin on Haematobiochemical and Histological Changes in the Liver of Polychlorined Biphenyls-Induced Adult Male Wistar Rats. J. Biomark. 2013, 2013, 960125. [Google Scholar] [CrossRef]
- Onzawa, Y.; Kimura, Y.; Uzuhashi, K.; Shirasuna, M.; Hirosawa, T.; Taogoshi, T.; Kihira, K. Effects of 3-O-Methyldopa, L-3,4-Dihydroxyphenylalanine Metabolite, on Locomotor Activity and Dopamine Turnover in Rats. Biol. Pharm. Bull. 2012, 35, 1244–1248. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, R.-D.; Shen, L.-Y.; Yang, L.-L.; Yen, K.-Y.; Hou, W.-C. Monoamine Oxidase B and Free Radical Scavenging Activities of Natural Flavonoids in Melastoma candidum D. Don. J. Agric. Food Chem. 2001, 49, 5551–5555. [Google Scholar] [CrossRef] [PubMed]
- Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Monoamine Oxidase Inhibition by Rhodiola rosea L. Roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The Role of Executive Function and Attention in Gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Hoang, I.; Paire-Ficout, L.; Derollepot, R.; Perrey, S.; Devos, H.; Ranchet, M. Increased Prefrontal Activity during Usual Walking in Aging. Int. J. Psychophysiol. 2022, 174, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.; Delcroix, V.; Ettaki, W.; Derollepot, R.; Paire-Ficout, L.; Ranchet, M. Left and Right Cortical Activity Arising from Preferred Walking Speed in Older Adults. Sensors 2023, 23, 3986. [Google Scholar] [CrossRef]
- Bavithra, S.; Selvakumar, K.; Pratheepa Kumari, R.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated Biphenyl (PCBs)-Induced Oxidative Stress Plays a Critical Role on Cerebellar Dopaminergic Receptor Expression: Ameliorative Role of Quercetin. Neurotox. Res. 2012, 21, 149–159. [Google Scholar] [CrossRef]
- Dreher, J.K.; Jackson, D.M. Role of D1 and D2 Dopamine Receptors in Mediating Locomotor Activity Elicited from the Nucleus Accumbens of Rats. Brain Res. 1989, 487, 267–277. [Google Scholar] [CrossRef]
- Gupta, R.; Shukla, R.K.; Pandey, A.; Sharma, T.; Dhuriya, Y.K.; Srivastava, P.; Singh, M.P.; Siddiqi, M.I.; Pant, A.B.; Khanna, V.K. Involvement of PKA/DARPP-32/PP1α and β- Arrestin/Akt/GSK-3β Signaling in Cadmium-Induced DA-D2 Receptor-Mediated Motor Dysfunctions: Protective Role of Quercetin. Sci. Rep. 2018, 8, 2528. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Keil Stietz, K.P.; Valenzuela, A.E.; Klocke, C.R.; Silverman, J.L.; Puschner, B.; Pessah, I.N.; Lein, P.J. Developmental Exposure to a Human-Relevant Polychlorinated Biphenyl Mixture Causes Behavioral Phenotypes That Vary by Sex and Genotype in Juvenile Mice Expressing Human Mutations That Modulate Neuronal Calcium. Front. Neurosci. 2021, 15, 766826. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef]
- Formisano, L.; Guida, N.; Cocco, S.; Secondo, A.; Sirabella, R.; Ulianich, L.; Paturzo, F.; Di Renzo, G.; Canzoniero, L.M.T. The Repressor Element 1-Silencing Transcription Factor Is a Novel Molecular Target for the Neurotoxic Effect of the Polychlorinated Biphenyl Mixture Aroclor 1254 in Neuroblastoma SH-SY5Y Cells. J. Pharmacol. Exp. Ther. 2011, 338, 997–1003. [Google Scholar] [CrossRef] [PubMed]





| Forward Primer (5′→3′) | Reverse Primer (3′→5′) | |
|---|---|---|
| β-actin | AGGCGACAGCAGTTGGTTGGA | TTGGGAGGGTGAGGGACTTCCT |
| iNOS | TGGCTTGCCCCTGGAAGTTT | GCTGAGAACAGCACAAGGGG |
| TNF | AGCCCCCAGTCTGTATCCTT | CTCCCTTTGCAGAACTCAGG |
| IL6 | CGGAGAGGAGACTTCACAGAGG | GCAAGTGCATCATCGTTGTTCA |
| Control | Control + ALE | PCB | PCB + ALE | |
|---|---|---|---|---|
| % Entry into open arms (EPM) | 35.9 ± 3.1 | 33.5 ± 2.7 | 24.4 ± 6.4 | 33.7 ± 5.1 |
| % Time in center (OF) | 8.0 ± 1.2 | 7.1 ± 0.9 | 5.5 ± 0.9 | 5.3 ± 0.08 |
| % Time in the periphery (OF) | 92.0 ± 1.2 | 92.9 ± 0.9 | 94.5 ± 0.9 | 94.7 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, P.H.F.; Fernandes, J.I.; de Menezes, M.P.; Fontes-Dantas, F.L.; Freitas, A.L.N.; Correa, R.E.; de Araujo, U.C.; Ognibene, D.T.; da Costa, C.A.; Filgueiras, C.C.; et al. Alpinia zerumbet Extract Mitigates PCB 126-Induced Neurotoxicity and Locomotor Impairment in Adult Male Mice. Sci. Pharm. 2025, 93, 23. https://doi.org/10.3390/scipharm93020023
da Silva PHF, Fernandes JI, de Menezes MP, Fontes-Dantas FL, Freitas ALN, Correa RE, de Araujo UC, Ognibene DT, da Costa CA, Filgueiras CC, et al. Alpinia zerumbet Extract Mitigates PCB 126-Induced Neurotoxicity and Locomotor Impairment in Adult Male Mice. Scientia Pharmaceutica. 2025; 93(2):23. https://doi.org/10.3390/scipharm93020023
Chicago/Turabian Styleda Silva, Paula Hosana Fernandes, Jemima Isnardo Fernandes, Matheus Pontes de Menezes, Fabrícia Lima Fontes-Dantas, André Luiz Nunes Freitas, Rayane Efraim Correa, Ulisses Cesar de Araujo, Dayane Teixeira Ognibene, Cristiane Aguiar da Costa, Cláudio Carneiro Filgueiras, and et al. 2025. "Alpinia zerumbet Extract Mitigates PCB 126-Induced Neurotoxicity and Locomotor Impairment in Adult Male Mice" Scientia Pharmaceutica 93, no. 2: 23. https://doi.org/10.3390/scipharm93020023
APA Styleda Silva, P. H. F., Fernandes, J. I., de Menezes, M. P., Fontes-Dantas, F. L., Freitas, A. L. N., Correa, R. E., de Araujo, U. C., Ognibene, D. T., da Costa, C. A., Filgueiras, C. C., Manhães, A. C., Daleprane, J. B., Resende, A. d. C., & de Bem, G. F. (2025). Alpinia zerumbet Extract Mitigates PCB 126-Induced Neurotoxicity and Locomotor Impairment in Adult Male Mice. Scientia Pharmaceutica, 93(2), 23. https://doi.org/10.3390/scipharm93020023









