Abstract
Background: Colorectal cancer (CRC) is one of the most prevalent cancers worldwide, and current treatments have significant side effects. Cannabidiol (CBD), a compound derived from Cannabis sativa, has demonstrated promising anticancer properties. However, further investigation is required to elucidate its underlying molecular mechanisms. Methods: Network pharmacology and molecular docking analysis approaches were utilized. Molecular targets of CBD and CRC-associated genes were identified using the Swiss Target Prediction, Malacards, and DisGeNet databases. Protein–protein interactions were analyzed using the STRING and Cytoscape. Ontology enrichment was conducted using ShinyGO, and gene expression and immune infiltration were evaluated with UALCAN and TISIDB. Results: We found 95 common genes between CRC and CBD targets. Six major genes (ANXA5, IGF1R, JAK2, MAPK8, MDM2, and PARP1) were particularly interesting due to their high connectivity and role in relevant metabolic pathways. The results of the molecular docking analysis indicated that CBD interacts favorably with these genes, modulating critical pathways such as RAS/MAPK and PI3K-AKT/FoxO, which are involved in cell proliferation, apoptosis, and cell cycle regulation. ANXA5 and JAK2 were identified as particularly relevant, as they correlated significantly with immune cell infiltration, suggesting a role in the immunoregulation of the tumor microenvironment. Conclusions: CBD has the potential to modulate key molecular processes in CRC through specific pathways and core genes, presenting itself as a possible complementary therapy to improve efficacy and reduce the adverse effects of conventional treatments.
1. Introduction
Colorectal cancer (CRC) is the uncontrolled, cancerous growth of cells in the colon and rectum that form at the lower end of the digestive tract [1]. CRC is among the five most prevalent types of cancer globally. In 2022, an estimated 1.9 million new CRC cases and 900,000 deaths were reported [2]. In Mexico, the mortality rate increased annually by 1.3% in women and 2.7% in men between 1998 and 2018 [3].
The standard treatment for CRC is the classic or laparoscopic resection of the tumor with the removal of the surrounding lymph nodes. In some instances, radiation therapy may be an alternative. In addition, chemotherapy is used, using drugs such as 5-fluorouracil modulated with folinic acid, irinotecan, and oxaliplatin, as well as molecularly targeted drugs such as bevacizumab, cetuximab, and panitumumab, among others. Furthermore, both chemotherapy and radiotherapy have been documented to elicit a considerable number of adverse effects [4]. Consequently, pursuing alternative therapeutic modalities that mitigate these adverse effects and facilitate the management of CRC represents a vibrant and active area of investigation.
Alternative treatments include nanomedicine, which employs selenium and silver nanoparticles [5,6]. Additionally, the administration of vitamins, minerals, and plant extracts (e.g., Curcuma longa, Panax quinquefolius, Emilia sonchifolia, and Cannabis sativa) and their derived compounds (e.g., carotenoids and flavonoids) for the treatment of CRC has been investigated [7,8]. Several studies have demonstrated that a compound found in the Cannabis sativa plant, cannabidiol (CBD), has beneficial effects on CRC by regulating apoptosis, proliferation, autophagy, and tumor size [9,10,11]. Furthermore, it has been documented that CBD can modulate the activity of immune cells, including natural killer cells, in colorectal cancer cells [12]. Nevertheless, the precise molecular mechanism through which CBD exerts its effects remains unclear, underscoring the need for further investigation.
The application of network pharmacology to medicinal plants for drug discovery represents a significant departure from the traditional “one disease–one target–one drug” model. Network pharmacology permits the examination of plant bioactive compounds and their interactions with intricate biological networks, thereby facilitating the development of treatments that are more accessible and have fewer adverse effects. Concentrating on the modulation of signaling modules as opposed to a solitary molecular target enables the implementation of more exact and efficacious therapies by utilizing combinations of drugs that act synergistically on multiple targets. This methodology depends upon several databases and bioinformatics tools, including InterPro, KEGG, and PubChem, which are fundamental to analyzing protein–protein interaction networks and biological pathways [13,14].
Several other methodologies, including molecular docking, support the field of network pharmacology. Molecular docking is a computational technique that simulates the binding of a small molecule to a biological macromolecule, providing information about the structure and energy of the formed complex. It is also possible to predict interactions between two macromolecules, such as protein–protein or protein–DNA, through this technique. It is a crucial tool in drug discovery and nutraceutical analysis. Although less accurate than experimental methods, it is notable for its speed and low cost, with continuous improvements through machine learning [15,16].
Therefore, the objective of this research is to investigate the mechanism of action of cannabidiol on colorectal cancer through a network pharmacology approach, relying on molecular docking analysis.
2. Materials and Methods
2.1. Data Acquisition
The chemical structure and SMILE of CBD were obtained from the PubChem database [17]. Then, the targets of this molecule were inferred using the Swiss Target Prediction [18] and PharmMapper Server [19] databases. In the first database, the results were filtered for those with a probability ≥ 0.1, and, in the second database, a normalized fit score ≥ 0.5 filter was used. For both databases, only the targets for Homo sapiens were considered.
On the other hand, we searched for genes associated with CRC in three databases: Malacards [20], DisGeNet [21], and CTD (Comparative Toxicogenomics Database) [22]. In the case of DisGeNet, we filtered the results using a cutoff point of Score_gda ≥ 0.1; and, in all three databases, only the species Homo sapiens was considered.
Finally, common genes were obtained by calculating the intersection between CRC-associated genes and cannabidiol targets using the online tool venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/, accessed on 5 July 2024), once duplicate genes and targets were removed.
2.2. Enrichment of Gene Ontology and Metabolic Pathways
The enrichment analysis of the 95 common genes was conducted using the ShinyGO 0.81 platform [23]. The enrichment analysis encompassed biological process (BP), molecular function (MF), cellular component (CC), and Kyoto Encyclopedia of Genes and Genomes (KEGG). The results were limited to a maximum of 5000 pathways and a minimum of 2, in addition to the species Homo sapiens. The False Discovery Rate (FDR) adjustment of p values was conducted using the Benjamini–Hochberg method. Subsequently, the results were visualized and graphed using the SRplot platform (https://www.bioinformatics.com.cn/en, accessed on 5 November 2024) [24].
2.3. Construction of Protein–Protein Interaction Network
We constructed a protein–protein interaction (PPI) network for the identified drug targets using the STRING database [25], with a specific focus on the species Homo sapiens. To ensure the reliability of the information, we set a confidence score threshold of >0.7. Subsequently, we imported the results obtained from the PPI network into Cytoscape v 3.10.3 for network generation and further analysis [26]. To identify highly interconnected regions within the PPI network, we utilized the Cytoscape plugin cytoHubba [27].
2.4. Molecular Docking Analysis Between Cannabidiol and Hub Genes
In the present study, we employed molecular docking to assess the viability of potential targets for CRC, with a particular focus on CBD. We used two sources to obtain the 3D structures of the hub proteins and two pieces of software to perform molecular docking.
In the first analysis, the structure of the hub proteins was obtained from The Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) [28]. The files used were 6k22 for ANXA5, 3nw5 for IGFR1, 6vgl for JAK2, 3pze for MAPK8, 4hbm for MDM2, and 7kk2 for PARP1. The structures were prepared for docking by removing water molecules and co-crystallized ligands and adding polar hydrogens using BIOVIA Discovery Studio. The chemical structure of CBD was obtained from the PubChem database in SDF file format; then, it was imported into Open Babel within PyRx, and energy minimization was performed by adding charges and optimizing using the universal force field. The molecular docking process was executed using Autodock Vina using PyRx 0.8 [29]. By performing eight iterations and maximizing the grid spacing, we then select the best pose for further analysis.
We also obtained the PBD files of the targets from the AlphaFold Protein Structure Database [30], limiting the results to the Homo sapiens organism and considering only those structures that had undergone review. The CB-Dock2 platform was used to perform the blind molecular docking simulation [31], selecting the results with the lowest score. The binding energy values for the docked ligand structures were recorded in -kcal/mol. BIOVIA Discovery Studio viewer (v21.1.0.20298) was used to visualize the PDB files and generate 2D interaction images of the docking results.
2.5. Analysis of Expression and Infiltration of Immune Cells
The UALCAN database was utilized to examine the disparities in the expression of hub genes between CRC tissues and adjacent non-cancerous tissues [32]. Furthermore, the Tumor Immune System Interaction Database (TISIDB) tool was employed to conduct a correlation analysis between hub genes and the infiltration of specific immune cells, as well as the expression of select immunological molecules [33], both analyses for colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ). Comparison of expression between cancerous and non-cancerous tissue was performed using the Student’s t-test. Spearman’s test was used for correlations; a p < 0.05 was taken as significant in both analyses.
3. Results
3.1. Analysis of Cannabidiol Targets and CRC-Associated Genes
We used the PubChem database to obtain the structure of CBD (Figure 1A). We identified 204 targets from the Swiss Target prediction and PharmMapper Server databases. On the other hand, the genes associated with CRC were 1881, using the Malacards, DisGeNet, and CTD databases; by performing an intersection between these molecules, we found 95 common ones (Figure 1B).
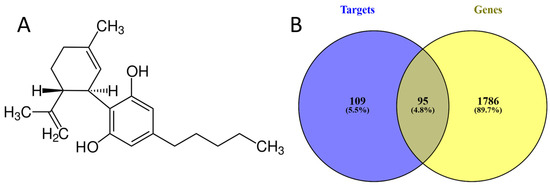
Figure 1.
(A) Molecular structure of cannabidiol. (B) Common molecules between cannabidiol targets and CRC-associated genes.
3.2. Gene Ontology Enrichment Analysis and KEEG Pathways
We performed a gene ontology analysis with the use of 95 common genes. The results showed that the enriched biological processes were mainly associated with apoptosis and a response to various substances, such as chemicals, organic substances, and organic cyclic compounds. Regarding the enrichment of cellular components, the results showed that common genes are mainly expressed in the cell membrane, such as raft and microdomains, as well as in vesicles. Finally, molecular functions were enriched primarily on kinase activity and nucleotide binding (Figure 2).
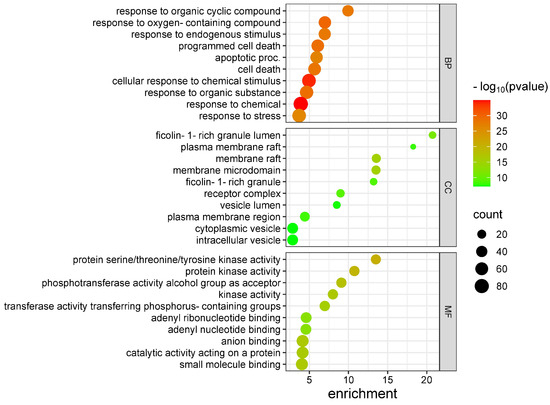
Figure 2.
Gene Ontology Enrichment Analysis. BP: biological process. CC: cellular component. MF: molecular function.
The analysis of KEEG pathway enrichment showed a wide variety of metabolic pathways, the most important of which are the FoxO, RAS, MAPK, and PI3K-AKT signaling pathways. These pathways are also related to the immune and endocrine systems, such as the IL-17 signaling pathway, lectin receptor, relaxin, progestin, and estrogens. Other signaling pathways are related to diseases, including Helicobacter pylori infection, atherosclerosis, and various types of cancer (Figure 3).
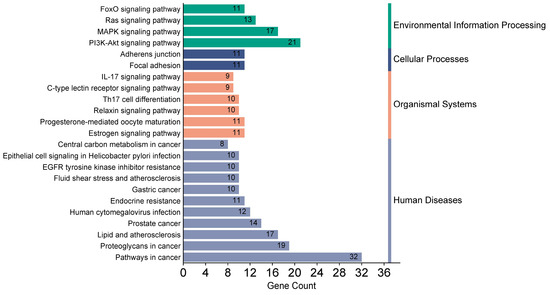
Figure 3.
Enrichment analysis of KEEG pathways.
3.3. Construction of the Protein–Protein Interaction Network and Prediction of Hub Genes
The protein–protein interaction network was analyzed on the Cytoscape platform. This network’s nodes represent proteins, and the edges represent interactions. The network had 1015 interactions, and Figure 4 shows the 95 proteins ordered from the highest to the lowest number of interactions.
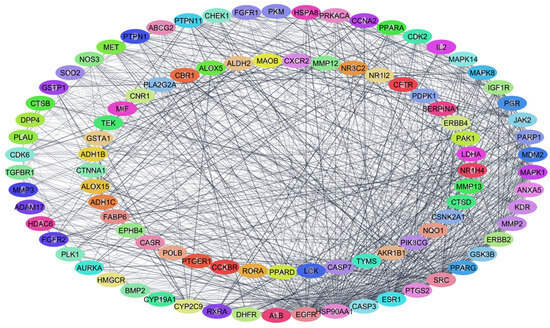
Figure 4.
Protein–protein interaction network constructed from common genes.
To find the proteins that could be the primary therapeutic targets of CBD in CRC, we filtered the proteins according to different topological algorithms, such as their number of interactions (degree), Maximum Neighborhood Component (MNC), Density of Maximum Neighborhood Component (DMNC), and Maximal Clique Centrality (MCC). These metrics help analyze biological networks to identify key nodes in biological processes. The degree calculates the total number of direct connections of a node. The MNC is a measure that evaluates the importance of a node in a biological network based on the size of the largest interaction group (neighborhood) in which it participates. The DMNC is an extension of the MNC that considers the size of the largest neighborhood and the density of that cluster. The MCC is a measure that evaluates the importance of a node based on its participation in clusters of nodes where all are connected, and no more nodes can be added without losing that property. In each of these analyses, we obtained the top 20 proteins. Then, we performed a Venn diagram, from which we obtained six proteins in common: annexin A5 (ANXA5), insulin-like growth factor 1 receptor (IGF1R), Janus kinase 2 (JAK2), mitogen-activated protein kinase 8 (MAPK8), MDM2 proto-oncogene, E3 ubiquitin-protein ligase (MDM2), and poly (ADP-ribose) polymerase 1 (PARP1). Figure 5 shows how the six hub genes were obtained.
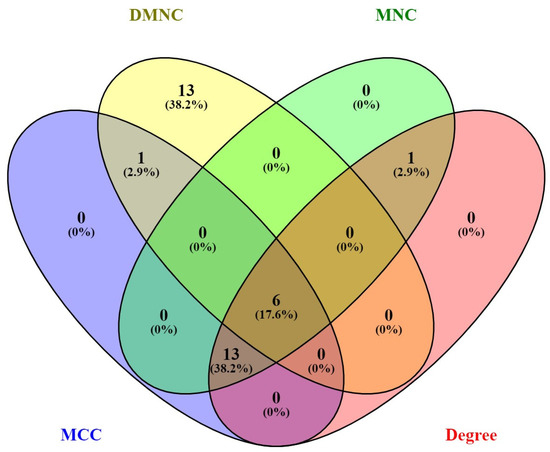
Figure 5.
Venn diagram to obtain the six hub genes.
3.4. Molecular Docking Between Cannabidiol and Hub Genes
We performed a molecular docking analysis between CBD and each hub protein (ANXA5, IGF1R, JAK2, MAPK8, MDM2, and PARP1). As illustrated in Table 1, the predicted binding energies (in kcal/mol) for the interaction between CBD and six target proteins were evaluated using two docking methods (AutodockVina and CB-Dock2) and two sources of protein structures (RCSB, experimental structures, and AlphaFold, computational models). Negative values indicate a higher binding affinity, and values lower than −5 are considered stable interactions. In the case of MDM2, the CB-Dock2 combination with RCSB structures obtained the most favorable binding energy (−8.2 kcal/mol). This contrasts with other combinations, where the differences between combinations vary in the range of 0.5–1 kcal/mol. We can infer that the six hub genes bind stably to CBD. Furthermore, we visualized the interactions between the molecules in a 2D diagram, as shown in Figure 6.

Table 1.
Coupling energies (kcal/mol) of cannabidiol with target proteins.
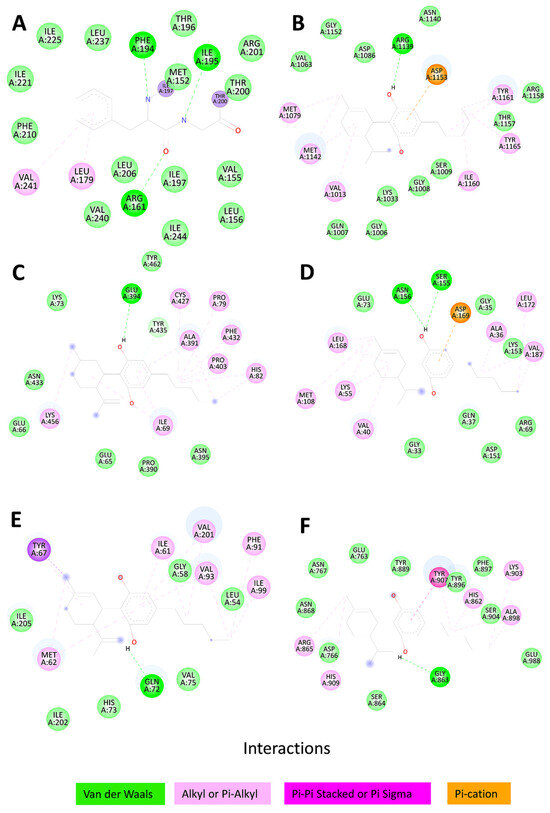
Figure 6.
2D diagram of interactions between cannabidiol and the six hub genes: (A) ANXA5, ΔG = −7.1; (B) IGF1R, ΔG = −6.8; (C) JAK2, ΔG = −7.8; (D) MAPK8, ΔG = −7.6; (E) MDM2, ΔG = −6.3; and (F) PARP1, ΔG = −8.1.
3.5. Analysis of Hub Gene Expression in CRC
The UALCAN database was employed to examine the expression of the hub genes in genomic data from the Cancer Genome Atlas (TCGA) project. In this analysis, colorectal cancer was classified into two distinct categories: colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) (Figure 7 and Figure 8).
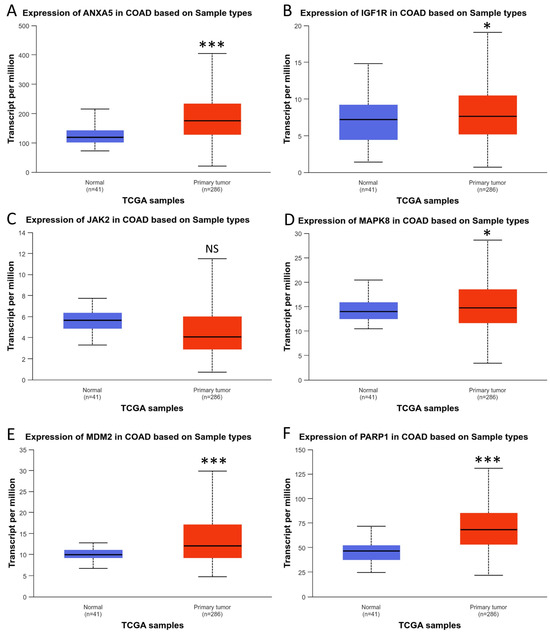
Figure 7.
Expression of hub genes in colon adenocarcinoma; (A) ANXA5, (B) IGF1R, (C) JAK2, (D) MAPK8, (E) MDM2, and (F) PARP1. * p < 0.05; *** p < 0.001. NS: Not significant.
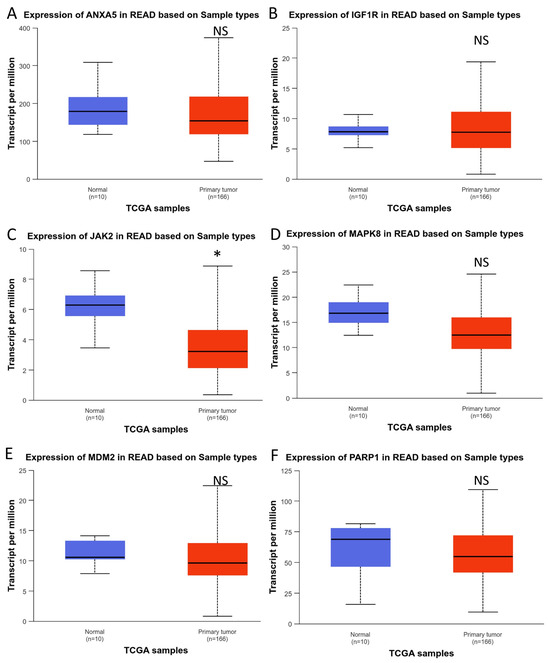
Figure 8.
Expression of hub genes in rectum adenocarcinoma: (A) ANXA5, (B) IGF1R, (C) JAK2, (D) MAPK8, (E) MDM2, and (F) PARP1. * p < 0.05. NS: Not significant.
As can be observed in Figure 7, five of the six hub genes were differentially upregulated in colon tumor tissue, except for JAK2, which did not show differential expression. When we performed the analysis on rectal tumor tissue, we found results opposite to those in the colon. Only one of the five hub genes, JAK2, was found to be differentially expressed. In any case, these results show that the six hub genes are differentially expressed in CRC.
3.6. Immune Cell Infiltration Analysis
A correlation analysis was conducted between the expression of hub genes and immune cells, including CD8 T cells, Th1, Th2, Th17, macrophages, and others. In addition, the analysis considered molecules such as IL10, TGB1, IL6, and CXCL8. The study was performed in COAD and READ, with the TISIDB database as the data source (Figure 9 and Figure 10).
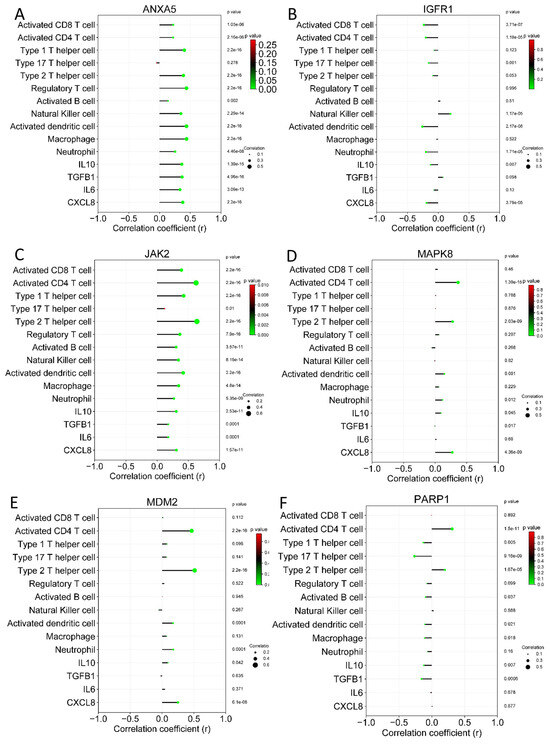
Figure 9.
Correlation analysis between hub genes and immune cells on colon adenocarcinoma. (A) ANXA5, (B) IGF1R, (C) JAK2, (D) MAPK8, (E) MDM2, and (F) PARP1.
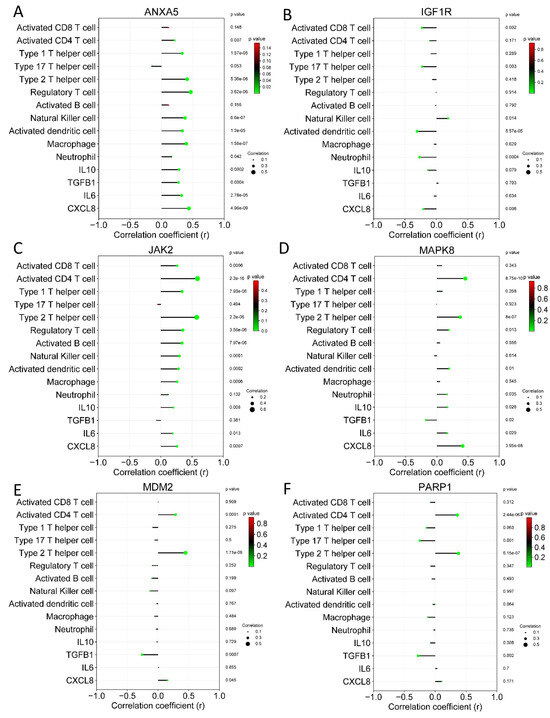
Figure 10.
Correlation analysis between hub genes and immune cells on rectum adenocarcinoma. (A) ANXA5, (B) IGF1R, (C) JAK2, (D) MAPK8, (E) MDM2, and (F) PARP1.
As shown in Figure 9, the ANXA5 and JAK2 genes showed the most significant correlations with immune cells and molecules in colon adenocarcinoma. These results were similar to those shown in rectum adenocarcinoma; once more, ANXA5 and JAK2 were the genes with the most significant correlations. With these results, it is inferred that cannabidiol can also mediate the antitumor immune response through ANXA5 and JAK2.
4. Discussion
The objective of this study was to investigate the mechanism of action of cannabidiol in colorectal cancer. The results demonstrated that CBD interacts with 95 distinct molecular targets in CRC, influencing processes such as apoptosis, chemical response, and kinase activity. These interactions are predominantly localized to the cell membrane. Furthermore, they are involved in the metabolic pathways of RAS, MAPK, FoxO, and PI3K-AKT and with the immune response through IL-17. Six hub genes, ANXA5, IGF1R, JAK2, MAPK8, MDM2, and PARP1, which bind favorably to CBD, are differentially expressed in CRC tumor tissue and correlate with immune cell infiltration.
Several studies have employed a network pharmacology approach to investigate the effects of CBD. Previous research on Cannabis sativa, particularly CBD, has indicated that CBD may exert beneficial effects on various types of cancer, including CRC [34,35]. In addition, in silico research has demonstrated that CBD can regulate inflammation, a process closely associated with cancer, suggesting its potential to impact several types of cancer, including CRC [36,37]. Research employing a network pharmacology approach to examine the effects of CBD on ovarian cancer has also been conducted. This study reports that CBD can regulate ovarian cancer through the PARP1 protein, a hub protein identified in our research [38]. Consequently, we can infer that CBD acts on CRC and other types of cancer or cancer-related biological processes, such as inflammation, using silico techniques.
A Gene Ontology enrichment analysis of the 95 molecular targets of cannabidiol revealed an enrichment in apoptotic processes. Previous reports have indicated that CBD can reduce anti-apoptotic molecules’ expression and enhance pro-apoptotic molecules’ expression in CRC cell lines. This mechanism is potentially mediated by reactive oxygen species [9,10,39]. Another mechanism by which CBD can induce CRC cell apoptosis is through the regulation of protein kinases, including, but not limited to, cyclin-dependent kinase 2 (CDK2), CDK4, CDK6, mitogen-activated protein kinase (MAPK), and protein kinase R (PERK) [9,10,40]. In addition, an enrichment analysis showed that the therapeutic targets of CBD are expressed in the cell membrane. A previous study showed that cannabidiol could exert its effect through molecules expressed in the cell membrane of CRC cells [12].
We also found an enrichment of the RAS and MAPK pathways. These pathways regulate cell cycle, cell growth, differentiation, metabolism, and senescence. An in silico and experimental analysis concluded that CBD can inhibit the MAPK pathway through RAS and bind to EGFR [10,41]. The PI3K-AKT signaling pathway, along with FoxO activation, is involved in the regulation of cell growth, proliferation, and survival. CBD exerts anticancer effects against colon cancer through down-regulating Akt and, thus, FoxO. Therefore, our results show that the anticancer effect of CBD is mediated through the RAS/MAPK and PI3K-AKT/FoxO metabolic pathways, which is supported by previous research [42].
Regarding immune-related metabolic pathways, we found the IL-17 signaling pathway to be enriched. It is well-known that IL-17 plays an important role in CRC metastasis and prognosis [43]. CBD reduces IL-17A-mediated mucosal damage, providing evidence that endocannabinoids may offer mucosal protection in inflammation [44]. It can be reasonably inferred that CBD exerts a protective effect on CRC through IL-17.
A protein–protein interaction network analysis yielded six hub genes. The initial candidate was ANXA5. This molecule has been linked to the advanced tumor stage and an unfavorable prognosis in colorectal adenocarcinomas [45]. To the best of our knowledge, no reports indicate that CBD exerts regulatory effects on ANXA5. However, our molecular docking analysis revealed a favorable binding interaction between these two molecules. It may, therefore, be speculated that CBD exerts its regulatory effect on CRC through ANXA5. Another hub gene, IGF1R, has been identified as a potential therapeutic target in CRC [46]. Previous studies have demonstrated that co-treatment with CBD and IGF-1 partially attenuates the cannabidiol-mediated antiproliferative effect, likely disrupting the interaction between CBD and IGF-1R [47]. JAK2 represents another gene hub, and it has been proposed that the blockade of the IL-6/JAK2/STAT3 signaling axis and its biological effects may constitute a potential treatment strategy for CRC [48]. To the best of our knowledge, there are no reports indicating that cannabidiol regulates JAK2. However, it has been proposed that CBD alleviates psoriasis-like symptoms in mice and may serve as a promising therapeutic agent for psoriasis by targeting the JAK2-STAT3 pathway [49]. It can thus be postulated that CBD exerts an antitumor effect through IGF1R and JAK2.
Several bioinformatics studies have indicated that MAPK8, another hub gene, may represent a potential therapeutic target in CRC [50,51]. Additionally, in silico and in vitro studies have suggested that MAPK8 may be regulated by CBD [10,52]. A genetic variant, rs2279744, of MDM2 has been associated with an increased risk of CRC [53]. While no reports indicate a link between CBD and cancer, research in human gingival mesenchymal stem cells demonstrated CBD can reduce MDM2 expression [54]. Conversely, evidence suggests CBDl only markedly elevates ROS production in cells with wild-type p53 (HCT116 and LS174T), linked to an accumulation of PARP1 [55]. Therefore, CBD can exert its regulatory effect on CRC through MAPK8, MDM2, and PARP1, a hypothesis corroborated by our molecular docking analysis results.
Subsequently, the expression of hub genes in tumor and non-tumor tissue was validated using the UALCAN database. It is noteworthy that JAK2 was not found to be differentially expressed in colon adenocarcinoma; however, it was found to be differentially expressed in rectum adenocarcinoma. In light of these findings, it can be concluded that the six hub genes are differentially expressed in CRC.
The molecular docking results suggest that the origin of the protein structure (experimental vs. predicted model) can influence the molecular docking results. In some cases, as in IGF1R and MDM2, RCSB structures favor a higher binding affinity, possibly due to a higher fidelity in the representation of the active sites. Conversely, AlphaFold structures yield superior outcomes in other instances, such as with ANXA5 and MAPK8. This may be attributable to enhanced predictions of flexible regions or more open conformations within the binding pocket. Conversely, CB-Dock2, in conjunction with RCSB or AlphaFold structures, often produces more negative values in certain instances (e.g., MDM2 and PARP1), indicating that its scoring function or search algorithm may identify interactions that AutodockVina does not evaluate as accurately in those combinations. However, it is important to recognize that discrepancies within the 0.5–1 kcal/mol range may arise from the margin of error inherent in molecular docking methods. Therefore, the available data offer a valuable opportunity to explore the potential influence of software and protein sources on the accuracy of binding affinity predictions for CBD. It is not possible to make an unequivocal assertion that one software is superior to another or that a particular protein structure source (AlphaFold or RCSB) is inherently superior. The results demonstrate that the prediction’s effectiveness depends on the specific protein under evaluation and the combination employed.
A correlation analysis of hub genes with immune cells and molecules in COAD and READ demonstrated that ANXA5 and JAK2 exhibited the highest and most significant correlations. ANXA5 functions as a mediator between the innate and adaptive immune systems, thereby contributing to an immunostimulatory profile within the antitumor environment. To date, no reports have documented the role of ANXA5 in the antitumor response in CRC. Nevertheless, in other forms of cancer, such as hepatocellular carcinoma and head and neck squamous cell carcinoma, ANXA5 seems to be a crucial element in the antitumor immune response [56,57]. The observed correlations between JAK2 and immune cells and molecules are not unexpected, given the involvement of this molecule in the cytokine signaling pathway, which plays a role in cancer, including CRC [48]. Although CBD is a molecule widely recognized as a regulator of the immune system [58,59], what is particularly noteworthy about these results is that it exerts this regulatory effect through ANXA5 and JAK2, particularly in the context of CRC.
Ultimately, the results have led to the formulation of a molecular mechanism of action through which CBD influences CRC. This mechanism centers on the hub genes and the most illustrative metabolic pathways. The mechanism mentioned above is illustrated in Figure 11.
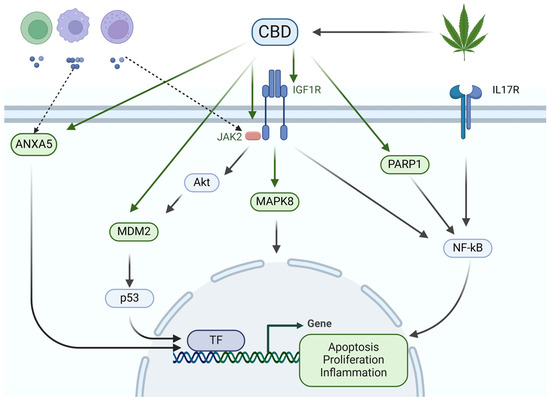
Figure 11.
Molecular mechanism of action of cannabidiol on colorectal cancer. Cannabis sativa contains cannabidiol (CBD), which can bind to ANXA5 and MDM2, thereby regulating the apoptosis of colorectal cancer (CRC) cells. Additionally, CBD can bind to IGF1R, with the assistance of JAK2 and Akt, regulating MAPK8 and MDM2. This, in turn, activates various transcription factors (TFs) that regulate CRC cell apoptosis and proliferation. Similarly, CBD binds to PARP1 to regulate NF-κB, part of the IL-17 signaling pathway, controlling the inflammatory process in CRC. Ultimately, ANXA5 and JAK2 are associated with infiltrating immune cells and molecules into cancerous tissue. The hub molecules are highlighted in green.
While this work presents novel data, it is essential that we acknowledge the inherent limitations of in silico studies. A primary limitation of the study is its exclusive reliance on silico methods, which, while enabling the identification of potential molecular interactions between CBD and genes associated with colorectal cancer, do not substitute for experimental validation to substantiate these findings. Additionally, while protein–protein interaction analysis and molecular docking techniques offer valuable predictions on the affinity and modulation of pathways such as RAS/MAPK and PI3K-AKT/FoxO, these results require confirmation by in vitro and in vivo studies to assess their functional and clinical relevance. Moreover, the computational tools may not fully capture the inherent complexity of the tumor microenvironment and heterogeneity of colorectal cancer, limiting the extrapolation of these results to more complex biological contexts. Additionally, further investigation is required to ascertain the optimal route of administration of CBD and to determine whether there are any interactions with other drugs or foods. These issues have been previously explored in other research. Some investigators suggest that intranasal administration and inhalation allow for faster release to the plasma and brain, whereas transmucosal, oral, and transdermal routes allow for a slower release [60]. Additionally, when CBD is administered orally, its absorption can be increased by food, possibly due to lipids in the food [61,62]. It is also important to note that this type of compound should be used as an adjuvant rather than a first-line drug. Finally, our results contribute to the growing body of evidence elucidating the molecular mechanism of cannabidiol in the context of colorectal cancer.
5. Conclusions
The study showed that cannabidiol interacts with 95 different molecular targets in colorectal cancer, influencing processes like programmed cell death, chemical response, and kinase activity. These interactions are mainly found at the cell membrane. Furthermore, they are implicated in the RAS, MAPK, FoxO, and PI3K-AKT metabolic pathways and the immune response through IL-17. Cannabidiol interacts favorably with six key genes—ANXA5, IGF1R, JAK2, MAPK8, MDM2, and PARP1—expressed differently in colorectal cancer. This variation is associated with immune cell infiltration, notably with ANXA5 and JAK2.
Author Contributions
Conceptualization, J.M.G.-F.; methodology, J.M.G.-F., F.M.-E., A.A.-A., G.T.A.-R. and M.F.G.-A.; software, J.M.G.-F., F.M.-E., A.A.-A., G.T.A.-R. and M.F.G.-A.; formal analysis, J.M.G.-F., F.M.-E., A.A.-A., G.T.A.-R. and M.F.G.-A.; investigation, J.M.G.-F., F.M.-E., A.A.-A., G.T.A.-R. and M.F.G.-A.; writing—original draft preparation, J.M.G.-F.; writing—review and editing, J.M.G.-F., F.M.-E., A.A.-A., G.T.A.-R. and M.F.G.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
The authors acknowledge the support from Universidad de Guadalajara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Z.; Wang, X.; Zhou, H.; Jiang, M.; Wang, J.; Sui, B. Molecular Complexity of Colorectal Cancer: Pathways, Biomarkers, and Therapeutic Strategies. Cancer Manag. Res. 2024, 16, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Espinosa-Tamez, P.; Suazo-Zepeda, E.; Sanchez-Blas, H.; Meneses-Medina, M.; Huitzil-Melendez, F.D.; Van Loon, K.; Potter, M.; Lajous, M. National and state-level colorectal cancer mortality trends in Mexico, 1998–2018. Salud Publica Mex. 2021, 64, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Religioni, U.; Czerw, A.; Deptala, A.; Karakiewicz, B.; Partyka, O.; Pajewska, M.; Sygit, K.; Cipora, E.; Kmiec, K.; et al. Nutritional Treatment of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 6881. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esquivias, F.; Gutierrez-Angulo, M.; Becerra-Ruiz, J.S.; Martinez-Perez, L.A.; de la Cruz-Ahumada, C.J.; Guzman-Flores, J.M. Bioinformatic Analysis of the Effect of Silver Nanoparticles on Colorectal Cancer Cell Line. Biomed. Res. Int. 2022, 2022, 6828837. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esquivias, F.; Gutierrez-Angulo, M.; Perez-Larios, A.; Sanchez-Burgos, J.A.; Becerra-Ruiz, J.S.; Guzman-Flores, J.M. Anticancer Activity of Selenium Nanoparticles In Vitro Studies. Anticancer Agents Med. Chem. 2022, 22, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef] [PubMed]
- Silva-Reis, R.; Silva, A.M.S.; Oliveira, P.A.; Cardoso, S.M. Antitumor Effects of Cannabis sativa Bioactive Compounds on Colorectal Carcinogenesis. Biomolecules 2023, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Tamia, G.; Song, H.J.; Amarakoon, D.; Wei, C.I.; Lee, S.H. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int. Immunopharmacol. 2022, 108, 108865. [Google Scholar] [CrossRef]
- Kim, N.Y.; Mohan, C.D.; Sethi, G.; Ahn, K.S. Cannabidiol activates MAPK pathway to induce apoptosis, paraptosis, and autophagy in colorectal cancer cells. J. Cell Biochem. 2024, 125, e30537. [Google Scholar] [CrossRef]
- Orrego-Gonzalez, E.; Londono-Tobon, L.; Ardila-Gonzalez, J.; Polania-Tovar, D.; Valencia-Cardenas, A.; Velez-Van Meerbeke, A. Cannabinoid Effects on Experimental Colorectal Cancer Models Reduce Aberrant Crypt Foci (ACF) and Tumor Volume: A Systematic Review. Evid. Based Complement. Alternat. Med. 2020, 2020, 2371527. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bashiri Dezfouli, A.; Multhoff, G. The immunomodulatory effects of cannabidiol on Hsp70-activated NK cells and tumor target cells. Mol. Immunol. 2024, 174, 1–10. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Tahir Ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The Art and Science of Molecular Docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Iny Stein, T.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017, 45, D877–D887. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Ramirez-Anguita, J.M.; Sauch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative toxicogenomics database’s 20th anniversary: Update 2025. Nucleic Acids Res. 2024, 53, D1724. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhatt, R.; Bhikadiya, C.; Bi, C.; Biester, A.; Biswas, P.; Bittrich, S.; Blaumann, S.; Brown, R.; Chao, H.; et al. Updated resources for exploring experimentally-determined PDB structures and Computed Structure Models at the RCSB Protein Data Bank. Nucleic Acids Res. 2025, 53, D564–D574. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Madhukar Kudke, A.; Joseph Nepveux V, F.; Xu, Y. Network-Based Pharmacology Study Reveals Protein Targets for Medical Benefits and Harms of Cannabinoids in Humans. Appl. Sci. 2022, 12, 2205. [Google Scholar] [CrossRef]
- Bian, Y.M.; He, X.B.; Jing, Y.K.; Wang, L.R.; Wang, J.M.; Xie, X.Q. Computational systems pharmacology analysis of cannabidiol: A combination of chemogenomics-knowledgebase network analysis and integrated in silico modeling and simulation. Acta Pharmacol. Sin. 2019, 40, 374–386. [Google Scholar] [CrossRef]
- He, M.; Shi, J.; Wu, C.; Xu, Y.J.; Liu, Y. Integrating Lipidomics, Metabolomics, and Network Pharmacology to Reveal the Mechanism of Cannabidiol against Inflammation in High-Fat, High-Cholesterol Diet-Induced Mice. J. Agric. Food Chem. 2024, 72, 19246–19256. [Google Scholar] [CrossRef]
- Ma, H.; Xu, F.; Liu, C.; Seeram, N.P. A Network Pharmacology Approach to Identify Potential Molecular Targets for Cannabidiol’s Anti-Inflammatory Activity. Cannabis Cannabinoid Res. 2021, 6, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lin, Y.; Lin, C.; Xu, M.; Wang, J.; Zeng, Z.; Wang, P.; Li, Q.; Wang, X.; Wang, W.; et al. Cannabidiol Inhibits Epithelial Ovarian Cancer: Role of Gut Microbiome. J. Nat. Prod. 2024, 87, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, J.; Deroubaix, A.; Omar, A.; Penny, C. A Bioinformatic Analysis Predicts That Cannabidiol Could Function as a Potential Inhibitor of the MAPK Pathway in Colorectal Cancer. Curr. Issues Mol. Biol. 2024, 46, 8600–8610. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of cancer metabolism: The therapeutic potential of cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef]
- Razi, S.; Baradaran Noveiry, B.; Keshavarz-Fathi, M.; Rezaei, N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine 2019, 116, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.S.; Sia, T.C.; Wattchow, D.A.; Smid, S.D. Interleukin 17A evoked mucosal damage is attenuated by cannabidiol and anandamide in a human colonic explant model. Cytokine 2014, 65, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hao, L.Q.; Ding, F.X.; Mei, Q.; Huang, J.J.; Fu, C.G.; Yan, H.L.; Sun, S.H. Expression of annexin a5 is associated with higher tumor stage and poor prognosis in colorectal adenocarcinomas. J. Clin. Gastroenterol. 2009, 43, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Shali, H.; Ahmadi, M.; Kafil, H.S.; Dorosti, A.; Yousefi, M. IGF1R and c-met as therapeutic targets for colorectal cancer. Biomed. Pharmacother. 2016, 82, 528–536. [Google Scholar] [CrossRef] [PubMed]
- D’Aloia, A.; Ceriani, M.; Tisi, R.; Stucchi, S.; Sacco, E.; Costa, B. Cannabidiol Antiproliferative Effect in Triple-Negative Breast Cancer MDA-MB-231 Cells Is Modulated by Its Physical State and by IGF-1. Int. J. Mol. Sci. 2022, 23, 7145. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.H.; Kim, S.W.; Bang, C.H. Cannabidiol Alleviates Imiquimod-Induced Psoriasis by Inhibiting JAK2-STAT3 in a Mouse Model. Biomedicines 2024, 12, 2084. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Malekzadeh, R.; Hosseini, S.A.; Yaghoobi, H. Investigation of the therapeutic role of native plant compounds against colorectal cancer based on system biology and virtual screening. Sci. Rep. 2023, 13, 11451. [Google Scholar] [CrossRef]
- Shi, H.; Tian, S.; Tian, H. Network Pharmacology Interpretation of Fuzheng-Jiedu Decoction against Colorectal Cancer. Evid. Based Complement. Alternat. Med. 2021, 2021, 4652492. [Google Scholar] [CrossRef]
- Guzman-Flores, J.M.; Perez-Vazquez, V.; Martinez-Esquivias, F.; Isiordia-Espinoza, M.A.; Viveros-Paredes, J.M. Molecular Docking Integrated with Network Pharmacology Explores the Therapeutic Mechanism of Cannabis sativa against Type 2 Diabetes. Curr. Issues Mol. Biol. 2023, 45, 7228–7241. [Google Scholar] [CrossRef] [PubMed]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; Kandil, E.; Fawzy, M.S. Genetic polymorphisms of TP53 (rs1042522) and MDM2 (rs2279744) and colorectal cancer risk: An updated meta-analysis based on 59 case-control studies. Gene 2020, 734, 144391. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dezfouli, A.B.; Khosravi, M.; Sievert, W.; Stangl, S.; Schwab, M.; Wu, Z.; Steiger, K.; Ma, H.; Multhoff, G. Cannabidiol-induced crosstalk of apoptosis and macroautophagy in colorectal cancer cells involves p53 and Hsp70. Cell Death Discov. 2023, 9, 286. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Yao, J.; Yuan, Z.; Yan, L.; Cao, B. ANXA5: A Key Regulator of Immune Cell Infiltration in Hepatocellular Carcinoma. Med. Sci. Monit. 2024, 30, e943523. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, H.; Zhou, Z.; Guo, J. ANXA5 predicts prognosis and immune response and mediates proliferation and migration in head and neck squamous cell carcinoma. Gene 2024, 931, 148867. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Jensen, S.S.; Kolli, A.R.; Nikolajsen, G.N.; Bruun, H.Z.; Hoeng, J. Strategies to Improve Cannabidiol Bioavailability and Drug Delivery. Pharmaceuticals 2024, 17, 244. [Google Scholar] [CrossRef]
- Mozaffari, K.; Willette, S.; Lucker, B.F.; Kovar, S.E.; Holguin, F.O.; Guzman, I. The Effects of Food on Cannabidiol Bioaccessibility. Molecules 2021, 26, 3573. [Google Scholar] [CrossRef] [PubMed]
- Abbotts, K.S.S.; Ewell, T.R.; Butterklee, H.M.; Bomar, M.C.; Akagi, N.; Dooley, G.P.; Bell, C. Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function. Nutrients 2022, 14, 2152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).