Synthesis and Catalytic Activity of Novel Complexes Based on Cyano-Substituted Phthalocyanines as Promising Drug Conversion Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalytic Properties
2.2. Synthesis
3. Results
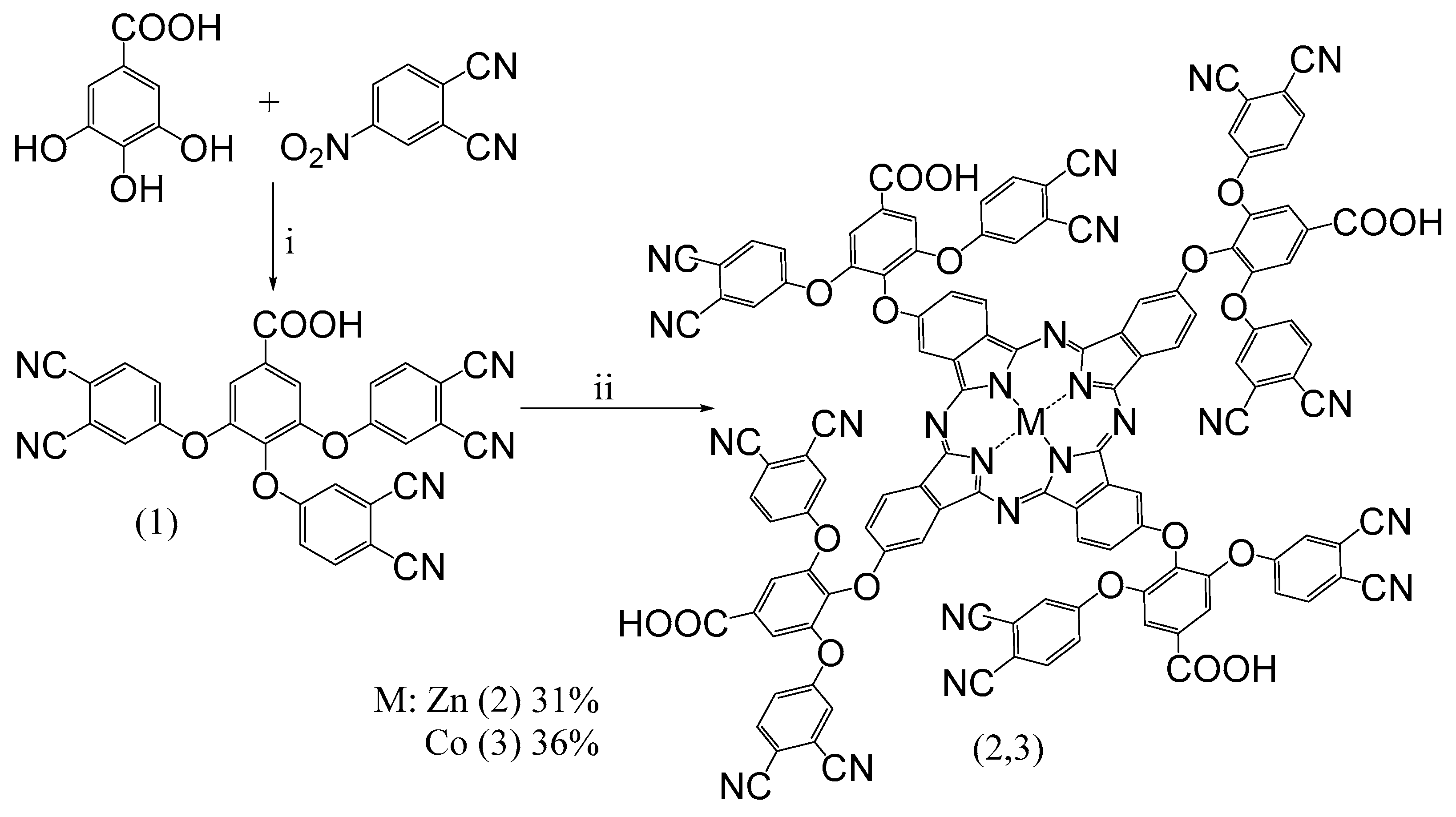
3.1. Synthesis of 3,4,5-Tris(3,4-dicyanophenoxy)benzoic Acid
3.2. Synthesis of d-Metals Phthalocyanine Complexes
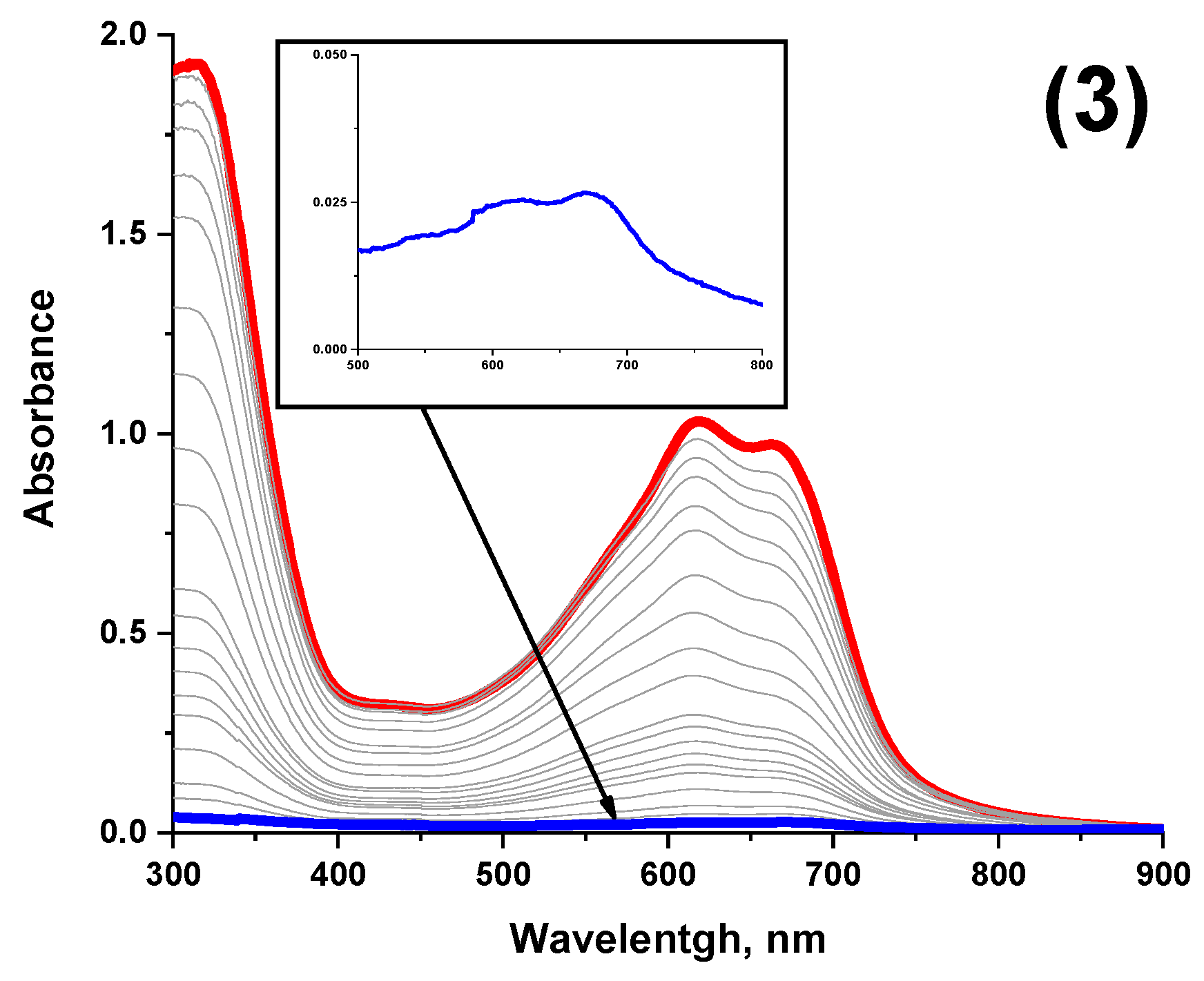
3.3. Spectroscopic Properties
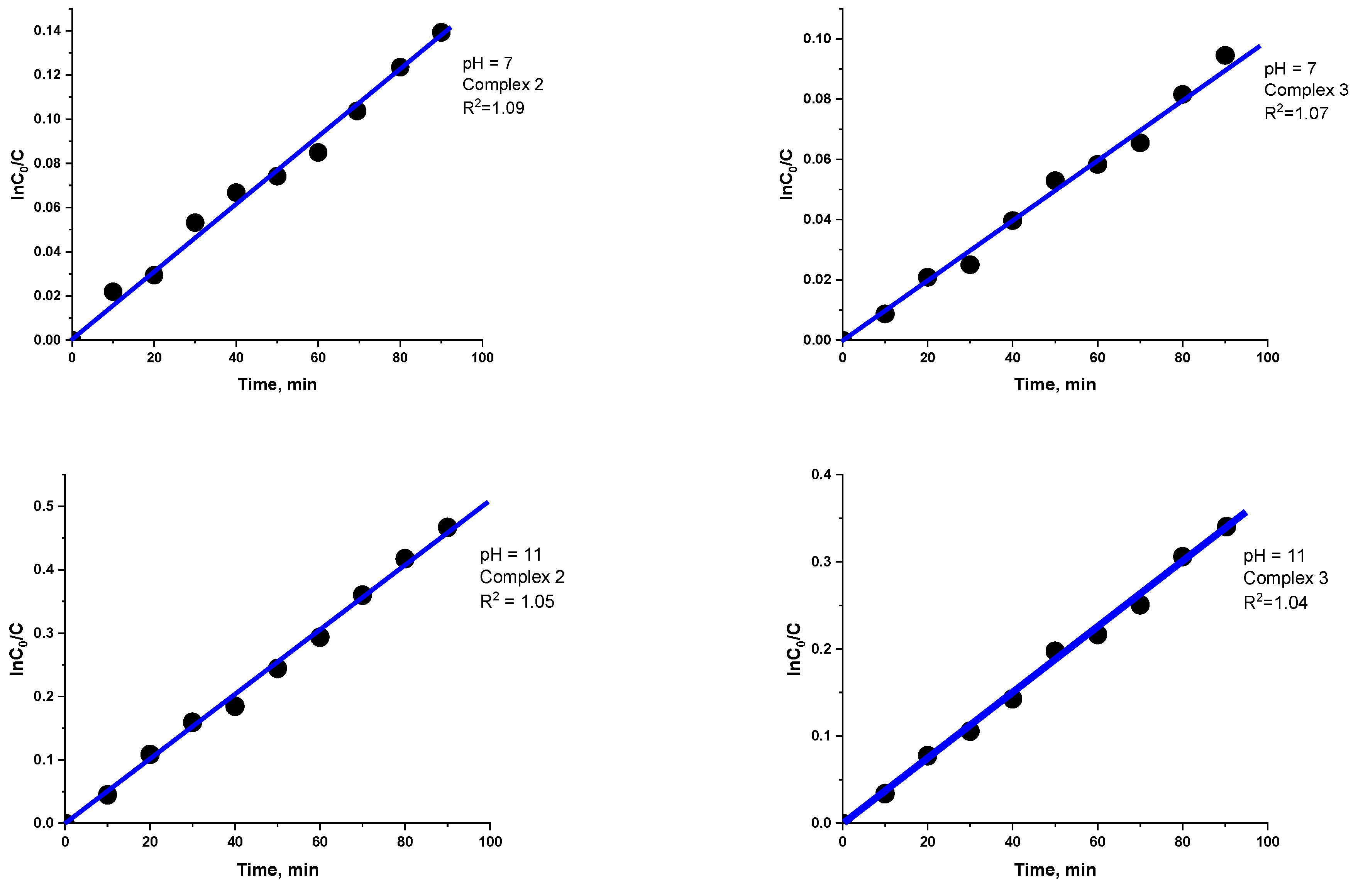
3.4. Catalytic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chick, J.; Gough, K.; Fakkowski, W.; Kerrshaw, P.; Hore, B.; Mehta, B.; Ritson, B.; Ropner, R.; Torley, D. Disulfiram treatment of alcoholism. Br. J. Psychiatry 1992, 161, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tamburin, S.; Mantovani, E.; De Bernardis, E.; Zipeto, D.; Lugoboni, F.; Agostoni, C.; Almasio, R.; Avveduti, P.; Baisini, O.; Ballerio, M.; et al. COVID-19 and Related Symptoms in Patients under Disulfiram for Alcohol Use Disorder. Intern. Emerg. Med. 2021, 16, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Voronina, A.A.; Filippova, A.A.; Znoiko, S.A.; Vashurin, A.S.; Maizlish, V.E. Effect of the Solvation Properties of the Solvent on the Formation of Associated Structures of Water-Soluble Co(II) Phthalocyanines. Russ. J. Inorg. Chem. 2015, 60, 1407–1414. [Google Scholar] [CrossRef]
- Voronina, A.A.; Kuzmin, I.A.; Vashurin, A.S.; Shaposhnikov, G.P.; Pukhovskaya, S.G.; Golubchikov, O.A. Self-Association of Sulfo Derivatives of Cobalt Phthalocyanine in Aqueous Solution. Russ. J. Gen. Chem. 2014, 84, 1777–1781. [Google Scholar] [CrossRef]
- Vashurin, A.; Marfin, Y.; Tarasyuk, I.; Kuzmin, I.; Znoyko, S.; Goncharenko, A.; Rumyantsev, E. Sulfonated Octa -Substituted Co(II) Phthalocyanines Immobilized on Silica Matrix as Catalyst for Thiuram E Synthesis. Appl. Organomet. Chem. 2018, 32, e4482. [Google Scholar] [CrossRef]
- Basova, T.V.; Belykh, D.V.; Vashurin, A.S.; Klyamer, D.D.; Koifman, O.I.; Krasnov, P.O.; Lomova, T.N.; Loukhina, I.V.; Motorina, E.V.; Pakhomov, G.L.; et al. Tetrapyrrole Macroheterocyclic Compounds. Structure–Property Relationships. J. Struct. Chem. 2023, 64, 766–852. [Google Scholar] [CrossRef]
- Chatti, I.; Ghorbel, A.; Grange, P.; Colin, J.M. Oxidation of Mercaptans in Light Oil Sweetening by Cobalt(II) Phthalocyanine-Hydrotalcite Catalysts. Catal. Today 2002, 75, 113–117. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ogumi, K.; Matsuo, Y.; Jeon, I.; Nakagawa, T.; Matsuo, Y.; Wang, H.; Ogumi, K. Recent Progress in Porphyrin- And Phthalocyanine-Containing Perovskite Solar Cells. RSC Adv. 2020, 10, 32678–32689. [Google Scholar] [CrossRef]
- Liu, X.; Qi, C.; Bing, T.; Cheng, X.; Shangguan, D. Highly Selective Phthalocyanine-Thymine Conjugate Sensor for Hg2+ Based on Target Induced Aggregation. Anal. Chem. 2009, 81, 3699–3704. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Rhee, K.Y. Phthalocyanine, Naphthalocyanine and Their Derivatives as Corrosion Inhibitors: A Review. J. Mol. Liq. 2021, 334, 116441. [Google Scholar] [CrossRef]
- Ghadari, R.; Sabri, A.; Saei, P.S.; Kong, F.T.; Marques, H.M. Phthalocyanine-Silver Nanoparticle Structures for Plasmon-Enhanced Dye-Sensitized Solar Cells. Sol. Energy 2020, 198, 283–294. [Google Scholar] [CrossRef]
- Hamad, O.A.; Kareem, R.O.; Omer, P.K. Recent Developments in Synthesize, Properties, Characterization, and Application of Phthalocyanine and Metal Phthalocyanine. J. Chem. Rev. 2024, 6, 39–75. [Google Scholar] [CrossRef]
- Ju, L.; Wan, Y.; Wang, X.; Liang, Q.; Li, Z.; Xu, S. Efficient Visible Light Photocatalytic Activitiy of Tetranitro Substituted Cobalt Phthalocyanines-Attapulgite Hybrid Materials Fabricated by Ultrasonic Impregnation Method. Optik 2016, 127, 4127–4130. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Pintus, V.; Schreiner, M. Photostability and Influence of Phthalocyanine Pigments on the Photodegradation of Acrylic Paints under Accelerated Solar Radiation. Polym. Degrad. Stab. 2017, 146, 13–23. [Google Scholar] [CrossRef]
- Duong, T.; Peng, J.; Walter, D.; Xiang, J.; Shen, H.; Chugh, D.; Lockrey, M.; Zhong, D.; Li, J.; Weber, K.; et al. Perovskite Solar Cells Employing Copper Phthalocyanine Hole-Transport Material with an Efficiency over 20% and Excellent Thermal Stability. ACS Energy Lett. 2018, 3, 2441–2448. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Z.; Zhang, J.; Jia, X.; Jiang, J.; Chen, Y.; Lin, B.; Jiang, H.; Fang, B.; Yuan, N.; et al. Interface Engineering via Phthalocyanine Decoration of Perovskite Solar Cells with High Efficiency and Stability. J. Power Sources 2019, 438, 226987. [Google Scholar] [CrossRef]
- Arokiyanathan, A.L.; Lakshmipathi, S. Impact of Functional Groups Substitution on the Molecular Properties of Magnesium and Scandium Phthalocyanines. Inorg. Chim. Acta 2018, 483, 203–210. [Google Scholar] [CrossRef]
- Kobayashi, N.; Ogata, H.; Nonaka, N.; Luk’yanets, E.A. Effect of Peripheral Substitution on the Electronic Absorption and Fluorescence Spectra of Metal-Free and Zinc Phthalocyanines. Chem. A Eur. J. 2003, 9, 5123–5134. [Google Scholar] [CrossRef]
- Kaur, P.; Sachdeva, R.; Singh, R.; Soleimanioun, N.; Singh, S.; Saini, G.S.S. Effect of Asymmetrical Peripheral Substitution of Sulfonic Acid Group on the Geometric and Electronic Structures and Vibrations of Copper Phthalocyanine Studied by Computational and Experimental Techniques. J. Mol. Struct. 2019, 1175, 314–334. [Google Scholar] [CrossRef]
- Huang, C.; Wang, K.; Sun, J.; Jiang, J. Planar Binuclear Phthalocyanine-Containing Sandwich-Type Rare-Earth Complexes: Synthesis, Spectroscopy, Electrochemistry, and NLO Properties. Eur. J. Inorg. Chem. 2014, 2014, 1546–1551. [Google Scholar] [CrossRef]
- Gorduk, S. Ferulic Acid Substituted Zn(II) Phthalocyanine: Synthesis, Characterization and Investigation of Photophysical and Photochemical Properties. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 903–918. [Google Scholar] [CrossRef]
- Leznoff, C.C. Phthalocyanines, Properties and Application; Leznoff, C.C., Lever, A.B.P., Eds.; VCH: New York, NY, USA, 1996; Volume 4, 536p. [Google Scholar]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From Outstanding Electronic Properties to Emerging Applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, Ü.; Göl, C.; Barut, B.; Bayrak, R.; Durmuş, M.; Kantekin, H.; Değirmencioğlu, İ. Peripherally and Non-Peripherally Tetra-Benzothiazole Substituted Metal-Free Zinc (II) and Lead (II) Phthalocyanines: Synthesis, Characterization, and Investigation of Photophysical and Photochemical Properties. J. Mol. Struct. 2017, 1130, 677–687. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Zhu, P.; Ng, D.K.P.; Kobayashi, N.; Jiang, J. Electron-Donating or -Withdrawing Nature of Substituents Revealed by the Electrochemistry of Metal-Free Phthalocyanines. Inorg. Chem. 2006, 45, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Klyamer, D.D.; Basova, T.V.; Krasnov, P.O.; Sukhikh, A.S. Effect of Fluorosubstitution and Central Metals on the Molecular Structure and Vibrational Spectra of Metal Phthalocyanines. J. Mol. Struct. 2019, 1189, 73–80. [Google Scholar] [CrossRef]
- Nwaji, N.; Mack, J.; Nyokong, T. Enhanced Nonlinear Optical Response of Benzothiazole Substituted Ball-Type Phthalocyanines in the Presence of Metallic Nanoparticles. Opt. Mater. 2018, 82, 93–103. [Google Scholar] [CrossRef]
- Baturhan Orman, E.; Sağlam, M.B.; Özkaya, A.R. Novel Peripherally Substituted Metal-Free, Zinc (II), and Cobalt (II) Phthalocyanines with 1,1’-Thiobis(2-Napthol) and Additional Tetraphthalonitrile Groups: Synthesis, Aggregation Behavior, Electrochemical Redox and Electrocatalytic Oxygen Reducing Prope. Synth. Met. 2020, 263, 116351. [Google Scholar] [CrossRef]
- Basova, T.; Hassan, A.; Durmuş, M.; Gürek, A.G.; Ahsen, V. Liquid Crystalline Metal Phthalocyanines: Structural Organization on the Substrate Surface. Coord. Chem. Rev. 2016, 310, 131–153. [Google Scholar] [CrossRef]
- Wang, K.; Qi, D.; Wang, H.; Cao, W.; Li, W.; Liu, T.; Duan, C.; Jiang, J. Binuclear Phthalocyanine-Based Sandwich-Type Rare Earth Complexes. Chem.-A Eur. J. 2013, 19, 11162–11166. [Google Scholar] [CrossRef]
- Erzunov, D.A.; Vashurin, A.S.; Koifman, O.I. Synthesis and Spectral Properties of Isomers of Cobalt Tetrakis (Dicyanophenoxy) Phthalocyaninate. Russ. Chem. Bull. 2018, 67, 2250–2252. [Google Scholar] [CrossRef]
- Vashurin, A.; Erzunov, D.; Kazaryan, K.; Tonkova, S.; Tikhomirova, T.; Filippova, A.; Koifman, O. Synthesis, Catalytic, Spectroscopic, Fluorescent and Coordination Properties of Dicyanophenoxy-Substituted Phthalocyaninates of d-Metals. Dye. Pigment. 2020, 174, 108018. [Google Scholar] [CrossRef]
- Erzunov, D.A.; Botnar, A.A.; Domareva, N.P.; Tikhomirova, T.V.; Vashurin, A.S. Synthesis, Spectroscopic Properties and Redox Behavior Kinetics of Rare-Earth Bistetrakis-4-[3-(3,4-Dicyanophenoxy)Phenoxy]Phthalocyaninato Metal Complexes with Er, Lu and Yb. Molecules 2021, 26, 2181. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Hong, A.P.K. Catalytic oxidation of reduced sulfur compounds by homogeneous and heterogeneous co(ii) phthalocyanine complexes. Sci. Total Environ. 1987, 64, 99–115. [Google Scholar] [CrossRef]
- Kumar, P.; Gill, K.; Kumar, S.; Ganguly, S.K.; Jain, S.L. Magnetic Fe3O4@MgAl–LDH Composite Grafted with Cobalt Phthalocyanine as an Efficient Heterogeneous Catalyst for the Oxidation of Mercaptans. J. Mol. Catal. A Chem. 2015, 401, 48–54. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-carmes, T. Dye-Sensitized Nanoparticles for Heterogeneous Photocatalysis: Cases Studies with TiO2, ZnO, Fullerene and Graphene for Water Purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Vashurin, A.; Kuzmin, I.; Mayzlish, V.; Razumov, M.; Golubchikov, O.; Koifman, O. Kinetics and Mechanism of the Oxidation of Dithiocarbamic Acids in the Presence of Co(II) Phthalocyaninetetacarboxylic Acid. J. Serbian Chem. Soc. 2016, 81, 1025–1036. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Mirzaeian, M.; Khodabakhshi, S. Synthesis of Carbon Nanotube-Supported Metallo Carboxyporphyrin as a Novel Nanocatalyst for the Mercaptan Removal. J. Nat. Gas. Sci. Eng. 2015, 25, 103–109. [Google Scholar] [CrossRef]
- Canlıca, M.; Ömür, B.C.; Salih, B. Synthesis, Photophysical, Photochemical and SO 2 Sensing Properties of Ball-Type Phthalocyanines Substituted with Carboxyl Groups. Inorg. Chem. Commun. 2019, 103, 75–81. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine Metal Complexes in Catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef]
- Vashurin, A.S.; Kuzmin, I.A.; Litova, N.A.; Petrov, O.A.; Pukhovskaya, S.G.; Golubchkov, O.A. Catalytic Properties of Cobalt Complexes with Tetrapyrazino Porphyrazine and Phthalocyanine Derivatives. Russ. J. Phys. Chem. 2014, 88, 2064–2067. [Google Scholar] [CrossRef]


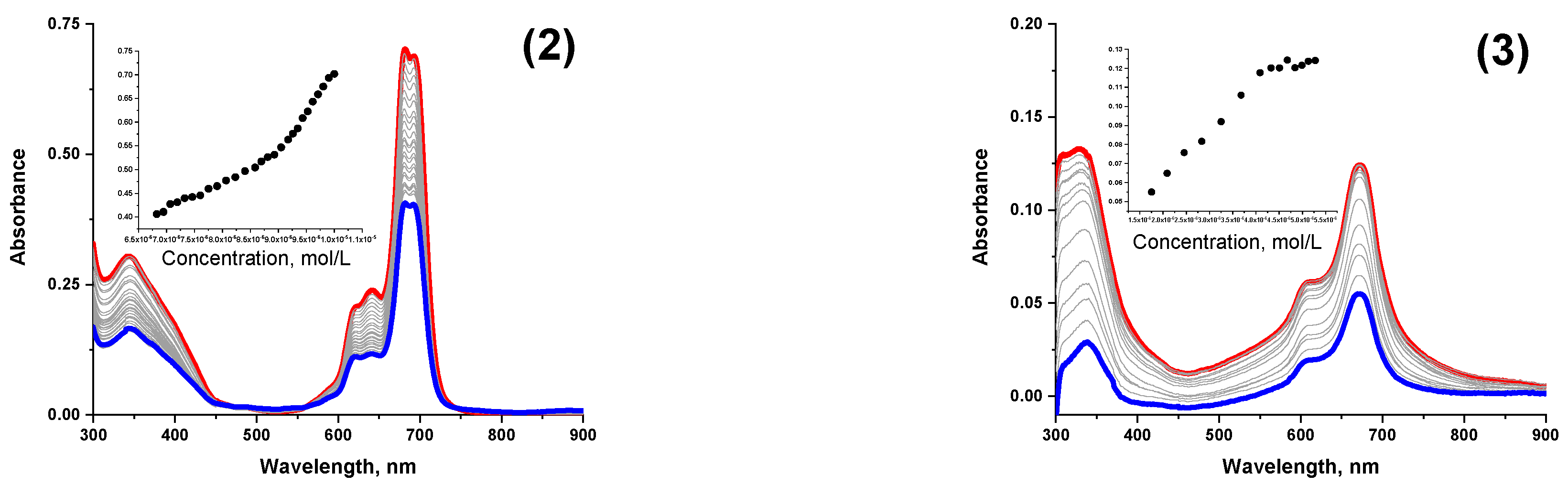

| Compound | Absorption Maximum, nm (lgε) | |||
|---|---|---|---|---|
| THF | DMF | DMSO | Pyridine | |
| 2 | 680–693 (4.83) | 686–702 (4.29) | 685 (4.61) | 688–702 (4.86) |
| 3 | 673 (4.31) | 675 (4.09) | 671 (4.18) | 680 (4.38) |
| Compounds | k, 1/min | |
|---|---|---|
| 7 pH | 11 pH | |
| 2 | 13 | 40 |
| 3 | 0.0014 | 0.0055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzunov, D.; Tonkova, S.; Sarvin, I.; Vashurin, A. Synthesis and Catalytic Activity of Novel Complexes Based on Cyano-Substituted Phthalocyanines as Promising Drug Conversion Agents. Sci. Pharm. 2024, 92, 47. https://doi.org/10.3390/scipharm92030047
Erzunov D, Tonkova S, Sarvin I, Vashurin A. Synthesis and Catalytic Activity of Novel Complexes Based on Cyano-Substituted Phthalocyanines as Promising Drug Conversion Agents. Scientia Pharmaceutica. 2024; 92(3):47. https://doi.org/10.3390/scipharm92030047
Chicago/Turabian StyleErzunov, Dmitry, Svetlana Tonkova, Ilya Sarvin, and Arthur Vashurin. 2024. "Synthesis and Catalytic Activity of Novel Complexes Based on Cyano-Substituted Phthalocyanines as Promising Drug Conversion Agents" Scientia Pharmaceutica 92, no. 3: 47. https://doi.org/10.3390/scipharm92030047
APA StyleErzunov, D., Tonkova, S., Sarvin, I., & Vashurin, A. (2024). Synthesis and Catalytic Activity of Novel Complexes Based on Cyano-Substituted Phthalocyanines as Promising Drug Conversion Agents. Scientia Pharmaceutica, 92(3), 47. https://doi.org/10.3390/scipharm92030047







