Abstract
The aim of this review is to discuss the numerous health-promoting properties of Cichorium intybus L. and bring together a range of publications to broaden knowledge and encourage further research and consideration of the plant use as treatment for a range of conditions. A comprehensive search of articles in Polish and English from 1986–2022 years was carried out in PubMed, Google Scholar and ScienceDirect using the keywords chicory, Cichorium intybus L., sesquiterpene lactones and their synonyms. Articles were checked for titles, abstracts, and full-text reviews. The first part of the review article discusses chicory, the countries in which it is found, its life cycle or modern cultivation methods, as well as its many uses, which will be discussed in more detail later in the article. The increased interest in plants as medicines or supplements is also briefly mentioned, as well as some limits that are associated with the medical use of plants. In the Results and Discussion section, there is a discussion of the numerous health-promoting properties of Cichorium intybus L. as a whole plant, with its collection of all the components, and we then examine the structure and the individual constituents of Cichorium intybus L. Among these, this article discusses those that can be utilized for causal applications in medicine, including sesquiterpene lactones and polyphenols, mainly known for their anti-cancer properties, although, in this article, their other health-promoting properties are also discussed. The article also examines inulin, a major component of Cichorium intybus L. The Discussion and the Conclusions sections propose directions for more detailed research and the range of factors that may affect specific results, which may have safety implications when used as supplements or medications.
1. Introduction
Plants have accompanied us since time immemorial. Among these is chicory, which is not only a food source but also offers significant applications in medicine, and oncology in particular. Such properties were usually discovered by experimentation. Hippocrates, the father of medicine, used various herbs in his therapies [1]. This article presents how different parts of chicory and their extracts can benefit human health.
As an example, it is worth taking a closer look at common chicory (Cichorium intybus L.), which is a versatile plant with multiple applications in herbal medicine. Both the leaves and roots are used worldwide [2]. The leaves are consumed in Poland, southern India, Italy and Greece, while the root is used as a coffee substitute. Cichorium intybus L. has numerous properties that affect the human metabolism, with various anti-fungal, anti-bacterial, analgesic, anti-cancer and anti-diabetic properties [3].
It is also important to look briefly at life cycle of this plant. Cichorium intybus L. undergoes two distinct production stages over its two-year life cycle [4]. During the initial vegetative phase, Cichorium intybus L. plants are sown in open fields in late spring, resulting in the growth of a rosette of green leaves with a fleshy taproot. This taproot is harvested in late autumn and subsequently stored at low temperatures to induce generative growth. In the second stage, the vernalized root is stimulated to develop an etiolated apical bud in a dark, moist environment. This process is referred to as forcing, and the period during which yield and quality are optimal is referred to as the forcing window. Typically, the roots will be stored until a suitable window opens [5]. Traditional growers covered the root with soil to stimulate growth; however, most chicory is now grown hydroponically in a multi-variety system and in dark growth chambers [4]. The combination of the introduction of hybrid varieties, hydroponic forcing systems, and a well-balanced long-term storage programme enables chicory production to continue throughout the year [6].
Cichorium intybus L. also has numerous health-promoting properties which definitely qualify it as a herbal medicine. Herbal medicine has a vibrant tradition and continues to find a place in modern medicine as a means of complementing traditional treatments for diseases, or at least mitigating their effects [7].
It should also be mentioned that, for many people, traditional treatments and herbal medicines are the main and sometimes even the only source of health care. They are supported by their proximity to home, affordability and accessibility; they are also culturally acceptable and highly trusted. Traditional medicine is growing in popularity as a way of dealing with the continuing rise in chronic non-communicable diseases, and it can be significantly less expensive than conventional treatments. Regulation of herbal products is emerging in most WHO Member States, and new legislation is being developed and implemented as needed. This aims to protect the health of consumers by confirming the safety and high quality of medicines [8].
However, the other side of using herbal medicine should not be forgotten. Despite their extraordinary potential, substances of plant origin are often toxic or can demonstrate poor water solubility and limited bioavailability, which may render a given compound in such a form useless for clinical purposes [2,9]. However, analogs of these compounds with increased efficacy and lower toxicity can be synthesized [10,11]. Secondary metabolites are typically present in plants at lower levels than those needed for therapeutic properties; nevertheless, it is feasible to enhance the bioavailability of medicinal substances to maintain low dosages [12,13].
The paper presents the current state of knowledge regarding the constituents present in common chicory (Cichorium intybus L.) and their uses, as well as the evidential base for the health-promoting properties of chicory. Due to the increasing prices of modern drugs, as well as their occasional ineffectiveness and potential side effects, interest has recently grown regarding the use of plants and their medicinal properties. Plants are a valuable yet inexpensive source of natural secondary metabolites which can play an essential role in fighting and treating lifestyle diseases and can even be used in vaccines and medicines. They are often less expensive because such treatments can often employ by-products from a given technological process. While the drugs derived from plants may have limitations, they nevertheless have made a considerable contribution to medicine in general.
To summarize the introduction, parts of which will be expanded upon later in the discussion, the overall aim is to answer the following question: what are the potential medical uses of chicory, what substances present in chicory are responsible for this, and, if possible, what mechanisms underlie such functioning?
2. Materials and Methods
2.1. Design, Location and Time
A systematic review was conducted to identify numerous health-promoting properties of Cichorium intybus L. from scientific journals published between 1986 and 2022 using the electronic databases PubMed, Google Scholar and Science Direct. The articles were selected based on criteria such as review articles and studies on the use of chicory extracts for various medical properties. Articles were used in various languages, the largest part being articles written in Polish and English. Chemical structures were drawn using ChemDraw according to the journal’s guidelines.
2.2. Data Collection
The research problem concerned the health-promoting properties of Cichorium intybus L. The following keywords were used: chicory AND health-promoting properties, polyphenols, sesquiterpene lactones, herbalism, herbal medicine, Cichorium intybus L. and their synonyms. Additionally, we also used citations of selected scientific works, which led to the enrichment of the bibliography.
2.3. Data Analysis
The results section presents all the data collected, which describe the properties of bioactive substances present in all morphological parts of Cichoriun intybus L., including sesquiterpene lactones and polyphenols and also a brief discussion of inulin. Of the 236 articles, 92 met the criteria adopted for this review.
3. Results
Chicory, a perennial member of the Asteraceae family, is undoubtedly an underestimated vegetable. Currently, its primary usage is concentrated in India, Greece, Italy, and Poland. Two primary species of chicory are cultivated: chicory endive (Cichorium edivia L.) and common chicory (Cichorium intybus L.). Among the characteristic features of chicory are the aster-shaped flowers (usually of blue color, more rarely white or pink) and the lanceolate leaves. The plant consists of a thick root with a growing stem that reaches 1−1.5 m in height. The central edible part of chicory is the flower and shoot bud [7,14].
3.1. Health-Promoting Properties of Chicory
Most medicinal chicory is fresh or dried. Its extracts have a wide range of pharmacological and biological properties that include various antimicrobial, hepatological, anti-diabetic and analgesic effects [15].
3.1.1. Antiviral Properties
Extracts obtained from Chicorium intybus L. showed high antiviral activity against Herpes simplex virus type 1 (HSV-1) and partial activity against adenovirus at higher concentrations [15]. A study by Zhang et al. confirmed that chicoric acid obtained from chicory leaves has antiviral properties. Chicory may offer some therapeutic potential against the SARS-CoV-2 virus, the source of the COVID-19 pandemic, if only because of its rich caffeic acid content, which has antiviral properties; however, further studies are needed in vitro and in vivo [16,17,18,19,20].
According to a study by Zhang et al. on d-galactosamine, Duck Hepatis B Virus (DHBV) is similar in genome organization and replication mechanism to Hepatitis B Virus (HBV). DHBV is generally accepted as a surrogate for HBV because of its many similarities. The natural transmission route is for the virus to enter eggs from the bloodstream of infected ducks, resulting in congenital infection. Ducks with congenital infection are at risk of developing secondary amyloidosis or hepatoma, which results from chronic stimulation of the immune system. The hepatocyte pattern of primary DHBV-infected ducks is a promising model for viral infection, as it demonstrates high efficacy and reproducibility for evaluating new substrates against HBV. Chicoric acid significantly inhibit the replication of DHBV viral DNA in infected hepatocytes; in addition, as chicoric acid stimulates cellular immunity while inhibiting hyaluronidase activity, as well as HIV infection, these “anti-DHBV” properties may be due to its immunomodulatory properties and blockage of viral DNA replication. The compound could achieve its hepatocyte-protective effects by stimulating phagocytosis, as well as through its anti-hyaluronidase and antioxidant properties. The antiviral effect could block the steps of viral protein synthesis and DNA replication, as well as immunomodulation [15,20,21,22]. Chicoric acid can therefore be used to design antiviral agents.
3.1.2. Anti-Fungal and Anti-Bacterial Properties
Common chicory is active against both Gram-negative and Gram-positive bacteria, as well as yeast. Methanolic and aqueous extracts from the aerial parts of the plant were tested on a group of microorganisms that cause food spoilage, including Staphylococcus epidermidis, Staphylococcus aureus, Staphylococus pyogenes and Pseudomonas aeruginosa. The methanolic extracts possess a broader antimicrobial spectrum than the aqueous extracts and the classical antibiotics tobramycin and gentamicin. The aqueous extracts, on the other hand, showed more potent activity than classical antibiotics in the case of Staphylococcus spp. Chicorium intybus L. can inhibit the growth of other microorganisms, e.g., Escherichia coli, Bacillus subtilis, Micrococcus lateus or Salmonella typhi. Tests were carried out on aqueous, ethanolic, chloroform and hexane extracts from chicory seeds against Staphylococcus aureus and Escherichia coli. All the extracts used showed activity against both microorganisms, although a greater degree of inhibition was observed against Staphylococcus aureus in the case of the aqueous extracts [15,23,24,25].
In the case of bacteria, Gram-positive strains are more sensitive than Gram-negative strains and fungi; this is influenced by the cell wall structure, in that Gram-negative bacteria possess an extra hydrophilic outer membrane containing lipopolysaccharides, which hinder the accumulation of phenolic compounds in the cell wall, rendering them less susceptible to the effects of plant extracts. Nevertheless, numerous studies have found chicory to have anti-bacterial properties against Gram-negative and Gram-positive bacteria, as well as anti-fungal properties against yeast or filamentous fungi [26,27,28].
Solvent extracts obtained from chicory seeds have demonstrated significantly higher activity than those obtained from chicory leaves and roots [28]; this is most likely due to differences in their phytochemical composition. Different proportions of bioactive compounds may also have divergent effects on microorganisms. Jurgoński et al. found the seed extract to be the richest source of proteins, fats, and minerals, as well as phenolic compounds. Phenols from plant extracts function as antimicrobial agents, effectively disrupting cell membranes by modifying their permeability, leading to inhibition of microbial growth [29]. Chicory exhibits antimicrobial activity against a broad spectrum of pathogens.
3.1.3. Anti-Cancer Properties
Chicory has also demonstrated anti-cancer potential through various known anti-cancer metabolites being present within it, such as eudesmanolides, polyacetylene, guaiano-lides, 6-methoxyflavone, sterol, delphinidin, 3,4-dihydroxyphenethyl and anthocyanin [30]. These phytometabolites have shown anti-cancer effects in vivo and cytotoxic effects in vitro in clinical trials, which signal the potential of Cichorium intybus L. as analgesics [31]. Chicory preparations have cytotoxic effects on the following cancers: vitreous melanoma (C32), adenocarcinoma of the kidney (ACHN), breast cancer (MCF-7) and leukemia cells [15]. A study on mice with ascites tumors showed a significant reduction in tumor progression using an ethanolic extract of the root, and aqueous-ethanol tincture inhibited proliferation of C32 amelanotic melanoma cells [15]. Ethanolic and methanolic extracts of Cichorium intybus L., have shown antioxidant and cytotoxic effects in studies. Extracts obtained from chicory can therefore serve as pharmaceutical material for treating cancer cells [32].
3.1.4. Analgesic Properties
Pain medications are used based on regimens developed by the WHO. The most commonly used drugs right next to antibiotics are paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs), followed by weak opioids (codeine, tramadol and dihydrocodeine) and potent opioids (fentanyl, methadone, morphine). NSAIDs stimulate pain receptors at the site of inflammation. Drugs of this type inhibit the production of the enzyme cyclooxygenase, which is necessary for the synthesis of prostaglandins (molecules that are found in all tissues and body fluids). Chicory extracts obtained from leaves with 1% hydrochloric acid were tested for inhibition of cyclooxygenase (COX-1 and COX-2) and lipid peroxidation (LPO) activity, enzymes that catalyze lipid peroxidation reactions in vitro. The extracts possessed cyanidin (cyanidin-3-O-(6-O-malonyl-β-glucopyranoside)) in their composition. The results confirmed that the treatment inhibited LPO (lipid peroxidation) activity by 88%, COX-1 (a constitutive isoform of cyclooxygenase) activity by 64% and COX-2 (induced cyclooxygenase, induced cyclooxygenase) activity by 79% compared with control samples [7].
Previous research has also examined the analgesic properties of three active substances derived from chicory: lactucin and its derivatives lactucopicrin and 11β,13-dihydrolactucin. The characteristic bitter taste of these sesquiterpene lactones was evaluated for sedative and analgesic properties using a mouse model. At doses of 15 and 30 mg/kg body weight, the compounds showed effects similar to ibuprofen (30 mg/kg body weight) in the hot plate test. In addition, the 30 mg/kg body weight dose gave a similar effect as 60 mg/kg body weight ibuprofen in the tail hugging test. Lactucopicrin showed the most substantial analgesic properties of all the compounds tested, while lactucin and lactucopicrin also showed sedative properties using the spontaneous motor activity test [33].
3.1.5. Anti-Neurotoxic Properties
Common chicory extracts were found to have beneficial effects on peripheral neuropathy induced by pyridoxine administered at 800 mg/kg body weight over 14 days [34]. Common chicory (Cichorium intybus L.) contains triterpenoids and glycosides that enhance GABA-ergic transmission and inhibit glutamatergic transmission. It is hence possible that the chicory extracts exerted their anti-neurotoxic effects through the GABA-ergic system, which modulate the transmission of pain-related signaling from the peripheral sensory nerves to the central nervous system. It was found that the root extract can affect inflammation in two ways: (1) inhibition of direct COX-2 activity and reduction in COX enzyme, and (2) inhibition of TNF-α with subsequent reduction in COX-2. The flavonoids and glycosides found in chicory can alter the function of the ionotropic GABA receptor. They increase the level of GABA receptor expression, especially GABA type A receptors. In contrast, substances such as glycosides, polyphenols, flavonoids, terpenoids, sterols, tannins or terpenoids show anti-inflammatory properties by reducing the levels of inflammatory mediators, prostaglandins, TNF-α (tumor necrosis factor), IL-6 (Interleukin 6) and IL-1 (Interleukin 1) and NO (nitric oxide). Also, sesquiterpene lactones and triterpenoids found in chicory root have the potential to inhibit COX-2, thus controlling inflammation, and increase the activity of GABA-ergic neurons. Studies indicate that chicory extracts have beneficial effects on peripheral neuropathy induced by pyridoxine. Hence, the neuroprotective and anti-neurotoxic effects of chicory may be mediated by the modulation of a GABA-ergic neurotransmitter, which in turn mediates the reduction of TNF-α [34,35,36].
3.1.6. Anti-Diabetic Properties
Aqueous chicory seed extract was able to significantly reduce glucose and triglycerides levels in blood serum in rats. It is worth noting that consumption of chicory seed extracts also reduced oxidative stress, inflammation and hypertriglyceridemia in another study on 150 patients diagnosed with type 2 diabetes [37]. In another study, whole plant ethanolic extract reduced serum glucose, triglyceride and cholesterol levels in rats with streptozocin-induced diabetes mellitus, and it also reduced glucose-6-phosphotase levels when compared to the control group [38]. It was also observed that an aqueous extract of chicory seeds can prevent weight loss in the treated animals [3].
Diabetes mellitus and its complications are believed to be induced by long-term hyperglycemia and oxidative stress. In people with diabetes, oxidative stress can be increased by increased glucose autoxidation, metabolic stress and non-enzymatic glycolysis. Plant products are a rich source of antioxidants and may have applications in type 2 diabetes. Chicory seed extract is rich in flavonoids and phenolic acids, which may affect the oxidative activity of the extract. The hydroxyl group from the phenols enables free radical scavenging, which can reduce the inactivation of antioxidant enzymes caused by free radicals. In addition, such a reduction in free radical content and an increases in antioxidant enzymes level lower lipid peroxidation in tissues. For example, chia seed preparation was found to lead to a reduction in inflammation, hypertriglyceridemia and oxidative stress and increased adiponectin levels [3,37]. Cichorium intybus L. seeds can act as a supplement for people with and without diabetes at pharmacological dosages [37].
Table 1 (The therapeutic action of Cichorium intybus L. organs.) shows the various health-promoting properties of the different parts of a chicory plant. It is worth noting that the health effects may vary depending on the part of the plant used for extraction. Additionally, a mixture of different parts of chicory can generate synergistic effects, highlighting the importance of a comprehensive approach to the study and use of this plant. It is also important to note the variability in the content of active ingredients depending on environmental factors, such as season or growing conditions, which can have a significant impact on the content of substances and thus on the final health properties of chicory extracts.

Table 1.
The therapeutic action of Cichorium intybus L. organs.
3.2. Components Cichorium intybus L.
The industrial use of chicory is mainly based on the use of the leafy part of lettuce heads for the production of all kinds of salads or salad mixes. Chicory roots are used as animal feed and to obtain inulin. They are also used in the production of fructose syrups and, more rarely, in the production of ethanol [3,39,40].
Table 2, Table 3 and Table 4 show the amounts of each component in the leaves of Cichorium intybus L.

Table 2.
The main components of chicory (Cichorium intybus L.) leaves per 100 g fresh weight [29,41].

Table 3.
Minerals occurring in chicory (Cichorium intybus L.) leaves per 100 g fresh weight [29,41].

Table 4.
Polyphenols of chicory (Cichorium intybus L.) leaves and roots per 100 g fresh weight [29,41].
The composition of chicory includes a variety of nutrients, such as vitamins B1, B2, C, β-carotene, folic acid, manganese, potassium, sodium, copper, zinc, iron and magnesium. Various nutritionally diverse plant compounds have been detected in chicory, indicating a versatile and rich nutritional composition, some of which are presented in Table 2 (The main components of chicory (Cichorium intybus L.) leaves per 100 g fresh weight) Carbohydrates, phenolic compounds, fatty acids, flavonoids, amino acids, sex-quiterpene lactones, minerals and vitamins are all present in Cichorium intybus L. Carbohydrates, lipids and proteins are the structural units and primary reserve materials present in plants.
Carbohydrates play an essential role in the plant’s defense systems, and their content depends on the plant itself, the species itself, growing conditions, weather conditions, or even the individual parts of the plant (e.g., leaves and roots). Its various minerals (Table 3 (Minerals occurring in chicory (Cichorium intybus L.) leaves per 100 g fresh weight)) are necessary for growth, the normal functioning of the plant’s life cycle, or metabolic functioning. Various environmental stresses, such as drought, light conditions and extreme temperatures, affect the mineral content in many ways, according to the variety or species of a particular plant. Minerals are also essential for the metabolic process in humans [41].
Plants also produce a range of substances, known as phytochemicals, with broad biological functions, many of which have health-promoting properties; for example, many are known to have anti-inflammatory, anti-cancer or antioxidant activities. Some of these substances in Cichorium intybus L., are shown in Table 4 (Polyphenols of chicory (Cichorium intybus L.) leaves and roots per 100 g fresh weight). Many are structurally plant polyphenols and are stored as glycosides to make them less reactive and easier to store in the cell vacuole. Chicory contains various beneficial phytochemicals (Table 4), as noted previously [29]. Chicory has a bitter taste, caused by the presence of sesquiterpene lactones, predominantly lactucin and lactucopicrin. The leaves and roots of chicory contain inulin, which is an essential ingredient that helps reduce the development of diet-related diseases; the inulin content is typically around 1.1 g in leaves and 22.3 g in roots [29,41]. Chicory also contains various other health-promoting compounds, such as fructose, pectin, choline, polyphenols and various fatty acids [41,42,43,44,45,46].
3.3. Sesquiterpene Lactones
More than 5000 sesquiterpene lactones have been identified in various plants. Chemically, these substances can be classified as terpenes. The lactone molecules bear a cyclic ring, whose size depends on the location of the hydroxyl and carboxyl groups in the hydroxycarboxylic acid [47,48,49].
Sesquiterpene lactones are most often found in vegetables of the Asteraceae family. They are colorless lipophilic compounds possessing a characteristic bitter taste. Some of the main lactones found in chicory are lactucin and lactucopicrin (Figure 1). Sesquiterpene lactones have numerous beneficial properties for the human body, such as anti-inflammatory, anti-parasitic, anti-bacterial or even anti-cancer activities [15,50].
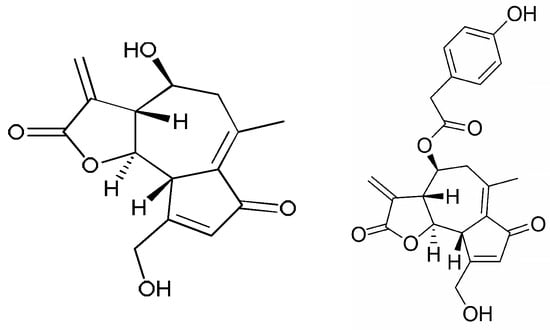
Figure 1.
Lactucine and Lactucopicrin.
3.3.1. Anti-Cancer Properties
A sesquiterpene lactone showing mainly anti-cancer properties, but also intense anti-inflammatory properties, is arglabin. The anti-cancer activity has been attributed to the presence of an α-methylene-γ-lactone group. Studies indicate that the cytotoxic effect may be due to the presence of a double exocyclic γ-lactone conjugated bond at C11–C13 [50,51]. Grech-Baran and Pietrosiuk [50] report that arglabin appears to be toxic to healthy human cells and, as such, merits further study.
3.3.2. Anti-Inflammatory Properties
Sesquiterpene lactones are known to have anti-inflammatory effects. These have been attributed to their cytotoxic potential to increase reactive oxygen species (ROS) levels and thus regulate p53 activity. However, some work suggests that p65 signaling is also possible. For example, it is more likely that a compound called helenalin modulates the molecule NF-κB (nuclear factor kappa-light chain enhancer of activated B cells) within the p65 subunit rather than preventing IκB degradation. A Western blot study of the alkylation of the p65 subunit in NF-κB and NF-κB translocation, and the possible degradation of IκB, found that IκB is not inhibited [52,53]. Although the level of efficacy cannot be generalized too broadly, it is safe to say that consuming sesquiterpene lactones with chicory will elicit similar effects to those obtained in in vivo studies and should be part of a balanced diet [52].
3.3.3. Anti-Parasitic Properties
The parasitoses leishmaniasis and trypanosomiasis pose a significant threat in developing countries and contribute to a colossal number of deaths per year. Many sesquiterpene lactones, such as peruvine, psylostachyin and helenalin, have anti-parasitic properties. Helenalin has activity against protozoa belonging to the Trypanosoma family, while peruvine and psylostachyin is active against worms belonging to the genus Leishmania. One natural source of sesquiterpene lactones with anti-parasitic activity is Chicorium intybus, or common chicory; its extract is used to treat infection by the gastrointestinal nematodes Haemonchus contortus or Teladorsagia circumcintat [54,55,56,57].
Feeding forage chicory to animals can contribute to a reduction in the fecal egg count (FEC) worm burdens of abosomal nermatodes. However, it does not protect against intestinal worm infection in ruminants. Bioactive feeds are believed to have direct and indirect anti-parasitic mechanisms. Direct effects refer to the chemical interaction between specific molecules found in parasite structures and plant compounds, resulting in the removal of parasites from the host or their destruction. Indirect effects include stimulation of the host organism, which in themselves may lead to expulsion of the worms or a supportive interaction of plant compounds with leukocytes. Guaianolides and other sesquiterpene lactones (SLs) have cytotoxic solid activity. This activity is mainly related to the presence of an α-methylene group (CH2) attached to a γ-lactone present in the SL molecule. The α-methylene group reacts with the sulfhydryl (thiol) groups of free cysteine or with cysteine-containing peptides, enzymes or other types of proteins by Michael addition (Michael 1,4 addition), i.e., a reaction between a Michael donor, such as an enolate, and a Michael acceptor, typically an α,β-unsaturated carbonyl. The result is the generation of a Michael adduct caused by the formation of a bond between carbons at the acceptor’s β-carbon, leading to alkylation of cellular macromolecules and disruption of cellular functions, e.g., impairment of mitochondrial respiration, cell signaling or cell replication. There is also emerging evidence that sesquiterpene lactones can interact with amino acids other than cysteine, and that lactones without an α-methylene group can still exhibit some activity in various biological systems, giving rise to the notion that there is no single mechanism of action for the entire group of sesquiterpene lactones. For example, artemisinin and its derivatives have antimalarial properties which seem to be based on the cleavage of the superoxide bridge (absent in the sesquiterpene lactones found in chicory). The substances take part in a heme-iron reaction in infected erythrocytes, leading to the formation of highly reactive free radicals and the subsequent death of the parasite [58,59,60].
3.3.4. Anti-Bacterial Properties
The threat of antibiotic-resistance continues to grow due to the overuse of such drugs. Antibiotics are also used in agriculture, which can adversely affect the quality of food products, such as meat or milk. Although the global market for antibiotics is growing steadily, few compounds are in development with entirely new mechanisms of action. Some strains of pathogens may eventually become resistant to all known antibiotics. In such cases, the antimicrobial compounds produced by plants such as chicory may be invaluable. Using chicory in this way also reduces industrial waste. From 1981 to 2019, as much as 67% of small molecules approved for therapeutic purposes had an active pharmacophore that was a natural product or derived from one. Although most currently used antibiotics are microbial metabolites, plant-derived compounds have also shown promise. Supercritical (SFE) chicory extract, as well as a purified fraction that included 11β,13-dihydro-8-deoxylactucin and 8-deoxylactucin, had an inhibitory effect on the growth of antibiotic-resistant bacteria such as S. aureus MRSA and P. aeruginosa IBRS P00. Antibiotic resistance is placing a significant burden on healthcare systems. To stop the spread of resistant bacterial strains, the focus should be on creating drugs of reduced selectivity by targeting less vital cellular processes, such as quorum sensing and bacterial virulence. Such drugs should also inhibit the ability to form biofilms, which allow bacteria to resist antibiotics in long-lasting infections. Chicory extracts obtained by SFE inhibited biofilm formation by P. aeruginosa IBRS P00. The extracts reduced the count of cells that make up the biofilm, as well as the accumulation of eDNA and EPS (extracellular polymeric substances) in the extracellular matrix myelin. Supercritical chicory extracts offer hope as future inhibitors of catheter-associated biofilms. A substance should also retain its activity against a specific target while maintaining low toxicity. In a preliminary acute toxicity study, supercritical fluid crude extracts were found to have reduced toxicity after SFE optimization combined with extract fractionation. Compounds with a higher mass had higher cytotoxicity related to Caco-2 and HaCaT cells, showing bioactivity or toxicity in a dose-dependent manner [61,62,63,64].
Hence, there is therapeutic potential for untapped plants and plant biomass generated as waste from industrial processes. Among these species, chicory is a plentiful source of bioactive compounds with antimicrobial properties.
Sesquiterpene lactones represent a large and important group of compounds for humans and their plant sources. Considering their myriad health-promoting properties, it would be beneficial to raise public awareness regarding fruit and vegetable consumption, as well as to improve the taste of vegetables containing sesquiterpene lactones, to encourage increased consumption.
3.4. Polyphenols
Chicory, especially its roots, is a rich source of polyphenols. Polyphenols are also receiving increasing interest among nutritionists, food scientists and consumers. These are secondary plant metabolites formed by the shikimic acid and acetic acid pathways. Recent studies indicate that polyphenols can play roles in the prevention of cancer, neurodegenerative or cardiovascular diseases [65]. They are known to have antioxidant properties that, together with ascorbic acid, carotenoids, tocopherols and physiological antioxidants, offer protection against oxidative stress [66]. Plants use such properties to protect themselves from UV radiation or to regulate metabolic processes during drought and low temperatures. During heat, water or mechanical stress, polyphenols are synthesized in higher concentrations [67].
The composition of polyphenols varies with plant material or plant species. Chicory contains many polyphenols, including chlorogenic acid or caffeic acid. Chlorogenic acid (Figure 2) is the best known-bound hydroxycinnamic acid, consisting of a combination of quinic acid and caffeic acid [68,69]. It is also vital to mention chicoric acid. Chlorogenic acid and chicoric acid (Figure 3) are potent antioxidants which also exhibit numerous antiviral, anti-inflammatory, antimicrobial, and anti-cancer properties [70,71].
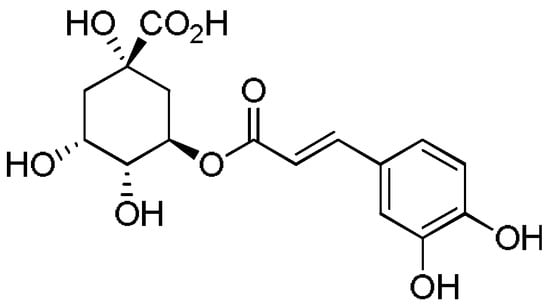
Figure 2.
Chlorogenic acid.
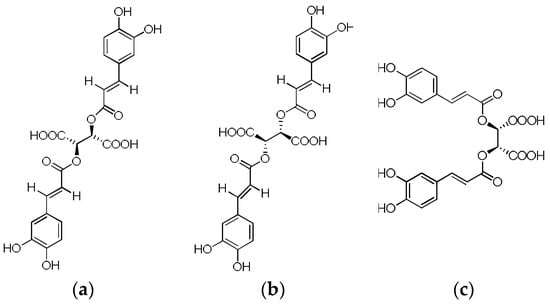
Figure 3.
(a) d-chicoric acid, (b) l-chicoric acid and (c) Meso-cichoric acid.
Chicoric acid (C22H18O12) is a derivative of caffeic acid that can be divided into d-chicoric acid (dextroro-tatory), l-chicoric acid (levorotatory) and mesochoric acid based on its two chiral carbon atoms. Chicoric acid is a precious functional food ingredient that has no side effects in overdose and no drug interactions [72].
3.4.1. Antiviral
Polyphenolic extracts can inhibit viruses by various routes depending on the type of virus, and the origin of the compound. Thus, for example, Herpes simplex virus (HSV-1, HSV-2) is inhibited via the ectodomain of the viral hemagglutinin or by inhibition of NS3 activity. It is worth noting that the mechanism can vary significantly between polyphenolic compounds, extracts containing several polyphenolic groups, and non-polyphenolic substances. Numerous studies indicate that antiviral activity takes place by limiting or inhibiting the replication of the virus during the initial stages of infection [73].
3.4.2. Anti-Cancer
Polyphenols, abundant in plants, exhibit anti-cancer properties in various ways by inhibiting cancer cell proliferation, angiogenesis, tumor growth or metastasis. Additionally, they can also protect healthy cells from free radical damage by influencing the immune system response. Polyphenols can influence many of the biochemical processes involved with tumor initiation, promotion and progression, as well as the pathways involved in carcinogenesis. One such mechanism involves its effect on nuclear factors, such as activator protein 1 (AP-1), or NF-κB; these have central roles in cell signaling cascades while regulating DNA transcription and gene expression in response to stimuli such as cell survival and proliferation. The bioavailability and therapeutic and prophylactic properties of polyphenols can be appropriately enhanced and expanded in combination therapies that include natural compounds from another or the same chemical class. However, it is essential to have the right combination of polyphenols with micronutrients in order to maintain the integrity and stability of the extracellular matrix while providing anti-cancer properties [74].
3.4.3. Anti-Inflammatory
Polyphenols regulate immunity by interfering with immune cell regulation, gene expression and pro-inflammatory cytokine synthesis. They inactivate NF-κB and modulate mitogen-activated protein kinase (MAPK) and arachidonic acid path-ways. Polyphenolic compounds inhibit phosphatidylinositide 3-protein kinase B (PI3K/AkT), an inhibitor of kappa/c-Jun amino-terminal kinases (IKK/JNK), and mammalian target of rapamycin complex 1 (mTORC1), a protein complex that controls protein synthesis. They can have an inhibitory effect on the expression of TLR (Toll-like) receptors, as well as pro-inflammatory genes. The anti-inflammatory properties demand effective antioxidant potential, as well as the ability to inhibit enzymes involved in the production of eicosanoids. They also inhibit some of the enzymes responsible for the production of ROS, such as NADPH oxidase (NOX), and xan-thine oxidase, which also increase the levels of the endogenous antioxidant enzymes catalase, superoxide dismutase (SOD) and glutathione peroxidase (GSH; Px). They also inhibit phospholipase A2 (PLA2), lipoxygenase (LOX) or cyclooxygenase (COX), thereby leading to a reduction in the production of leukotrienes (LTs) and prostaglandins (PGs). Such biologically active compounds have a positive effect on the immune system, especially in chronic inflammatory diseases. Polyphenols may play a significant role in the prevention and progression of chronic diseases associated with inflammation, such as obesity, cancer, cardiovascular disease and diabetes [75].
3.4.4. Antimicrobial
Chlorogenic acid is known to have anti-bacterial properties. Although the mechanism of antimicrobial activity has been described very rarely, it is believed to act by selectively disrupting the membranes of microorganisms. Herbs with anti-bacterial activity are often used commercially and are intended to improve food safety by preventing the growth of microorganisms. Natural ingredients are used as an alternative to preservatives or other food preservation components, which also protect from emerging antibiotic resistance among microorganisms. Chlorogenic acid is effective against both Gram-positive and Gram-negative bacteria. The outer membrane of Gram-negative bacteria consists of proteins and lipopolysaccharides, which are held together by electrostatic interactions with divalent cations, which are essential for stabilization. Anionic substances can eject divalent cations from their binding sites in lipopolysaccharides and thus disrupt the integrity of the outer membrane. Chlorogenic acid, possessing a negative charge, can bind to the outer membrane through electrostatic interactions, subsequently chelating Mg2+ and disrupting the membrane, leading to a loss of barrier function or the ability to block nutrient flow. When bacterial membranes are in a compromised state, small molecules can be missed. It was also observed that chlorogenic acid treatment resulted in significant releases of K+ ions from S. pneumoniae and Shigella dysenteriae. Chlorogenic acid was found to act on the membrane by increasing permeabilization. The effect of chlorogenic acid on bacterial membrane integrity and membrane potential was characterized by DiBAC43 [bis-(1,3-dibutylbarbituric acid)trimethine oxonol] and PI (propidium iodide) uptake registrations. PI was able to rapidly penetrate the cells of S. pneumoniae and Shigella dysenteriae after the prior addition of chlorogenic acid, while the membrane remained polarized. Chlorogenic acid disrupts the permeability of the cell membrane, thus causing its depolarization, most likely due to the loss of the cells’ ability to maintain metabolites and membrane potential. Chlorogenic acid increases membrane permeabilization and induces nucleotide leakage, but to a lesser degree than pore-forming peptides. Chlorogenic acid may act differently than peptides and other antibiotics, which can lead to the formation of pores in the cell membrane, resulting in the death of the microorganism. Chlorogenic acid kills bacteria, such as S. pneumoniae and Shigella dysenteriae, by causing irreversible permeability changes in the cell membrane and preventing the cell from maintaining cytoplasmic macromolecules, such as nucleotides, and membrane potential [76].
3.5. Inulin
When discussing Chicorium intybus L., inulin, which has a content of around 40%, should also be mentioned. Inulin is a naturally occurring storage polysaccharide with a wide range of applications in the food or pharmaceutical industries. It consists of linear chains of fructose residues linked by p-1,2-glycosidic bonds, often terminated by a glucose molecule. The number of fructose residues in inulin ranges from two to 70, depending on the origin (Figure 4). This polysaccharide is a class of dietary fiber formed from fructose monomers, i.e., a fructan, that is less sweet than sucrose [43,77,78].
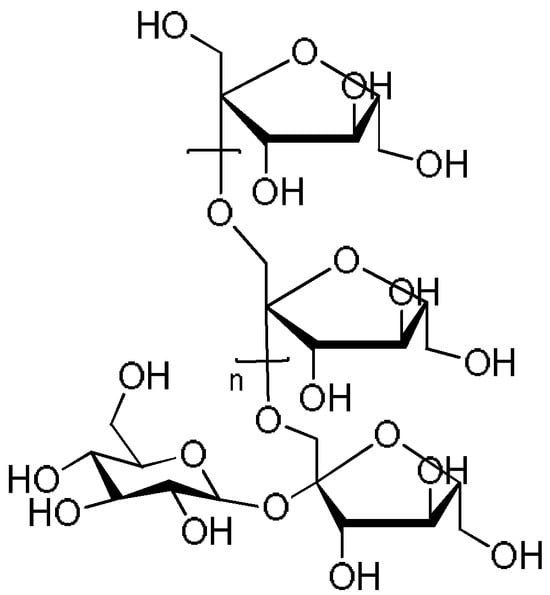
Figure 4.
Inulin.
In the case of chicory, inulin-type fructans consist of a sucrose molecule with variable numbers of fructose molecules bound using a β (2-1) linkage; these are known as fructose−glucose polymers. The typical degree of polymerization, i.e., the number of fructose residues, is 10, ranging from two to 65 [15,79]. Chicory-derived inulin can exist as the longer chain high-performance inulin, in which the degree of polymerization is between 10 and 65, and the shorter chain oligofructose, ranging from two to eight residues [80,81].
Inulin received a “generally recognized as safe” status (GRAS) as early as 2002, which contributed to its prominent use in the food industry (poultry products, meat products or baby products). In many countries, plants abundant in inulin are an integral part of the daily diet. Europeans typically consume 3–11 g per day, while Americans consume 1–4 g. Inulin also has several health-promoting properties, which is why it is also added to all sorts of foods as a functional additive. Among other things, it exhibits prebiotic effects against atherosclerosis, increasing the absorption of calcium absorption from meals [82,83,84].
Several mechanisms can be suggested for the effect of inulin on calcium absorption. Carbohydrates not digested in the small intestine act as substrates for the formulation of short-chain fatty acids (SCFAs) in the large intestine by intestinal microorganisms. Fermentation lowers the pH in the intestine, with a consequent increase in the steadiness of ionized calcium, and accelerates its diffusion. The accumulation of calcium phosphate in the large intestine and its subsequent dissolution by SCFAs most likely plays an essential role in enhancing calcium absorption. Likely, SCFAs may also influence calcium absorption by modifying Ca-H electrolyte exchange. Calcium can pass through the cell membrane in the form of a less-charged complex. The high rate of calcium absorption in the large intestine may trigger a feedback mechanism that involves inhibition of duodenal absorption, as endocrine factor-mediated control of dietary calcium balance occurs. Inulin is not very rich in minerals, but it can be used in products with high levels of such components (such as dairy products); this could help improve calcium availability by solubilization of calcium in the large intestine [85,86,87,88,89].
Inulin may be of value among diabetics, as it can reduce the glycemic index level of products. Its properties support the control of lipids and cholesterol in the blood. Although the human body does not digest inulin, it functions as a dietary fiber, thus supporting regular bowel movements and preventing constipation.
4. Discussion
In the pharmaceutical industry, the number of groups working on natural products has decreased significantly; in the USA, this is also linked to a reduction in government funding. Unfortunately, however, the prospects for the development of new antibiotics are, to say the least, not very optimistic, and despite the growing need for effective antibiotics, we lack central financial support for their discovery and development. In many areas, only fully synthetic drugs are used, whereas natural products and their derivatives do not appear adequate for treating specific diseases. However, the belief that nature actively contributes to the creation of medicine endures [90]. The ever-increasing costs for large pharmaceutical companies and their subsequent inadequate return on investment in the current system have led to little interest in seeking remedies from natural sources. Nevertheless, natural products, coupled with the knowledge and expertise of chemists and biologists, offer the opportunity to develop new structures, ultimately leading to the discovery of effective agents for a wide range of human diseases. [91]. Herbal medicine in some cases lacks sufficient experimental support and can be classified as alternative medicine or pseudo-medicine. However, when used in appropriate quantities, products of plant origin can have many beneficial pro-health properties, as illustrated by common chicory (Cichorium intybus L.). Its secondary metabolites, such as polyphenols or sesquiterpene lactones, are key here because of their properties, which give rise to the potential medical benefits discussed in this article. For the plants themselves, secondary metabolites can play an important role in survival in adverse environmental conditions. They can have a protective function against pathogens, soil salinity levels, herbivores and radiation, and they can also attract insects [92]. They may also have a function in basal metabolism [93]. As such, the mentioned traditional approaches should be viewed as both an integral component and a complementary approach to conventional medicine.
5. Conclusions
The effectiveness of chicory extract and its health-promoting properties can depend on the phytochemical profile of the plant organ, as well as the dosage and nature of the preparation, its interaction with other dietary ingredients, and the individual sensitivity, genetics and state of health of the recipient. All these should be taken into account when preparing medicines or supplements. Furthermore, such phytochemicals may interact with other medications, and this should always be considered during medical consultation. Nevertheless, herbal medicines and substances of plant origin offer immense potential in regard to treating diseases or mitigating their effects.
Author Contributions
Conceptualization, G.B. and Ł.D.; methodology, G.B. and Ł.D.; software, G.B., Ł.D. and A.J.; validation, Ł.D.; formal analysis, Ł.D., K.K.K. and G.B.; investigation, Ł.D., G.B. and A.J.; resources, Ł.D and K.K.K.; data curation, Ł.D., G.B. and K.K.K.; writing—original draft preparation, Ł.D., G.B.; writing—review and editing, Ł.D., K.K.K., D.K., Ż.K.-K. and G.B.; visualization, Ł.D., K.K.K., D.K., Ż.K.-K. and A.J.; supervision, G.B., K.K.K. and Z.W.P.; project administration, Ł.D., G.B., K.K.K. and Z.W.P.; funding acquisition, G.B., K.K.K. and Z.W.P. All authors have read and agreed to the published version of the manuscript.
Funding
Medical University of Lodz. Account number: 503/1-153-02/503-11-001.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giermaziak, W.; Przyłuska, I. Medycyna naturalna dawniej i dziś/Wojciech Germaziak, Izabella Przyłuska; Główna Biblioteka Lekarska Warszawa, Główna Biblioteka Lekarska. Oddział Zielona Góra. Forum Bibl. Med. 2017, 10, 548–560. [Google Scholar]
- Clark, G.C.; Casewell, N.R.; Elliott, C.T.; Harvey, A.L.; Jamieson, A.G.; Strong, P.N.; Turner, A.D. Friends or Foes? Emerging Impacts of Biological Toxins. Trends Biochem. Sci. 2019, 44, 365–379. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef]
- De Jaegere, I.; Cornelis, Y.; De Clercq, T.; Goossens, A.; Van de Poel, B. Overview of Witloof Chicory (Cichorium intybus L.) Discolorations and Their Underlying Physiological and Biochemical Causes. Front. Plant Sci. 2022, 13, 843004. [Google Scholar] [CrossRef]
- van Kruistum, G.; Zwanepol, S.; Alblas, J.; Titulaer, H.H.H.; Sukkel, W. Productie van witlof en roodlof. In Teelthandleiding/Praktijkonderzoek voor de Akkerbouw en de Vollegrondsgroenteteelt; PAV: Lelystad, The Netherlands, 1997. [Google Scholar]
- de Proft, M.; Van Stallen, N.; Veerle, N. Breeding and cultivar identification of Cichorium intybus L. var.foliosum Hegi. In EUCARPIA Leafy Vegetables 2003, Proceedings of the EUCARPIA Meeting on Leafy Vegetables Genetics and Breeding, Noordwijkerhout, The Netherlands, 19–21 March 2003; van Hintum, T.J.L., Lebeda, A., Pink, D.A., Schut, J.W., Eds.; CGN: Wageningen, The Netherlands, 2003; pp. 83–90. [Google Scholar]
- Mulabagal, V.; Wang, H.; Ngouajio, M.; Nair, M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009, 230, 47–53. [Google Scholar] [CrossRef]
- World Health Organization. Who Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 7 May 2024).
- Gurgul, A.; Lityńska, A. Substancje pochodzenia roślinnego w terapii nowotworów. Postępy Fitoter. 2017, 18, 203–208. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef]
- Ignat, M.; Mudura, E.; Coldea, T.; Salanță, L. Therapeutic properties of chicorium intybus. Hop and Medicinal Plants; Academic Press: Cambridge, MA, USA, 2019; Volume XXVII, pp. 76–86. [Google Scholar]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R. An Evaluation of Traditional Persian Medicine for the Management of SARS-CoV-2. Front. Pharmacol. 2020, 11, 571434. [Google Scholar] [CrossRef]
- Shawky, E.; Nada, A.A.; Ibrahim, R.S. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: Identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020, 10, 27961–27983. [Google Scholar] [CrossRef] [PubMed]
- Thota, S.M.; Balan, V.; Sivaramakrishnan, V. Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19. Phytother. Res. 2020, 34, 3148–3167. [Google Scholar] [CrossRef]
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Rafieian-Kopaei, M.; Naghdi, N. An ethnobotanical study of medicinal plants administered for the treatment of hypertension. J. Renal. Inj. Prev. 2016, 5, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Dai, L.H.; Wu, Y.H.; Yu, X.P.; Zhang, Y.Y.; Guan, R.F.; Liu, T.; Zhao, J. Evaluation of hepatocyteprotective and anti-hepatitis B virus properties of Cichoric acid from Cichorium intybus leaves in cell culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef]
- Facino, R.M.; Carini, M.; Aldini, G.; Saibene, L.; Pietta, P.; Mauri, P. Echinacoside and caffeoyl conjugates protect collagen from free radical-induced degradation: A potential use of Echinacea extracts in the prevention of skin photodamage. Planta Med. 1995, 61, 510–514. [Google Scholar] [CrossRef]
- Reinke, R.A.; Lee, D.J.; McDougall, B.R.; King, P.J.; Victoria, J.; Mao, Y.; Lei, X.; Reinecke, M.G.; Robinson, W.E., Jr. L-chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology 2004, 326, 203–219. [Google Scholar] [CrossRef]
- Petrovic, J.; Stanojkovic, A.; Comic, L.; Curcic, S. Antibacterial activity of Cichorium intybus. Fitoterapia 2004, 75, 737–739. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Liu, Y.; Chen, G.; Cui, J. Antimicrobial and antioxidant activities of Cichorium intybus root extract using orthogonal matrix design. J. Food Sci. 2013, 78, M258–M263. [Google Scholar] [CrossRef]
- Jasim, R.S. Antioxidant, Antimicrobial Activities and Phytochemical Constituents of Cichorium intybus L. Aerial Parts. Int. J. Bot. 2017, 14, 24–29. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I. Antibacterial properties of traditionally used Indian medicinal plants. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Stoia, M.; Coman, D. Effects of Extraction Conditions on Bioactive Anthocyanin Content of Vaccinium Corymbosum in the Perspective of Food Applications. Procedia Eng. 2012, 42, 489–495. [Google Scholar] [CrossRef]
- Amer, A.M. Antimicrobial Effects of Egyptian Local Chicory, Cichorium endivia subsp. pumilum. Int. J. Microbiol. 2018, 2018, 6475072. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [PubMed]
- Imam, K.; Xie, Y.; Liu, Y.; Wang, F.; Xin, F. Cytotoxicity of Cichorium intybus L. metabolites (Review). Oncol. Rep. 2019, 42, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Nasr, F.A.; Noman, O.M.; Alyhya, N.A.; Ali, I.; Saoud, M.; Rennert, R.; Dube, M.; Hussain, W.; Green, I.R.; et al. Cichorins D-F: Three New Compounds from Cichorium intybus and Their Biological Effects. Molecules 2020, 25, 4160. [Google Scholar] [CrossRef] [PubMed]
- Kandil, A.S.; Abou-Elella, F.; El Shemy, H.A. Cytotoxic profile activities of ethanolic and methanolic extracts of chicory plant (Cichorium intybus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 106–111. [Google Scholar] [CrossRef]
- Wesolowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wojcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Hasannejad, F.; Ansar, M.M.; Rostampour, M.; Mahdavi Fikijivar, E.; Khakpour Taleghani, B. Improvement of pyridoxine-induced peripheral neuropathy by Cichorium intybus hydroalcoholic extract through GABAergic system. J. Physiol. Sci. 2019, 69, 465–476. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, W.; Fayazuddin, M.; Shariq, S.; Singh, O.; Moin, S.; Akhtar, K.; Kumar, A. Anti-inflammatory activity of roots of Cichorium intybus due to its inhibitory effect on various cytokines and antioxidant activity. Anc. Sci. Life 2014, 34, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Jain, V.; Jabin, A.; Dwivedi, S.; Joshi, S.; Ahmad, S.; Jain, S.K. Effect of Cichorium intybus seeds supplementation on the markers of glycemic control, oxidative stress, inflammation, and lipid profile in type 2 diabetes mellitus: A randomized, double-blind placebo study. Phytother. Res. 2020, 34, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Chandra, K.; Khan, W.; Jetley, S.; Ahmad, S. Antidiabetic, toxicological, and metabolomic profiling of aqueous extract of Cichorium intybus seeds. Pharmacogn. Mag. 2018, 14, S377–S383. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Wimalasiri, K.M.; Silva, K.F.; Ajlouni, S. Comparison of properties of new sources of partially purified inulin to those of commercially pure chicory inulin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Ravishankar, G.A. Cichorium intybus L—Cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001, 81, 467–484. [Google Scholar] [CrossRef]
- Perovic, J.; Tumbas Saponjac, V.; Kojic, J.; Krulj, J.; Moreno, D.A.; Garcia-Viguera, C.; Bodroza-Solarov, M.; Ilic, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Su, Z.; Yang, Y.; Ba, H.; Aisa, H.A. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss. et Huet. by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1176, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889, Erratum in Adv. Nutr. 2021, 12, 1598. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Zdunczyk, Z.; Zary-Sikorska, E.; Krol, B.; Milala, J.; Jurgonski, A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on caecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011, 105, 710–720. [Google Scholar] [CrossRef]
- Giambanelli, E.; D’Antuono, L.F.; Ferioli, F.; Frenich, A.G.; Romero-González, R. Sesquiterpene lactones and inositol 4-hydroxyphenylacetic acid derivatives in wild edible leafy vegetables from Central Italy. J. Food Compos. Anal. 2018, 72, 1–6. [Google Scholar] [CrossRef]
- Mona, I.M.; Wafaa, A.A.; Elgindy, A.A. Chemical and Technological Studies on Chicory (Cichorium intybus L.) and Its Applications in Some Functional Food. J. Adv. Agric. Res. 2009, 14, 735–742. [Google Scholar]
- Surowiak, A.K.; Balcerzak, L.; Lochynski, S.; Strub, D.J. Biological Activity of Selected Natural and Synthetic Terpenoid Lactones. Int. J. Mol. Sci. 2021, 22, 5036. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Galindo, J.C.; Macias, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef] [PubMed]
- OlesiŃSka, K. Sesquiterpene lactones—Occurrence and biological properties. A review. Agron. Sci. 2018, 73, 83–95. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Pietrosiuk, A. Arglabina—Lakton Seskwiterpenowy O WŁaŚciwoŚciach Przeciwnowotworowych. Prospect. Pharm. Sci. 2010, 8, 22–26. [Google Scholar] [CrossRef]
- Zhang, S.; Won, Y.K.; Ong, C.N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Paco, A.; Bras, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Barrera, P.A.; Jimenez-Ortiz, V.; Tonn, C.; Giordano, O.; Galanti, N.; Sosa, M.A. Natural sesquiterpene lactones are active against Leishmania mexicana. J. Parasitol. 2008, 94, 1143–1149. [Google Scholar] [CrossRef]
- Barrera, P.; Sulsen, V.P.; Lozano, E.; Rivera, M.; Beer, M.F.; Tonn, C.; Martino, V.S.; Sosa, M.A. Natural Sesquiterpene Lactones Induce Oxidative Stress in Leishmania mexicana. Evid. Based Complement. Altern. Med. 2013, 2013, 163404. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Pines, M.; Hurwitz, S. Relationship between endogenous cyclic AMP production and steroid hormone secretion in chick adrenal cells. Comp. Biochem. Physiol. B 1986, 84, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Heckendorn, F.; Haring, D.A.; Maurer, V.; Senn, M.; Hertzberg, H. Individual administration of three tanniferous forage plants to lambs artificially infected with Haemonchus contortus and Cooperia curticei. Vet. Parasitol. 2007, 146, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Anastacio, J.D.; Nunes Dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Pena-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; Lopez-Munoz, R.; Williams, A.R. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review. Parasites Vectors 2018, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Tariq, K.A.; Wazir, V.S.; Singh, R. Antiparasitic efficacy of Artemisia absinthium, toltrazuril and amprolium against intestinal coccidiosis in goats. J. Parasit. Dis. 2013, 37, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Maslowiec, D. Antimicrobial Activity of Lactones. Antibiotics 2022, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Hakkinen, S.T.; Sokovic, M.; Nohynek, L.; Ciric, A.; Ivanov, M.; Stojkovic, D.; Tsitko, I.; Matos, M.; Baixinho, J.P.; Ivasiv, V.; et al. Chicory Extracts and Sesquiterpene Lactones Show Potent Activity against Bacterial and Fungal Pathogens. Pharmaceuticals 2021, 14, 941. [Google Scholar] [CrossRef]
- Duthie, G.G.; Brown, K.M. Reducing the Risk of Cardiovascular Disease. In Functional Foods; Springer: Berlin/Heidelberg, Germany, 1994; pp. 19–38. [Google Scholar]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, C.; Zhang, T.; Shi, L.; Li, J.; Liang, H.; Lv, X.; Jing, F.; Qin, L.; Zhao, T.; et al. Chicoric Acid: Natural Occurrence, Chemical Synthesis, Biosynthesis, and Their Bioactive Effects. Front. Chem. 2022, 10, 888673. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, A.; Van Den Ende, W. Inulin metabolism in dicots: Chicory as a model system. Plant Cell Environ. 2002, 25, 803–813. [Google Scholar] [CrossRef]
- Lopes, S.M.; Krausova, G.; Rada, V.; Goncalves, J.E.; Goncalves, R.A.; de Oliveira, A.J. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Hicks, P.M.D.; Heaney, R.P.; Abrams, S.A. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr. Res. 2003, 23, 901–909. [Google Scholar] [CrossRef]
- Seifert, S.; Watzl, B. Inulin and oligofructose: Review of experimental data on immune modulation. J. Nutr. 2007, 137, 2563S–2567S. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Choi, H.G.; Choi, Y.S.; Kim, J.H.; Lee, J.H.; Jung, E.H.; Lee, S.H.; Choi, Y.I.; Choi, J.S. Effect of Chicory Fiber and Smoking on Quality Characteristics of Restructured Sausages. Korean J. Food Sci. Anim. Resour. 2016, 36, 131–136. [Google Scholar] [CrossRef]
- Remesy, C.; Levrat, M.A.; Gamet, L.; Demigne, C. Cecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am. J. Physiol. 1993, 264, G855–G862. [Google Scholar] [CrossRef]
- Nilsson, U.; Bjorck, I. Availability of cereal fructans and inulin in the rat intestinal tract. J. Nutr. 1988, 118, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Levrat, M.A.; Remesy, C.; Demigne, C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J. Nutr. 1991, 121, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.; Hornick, J.L.; Baldwin, P.; Van Eenaeme, C.; Istasse, L. The influence of sugar-beet fibre, guar gum and inulin on nutrient digestibility, water consumption and plasma metabolites in healthy Beagle dogs. Res. Vet. Sci. 1998, 64, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Bellanger, J.; Castiglia-Delavaud, C.; Remesy, C.; Vermorel, M.; Rayssignuier, Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur. J. Clin. Nutr. 1997, 51, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Lucka, M.; Szemraj, J.; Sakowicz, T. Therapeutic potential of secondary metabolites produced in the hairy roots cultures. Postepy Hig. Med. Dosw. 2015, 69, 549–561. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).