Abstract
Concerns surrounding breast cancer have been increasing, as it leads to the current global cancer incidence and causes a high mortality rate in women. This study investigated the physiological effects of common dietary polyphenols that might prevent breast cancer progression. Quercetin, kaempferol, and rosmarinic acid were selected to explore their potential bioactivities. Each polyphenol was formulated into a microemulsion to improve its bioactivity and bioavailability. In vitro antioxidant and cytotoxicity activities of the selected polyphenols and their microemulsion forms were further investigated. The optimized microemulsion carrier with 1% oleic acid, 3% ethanol, 10% polysorbate 20, and 86% ultrapure water achieved more than 90% polyphenol encapsulation efficiency. The microemulsion was stable for more than 30 days when encapsulating polyphenol in the fluctuating temperature treatment. In vitro studies suggested that rosmarinic acid-loaded microemulsion had the best antioxidant activity compared with other polyphenol-loaded microemulsions (PL-MEs). Blank microemulsion and all PL-MEs significantly inhibited the proliferation of both hormone-dependent (T47D) and hormone-independent (MDA-MB-231) breast cancer cells. More studies are warranted to confirm the contribution of the microemulsion carrier components to the polyphenols’ improved antioxidant activity and high toxicity of PL-MEs on breast cancer cells.
1. Introduction
Breast cancer has become the most prevalent cancer in the world and is the main cause of cancer mortality among women, causing 685,000 deaths in 2020 [1,2]. It is predicted that the breast cancer incidence will increase by 31% over the next twenty years [2]. Despite these concerning facts, common treatments for breast cancer such as chemotherapy, surgery, hormone treatment, and radiotherapy have adverse effects that decrease patients’ quality of life during and after the treatment [3,4]. Therefore, prevention strategies are an ideal strategy rather than treatment to tackle increasing breast cancer burden [5]. Diet-related intervention is one of the most cost-effective strategies in the primary prevention effort of breast cancer [6]. A dietary modification trial by the Women’s Health Initiative (WHI) showed that in addition to reduced fat intake, increasing vegetable, fruit, and grain consumption in the long-term contributed to a 5% reduction in breast cancer risk [7].
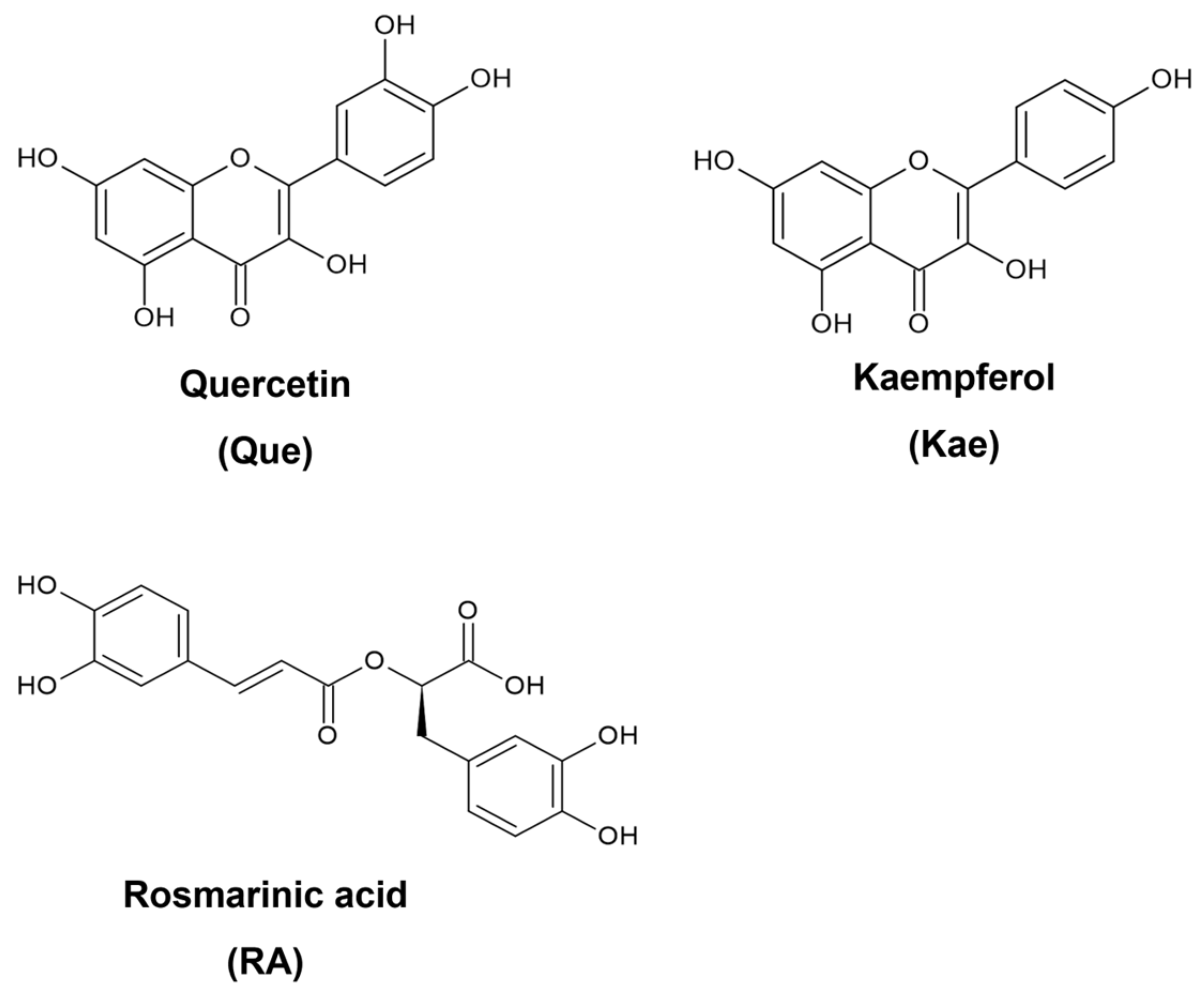
From 2000 to 2020, 42% of clinical trials were on polyphenol-rich foods, and 58% were focused on pure compounds or extracts [8]. These data indicate a continuous interest in the benefits of food bioactives, especially their implications for human health. Regarding breast cancer, a study showed that consuming an acute dose of polyphenol-rich cranberry beverages with low calories improved antioxidant status, and after 8 weeks of daily intake, it regulated glucose levels better, downregulated inflammation, and increased high-density lipoprotein (HDL) cholesterol [9]. Among dietary polyphenols, flavonoids and phenolic acid have been known as effective antioxidant and anti-inflammatory agents, two bioactivities that interfere with breast cancer progression [10]. Quercetin (Que) (Figure 1) is the most abundant flavonoid in vegetables and fruits [11]. Previous studies reported the cytotoxicity of Que in killing breast cancer cell lines, i.e., luminal A MCF-7 and triple-negative breast cancer (TNBC) MDA-MB-231 [12]. Kaempferol (Kae) (Figure 1), another flavonoid that differs from Que only by one hydroxyl group substituted on a phenyl ring, is also a dominant compound in green leafy vegetables [13]. Kae exhibits high radical scavenging activities and inhibits nitric oxide (NO) release [14]. Other studies also showed that Kae inhibited the proliferation of MCF-7 and MDA-MB-231 [15,16]. Rosmarinic acid (RA) (Figure 1), a phenolic acid, has two catechol moieties in its structure. It is commonly found in various herbs and fruits and has comparable intrinsic antioxidant activity to Que [17]. Moreover, RA induces cell cycle arrest in TNBC cells [18].
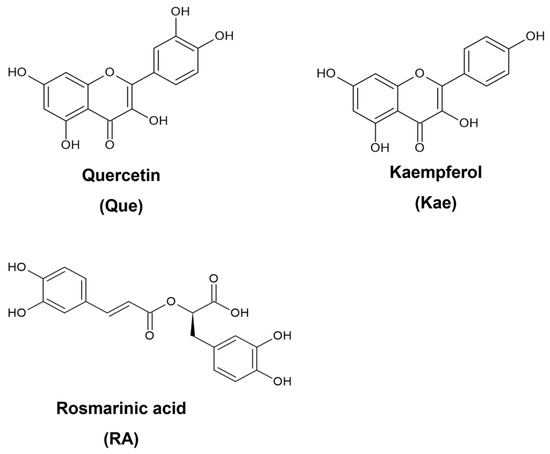
Figure 1.
Structures of selected dietary polyphenols.
Bioactivities of dietary polyphenols have an array of roles in breast cancer prevention. However, low bioavailability of dietary polyphenols in the human body (which is primarily composed of water) hinders their ability to provide health benefits to cancer patients. Nanotechnology has evolved over the decades to make various tiny carrier designs available for precisely carrying lipophilic compounds with low bioavailability, like polyphenols. Microemulsion is one of them, and this drug carrier is less than 100 nanometers in size [19]. Microemulsions have an oil core and surfactant layer that dissolve the lipophilic polyphenols and increase the polyphenol solubility in aqueous environment, respectively [20]. Encapsulation of polyphenols in microemulsion results in more precise compound delivery to cancer cells due to their nanometric size. The nanometric size allows better penetration to the targeted cells and improves the biological distribution of the compound [21]. Precise delivery prevents the polyphenols from damaging normal cells, which then lowers risk of side effects and may improve patients’ recovery after treatment [22,23].
In this study, considering their abundance in edible plants that showed their potential in cancer prevention, we chose Que, Kae, and RA to be formulated into microemulsions to study how the carrier system may improve cancer prevention-related bioactivities (i.e., antioxidant, cytotoxicity) of polyphenols. Microemulsion is a suitable drug carrier for non-invasive drug administration, such as topical and transdermal routes [24]. The selected polyphenols and formulated PL-MEs were tested on two notable breast cancer cell lines: T47D, which represents Luminal A, the most common type of breast cancer, and MDA-MB-231, a cell model for triple-negative breast cancer, which has the worst prognosis among other types of breast cancer.
2. Results
2.1. Polyphenol-Loaded Microemulsions Are Homogenous and Stable under Varied Temperatures
Spontaneous emulsification employs organic phase motion; a phase consists of carrier oil, surfactant, and solvent in an aqueous phase. Although the formulations’ physicochemical characteristics did not change drastically throughout the accelerated stability test, the effect of temperature change was observed in blank microemulsion (BM) and PL-ME formulations (Table 1). The size of all tested microemulsion systems stayed in the nanometric scale throughout the stability tests; BM and PL-MEs were less than 100 nm in size. BM and PL-ME formulations had decreased transmittance while their droplet size and polydispersity index (PDI) increased. Nonetheless, the final PDI values were still recorded at less than 0.5, meaning that the BM and PL-ME formulations were considered homogenous emulsions [25]. BM showed the highest size change (from 10.487 to 92.317 nm) after the stability test. Negative charge was detected in both BM and PL-MEs after the zeta potential measurement.

Table 1.
Particle analysis of PL-MEs before and after subsequent stability tests. All parameters measured in triplicate.
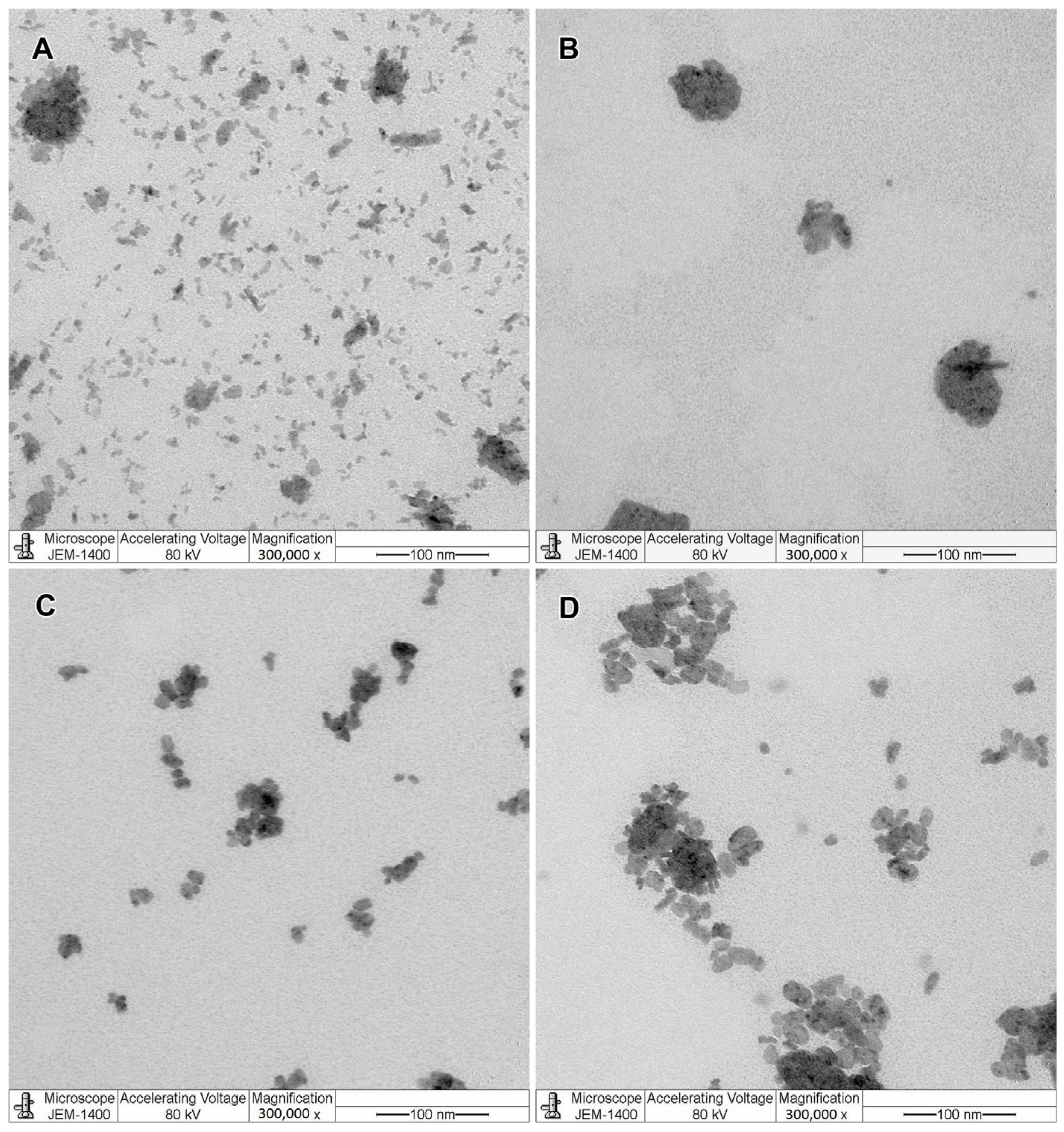
The TEM visualization showed that both BM and PL-MEs had non-spherical droplet morphology (Figure 2). All formulations had some aggregations, except for quercetin-loaded microemulsion (QM), which possessed big ellipsoid droplets (Figure 2B). In terms of pH stability, each formulation gradually decreased from week zero to week three, but no drastic change was observed (Figure S1).
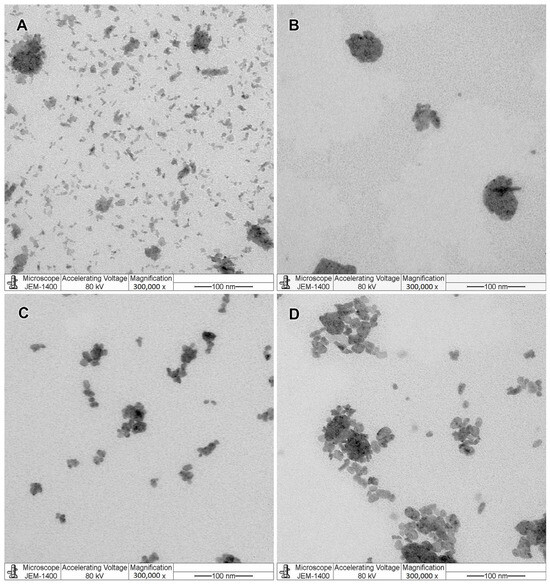
Figure 2.
Visualization of blank microemulsion (BM; (A)), quercetin-loaded microemulsion (QM; (B)), kaempferol-loaded microemulsion (KM; (C)), and rosmarinic acid-loaded microemulsion (RM; (D)) under a transmission electron microscope (300,000× magnification, negative staining).
All PL-ME formulations reached an encapsulation efficiency higher than 90% (Table 2). Among these three PL-MEs, rosmarinic acid-loaded microemulsion (RM) showed the lowest encapsulation efficiency compared with QM and kaempferol-loaded microemulsion (KM).

Table 2.
Percent of encapsulation efficiency of each polyphenol-loaded microemulsions.
2.2. Microemulsion Carrier Improves Antioxidant Activities of Dietary Polyphenols
In comparison with pure polyphenols, Kae was the one that had the lowest activity (Table 3). The activity of RA was similar to Que with no significant difference between their IC50 value. This trend was also observed in QM and RM. Kae and KM exhibited the lowest scavenging activity. Overall, the optimized microemulsion carrier did improve the bioactivity of all polyphenols in scavenging DPPH. For NO scavenging activity, the results showed that microemulsion carrier helped selected dietary polyphenols (except for Que) to achieve better NO scavenging activity, a trend that was also observed in the DPPH experiment. NO scavenging activity of RM was not significantly different to the positive control, gallic acid.

Table 3.
Antioxidant activity of all PL-MEs compared with polyphenols against DPPH and NO radicals.
2.3. Blank Microemulsion and PL-MEs Are More Toxic Than Pure Polyphenols on Breast Cancer Cells
Cytotoxicity tests were conducted to confirm the anti-proliferative potential of PL-MEs on breast cancer cells compared with pure polyphenol compounds. The polyphenols (Que, Kae, and RA) had no cytotoxic effects against T47D cells. After being loaded into microemulsion carrier to make PL-MEs, the PL-MEs exhibited their cytotoxicity. All pure polyphenols and PL-MEs, except Kae, were cytotoxic against MDA-MB-231 (Table 4).

Table 4.
Cytotoxicity of polyphenols and all PL-MEs on hormone-dependent breast cancer cell line (T47D) and hormone-independent breast cancer cell line (MDA-MB-231).
3. Discussion
Storing microemulsions in places with fluctuating temperatures can deteriorate the emulsion system. Oil and non-ionic surfactants have different tendencies in increasing temperature; oil becomes more soluble, but non-ionic surfactants become less soluble. This may lead to phase separation [26,27]. Ostwald ripening, flocculation, and/or coalescence may increase droplet size during storage, which then decreases the transmittance (clarity) of the formula, making it more turbid [19]. Increasing size of microemulsion droplet and PDI value have been reported in a curcumin-loaded microemulsion formula [28]. The notable change in BM size after the stability test could be attributed to the absence of polyphenols in BM [29]. Further study is required to confirm how loaded polyphenol may affect the size of a microemulsion droplet.
Zeta potential is an electrical charge difference between the charge possessed by fluid around nanometric particles and the charge in a layer made of ions surrounding the nanometric particle [30]. Zeta potential value predicts whether a nanometric particle does not undergo significant flocculation or aggregation in a long period of storage [31,32]. Polysorbate 20 is a non-ionic surfactant; it does not give any charge to microemulsions. The minus value in both BM and PL-MEs was possibly due to ionic impurities or fatty acids released upon formulation process or storage [33]. This is why the detected zeta potential values in all formulations were near zero, as the charge came from the byproduct of the side chemical reaction, not from the main emulsification process. Microemulsion with non-ionic surfactant is stabilized more via steric repulsion rather than electrostatic interaction, an interaction that stabilizes microemulsion with ionic surfactant. Steric repulsion occurs when two microemulsion droplets are in close proximity [34]. If the steric repulsion in a microemulsion system fails to overcompensate the Van der Waals interaction among the droplets, droplet aggregation will occur [35]. Aggregation of microemulsion droplets can lower measured transmittance. Given that transmittance in both BM and PL-MEs were maintained above 95% after storage and subsequent stability tests, this indicates the microemulsion carrier provided enough steric repulsion to prevent aggregation.
Non-spherical morphology of PL-MEs agrees with the description of oil-in-water microemulsion droplet made by McClements, which is a worm-like or ellipsoid droplet due to low interfacial tension of its surfactant monolayer [19]. BM exhibited a worm-like morphology, while QM, KM, and RM morphologies were more ellipsoid. Big ellipsoid droplets of QM might be formed from fused droplets, which was caused by a 1:10 dilution of the formulations in the sample preparation. Dilution could affect the microemulsion droplet as it changes component concentrations in the microemulsion system. Regarding the impact of different morphologies in BM and PL-MEs, some studies indicated that worm-like or elongated (rather than spherical) emulsion droplets tend to have improved photostability, better skin permeation, and a higher drug loading [36].
Decreasing pH after three weeks is expected, as fatty acid ester bond in polysorbate can be hydrolyzed and release free fatty acids during storage [37]. The acidity of a microemulsion formula should be considered before developing the formula for an intended application. For instance, to develop a microemulsion-based topical or transdermal therapeutic system, the recommended pH is around 4.1 to 5.8 [38,39]. With a pH that matches normal skin condition, the microemulsion formulation will possess lower risk of irritation and discomfort upon application onto the patient’s skin [38,40]. BM and PL-MEs had a pH of less than 4, which is too acidic for transdermal delivery. Therefore, to make the formulated PL-MEs suitable for development of non-invasive transdermal or topical breast cancer treatment, the pH might be slightly adjusted to reach pH 4 through dilution with aqueous phase or dispersion in buffer [41].
In the measurement of encapsulation efficiency, the concentration of unencapsulated polyphenol in the water phase reflected the performance of the microemulsion system to encapsulate polyphenol in its core [42]. Components of the microemulsion carrier affect its encapsulation efficiency. The components must protect the polyphenol from hydrolytic, oxidative, and light-induced chemical interactions. They also should be able to prevent the interaction between polyphenol and other elements in the dispersed microemulsion system [22]. RA is slightly soluble in water, and this might be the reason why RA was harder to attract into the oil core inside the microemulsion carrier, considering the dispersion system was hydrophilic and the microemulsion core was hydrophobic [43].
Natural antioxidant compounds may prevent or alleviate breast cancer [44]. A compound with high DPPH scavenging activity is better in neutralizing peroxyl radicals, which reduces proinflammatory mediator production and then halts chronic inflammation-mediated carcinogenesis. Nitric oxide (NO) is a water-soluble radical gas commonly found in the human body. Although it has a confusing double-faced role in tumor progression, NO has been reported to be involved in angiogenesis promotion and encouraging the progression of breast carcinoma to the metastasis stage, leading to grave patient prognosis [45]. The role of NO in cancer progression may be linked to its activity as a mediator in inflammation [46]. Hydrophilic surfactant like polysorbate 20 improves water dispersibility of polyphenols in the microemulsion system and protect them from degradation, hence the higher antioxidant activity of PL-MEs than pure polyphenols [47]. Que and RA exhibited comparable antioxidant activities, whereas Kae had the lowest activity, whether in its pure form or as a microemulsion (KM). Previous study reported similar results; Que is a stronger antioxidant than Kae because it has more hydroxyl groups than Kae, and Que has the same amount of hydroxyl as RA [48]. Nevertheless, the activity of Kae was enhanced after being carried into the microemulsion system. A study on myricetin microemulsion displayed similar results, where the bioactivity of the antioxidant compound was enhanced after microemulsion encapsulation [49]. When incorporated into a carrier system, antioxidants will be protected from oxidation and retain their radical scavenging activity compared with pure compounds without a carrier system [50]. BM exhibited poor antioxidant activities against both DPPH and NO radicals. This confirms that improved activity of PL-MEs was caused by the well-protected polyphenol in the optimized microemulsion carrier.
Each cancer cell line has its own unique molecular features that affect its drug response; hence, different cytotoxicity of drugs on different cell lines is expected [51]. In this study, all pure polyphenols showed no cytotoxicity on T47D, while on MDA-MB-231, Kae was the only noncytotoxic tested compound. The structure–activity relationship may also influence the cytotoxicity of the polyphenols. In flavonoids, cytotoxicity comes from an adequate amount of hydroxyl group, the presence of ortho-dihydroxy and 3′-OH in ring B, and the presence of a 2,3-double bond. Kae has the lowest hydroxyl number among the tested polyphenols, and its B ring has no ortho-dihydroxy and 3′-OH. Additionally, Kae has 5-OH, which has been reported before to lower the cytotoxicity of Kae [52].
Although BM did not carry any polyphenol, it showed comparable cytotoxicity to the PL-MEs in both cell lines. An additional study conducted on HaCaT cells also showed higher toxicity of all PL-MEs and BM than pure polyphenols (Figures S2 and S3). It could potentially be caused by components of the microemulsion carrier, i.e., polysorbate, oil, ethanol. Ethanol at concentrations ranging from 0.15% to 1.25% is non-toxic to MDA-MB-231 and MCF-7 cells [53]. Ethanol in PL-MEs was only 0.6% in 50 µg/mL PL-ME, meaning that the concentration was too low to induce a cytotoxicity effect on both cell lines. Polysorbate 20 has been reported to significantly decrease the viability of human colon adenocarcinoma, HT-29, at a concentration of 0.02% [54]. In 50 µg/mL PL-ME, the concentration of polysorbate 20 was 1%, which is higher than the reported safe concentration. This suggests the contribution of polysorbate 20 in the PL-ME toxicity. Several mechanisms allow surfactants to exhibit high toxicity in cells. First, as an emulsifier, a surfactant might break down the cell membranes, or change the position and function of active transporters located in the cell membranes. Second, in the in vitro studies (i.e., MTT assay), surfactants may compete with cells for surface attachment in culture plates, leading to detachment of the cells [54].
Another possible source of toxicity in BM is oleic acid. A study reported a high tumoricidal effect of oleic acid-modified liposomes on MDA-MB-231 [55]. Moreover, oleic acid enhances growth inhibitory effect of trastuzumab in SKBr-3 and BT-474 cells, which both are breast cancer cell lines [56]. Furthermore, cytotoxicity of microemulsion without drug (blank microemulsion) has also been reported in several studies. In a study on mitomycin-C microemulsion, a blank microemulsion that consisted of polysorbate 80, lecithin, soybean oil, ethanol, and water reduced viability of A549 carcinoma cells by 41% at 300 μM dose. The study further suggested that free polysorbate 80 concentration in the formula might lead to significant toxicity to cells. Therefore, enough excipient (i.e., co-surfactant like lecithin) may be necessary for the formula to bind the polysorbate 80 and reduce its toxicity [57]. Another study reported that drug-free microemulsion made from polysorbate 80, span 20, isopropyl myristate, and ethanol had comparable cytotoxicity with gemcitabine-loaded microemulsion on MCF-7 and HCT116 [58]. Furthermore, the morphology of BM seemed more elongated than that of QM, KM, and RM. This factor could also contribute to the cytotoxicity activity of BM, although to confirm this, an extensive investigation is needed. Considering the overall results and supporting data from the aforementioned studies, the microemulsion formulation in this study might lack a co-surfactant (e.g., lecithin) to mask the toxicity of polysorbate 20. This finding highlights the role of co-surfactants and other excipients in reducing the toxicity of microemulsion carriers. It also opens a potential anticancer activity of blank microemulsion itself after further optimization, without the need for drug loading.
4. Materials and Methods
4.1. Microemulsion Formulation
The low-energy method was utilized based on the previous study [59]. Que (≥95% purity; Sigma-Aldrich, St. Louis, MO, USA), Kae (>97%; Tokyo Chemical Industry, Tokyo, Japan), RA (≥96% purity; Sigma-Aldrich, USA) with concentration of 500 µg/mL were mixed with various concentrations of carrier oil: oleic acid (Tokyo Chemical Industry, Japan), olive oil (Rumah Atsiri Indonesia, Karanganyar, Indonesia), or MCT oil (Coco’ Care, Bangkok, Thailand). To the organic mixture, ethanol (AR grade; RCI Labscan, Bangkok, Thailand) and surfactant (polysorbate 80 or polysorbate 20; Sigma-Aldrich, USA) were added and homogenized using a magnetic stirrer (Corning Hot Plate Stirrer PC-351, Corning, Inc., New York, NY, USA) to make an organic phase (Tables S1–S3). Ultrapure water was added drop-by-drop to the organic phase mixture while stirring at 550 rpm for 30 min at room temperature. The exact concentration of surfactant, carrier oil, and ethanol of optimized microemulsion carrier was obtained after a series of component screening experiments (Tables S1–S3, Figure S4). The optimized microemulsion carrier was produced from 1% (v/v) oleic acid, 3% (v/v) ethanol, 10% (v/v) polysorbate 20, and 86% (v/v) ultrapure water.
4.2. Microemulsion Characterization
4.2.1. Particle Size
Particle size and polydispersity index (PDI) of BM and polyphenol-loaded microemulsions (PL-MEs), i.e., quercetin-loaded microemulsion (QM), kaempferol-loaded microemulsion (KM), and rosmarinic acid-loaded microemulsion (RM), were measured by the dynamic laser scattering method [60]. Each formula was diluted in ultrapure water (1:2 dilution) for the measurement. The analysis was carried out in triplicate at 25 °C by Zetasizer Nano ZSP (Malvern Instruments Ltd., Malvern, UK).
4.2.2. Zeta Potential
The electric charge on microemulsion droplets was determined [61]. BM and PL-MEs (3 mL, 1:2 dilution in ultrapure water) were analyzed with particle electrophoresis instrument (Zetasizer Nano ZSP, Malvern Instruments Ltd., Malvern, UK). The analysis was carried out thrice at 25 °C.
4.2.3. Transmission Electron Microscopy (TEM) Visualization
Negative staining was used to visualize BM and PL-ME droplets [61]. The microemulsion formulations (7 µL) were ten-fold diluted, then positioned on a copper grid, and washed with ultrapure water. A solution of 2% uranyl acetate (7 µL) was used to stain the droplets. The TEM (JEM-1400; JEOL Ltd., Tokyo, Japan) micrographs were acquired at 200 kV. A grating replica 3 mm grid calibrated the scales.
4.2.4. Physicochemical Stability Test
Optimized PL-MEs and BM were subjected to an accelerated stability test that subsequently started from centrifugation test (3500 rpm for 30 min; Velocity 14R Refrigerated Benchtop Centrifuge, Dynamica Scientific Ltd., Livingston, UK), six cycles of heating–cooling, to three cycles of freeze–thawing [62]. One cycle of heating–cooling needed 4 days to finish; it involved 48 h of microemulsion storage at 40 °C, followed by 48 h of storage at 4 °C. Similar to heating–cooling, one cycle of freeze–thawing also needed 4 days to be performed; it consisted of 48 h of storage at −19 °C, then the thawing process continued at 30 °C for 48 h. Before and after the stability test, particle size, PDI, and zeta potential were measured. Aggregation, turbidity, and transmittance were measured with ultraviolet–visible (UV-Vis) spectrophotometer (650 nm; Shimadzu UV-1800, Kyoto, Japan) after specific cycles. The pH stability was observed at 37 °C and measured by a pH meter (Apera PH700 Benchtop, Columbus, OH, USA) once a week during a 3-week observation.
4.2.5. Encapsulation Efficiency
Efficiency of the optimized microemulsion carrier to encapsulate selected polyphenols was analyzed [42]. BM and PL-ME (500 µL) were placed in ultrafiltration units with 5 KDa cut-off (Sartorius, Goettingen, Germany) and centrifuged for 30 min (10,000 rpm, 28 °C). The optical density of polyphenol in the filtrate was measured in a 96-well microplate at the maximum wavelength (λmax) of each polyphenol (Que = 374 nm, Kae = 363 nm, and RA = 325 nm; BioTek Synergy H1 plate reader, BioTek Instruments Inc., Winooski, VT, USA). Standard curves used in determination of polyphenol concentration were prepared by making separated two-fold dilution for Que, Kae, and RA in a known range of concentration from 125 to 0.488 µg/mL. The concentrations were measured using the same maximum wavelength of each compound mentioned before. The concentration of free polyphenol in the filtrate was estimated by standard curves. The final encapsulation efficiency was calculated by the following equation:
Total polyphenol mass was the initial amount of polyphenol that was added into the microemulsion formula (500 µg/mL), and free polyphenol mass was the estimated amount of polyphenol outside the microemulsion carrier.
4.3. In Vitro Antioxidant Activities
4.3.1. Free Radical Scavenging Activity against 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
The free radical scavenging ability of BM and PL-MEs against DPPH (Sigma-Aldrich, USA) was determined based on a previous study [63]. Diluted BM and PL-MEs in distilled water were made at concentrations ranging from 3.125 µg/mL to 100 µg/mL. Polyphenols (Que, Kae, and RA) were prepared in DMSO (RCI Labscan, Thailand) to compare the activity. Each solution (20 µL) was then placed into a 96-well plate and measured with a microplate reader (517 nm, Biotek Synergy H1 plate reader). A solution of 120 µM DPPH in MeOH (180 µL) was added into the wells, and the mixture was dark-incubated for 30 min (250 rpm, 37 °C). Ultrapure water and DMSO were used as blanks. The solution absorbance was measured at 517 nm, and the radical scavenging activity was calculated by the equation below:
A0 is expressed as an absorbance of negative control, and A is expressed as the absorbance of a tested sample after the reaction.
4.3.2. Free Radical Scavenging Activity against Nitric Oxide (NO)
Griess reaction from previous study was utilized to assess the scavenging activity of polyphenols, BM, and PL-MEs against NO [64]. Polyphenols (Que, Kae, and RA) were dissolved in MeOH and diluted in distilled water. BM and PL-MEs were diluted in distilled water. Gallic acid (Sigma-Aldrich, USA) was used as a positive control. All microemulsions and compounds were diluted in a range of concentrations (400, 200, 100, 50, 25, 12.5, and 0 µg/mL). Each solution (10 µL) was placed into a 96-well plate, followed by adding 10 mM SNP (Sigma-Aldrich, USA) solution (90 µL) under polychromatic light condition for 90 min in an open incubator shaker. A solution of 1% sulfanilamide was then added (50 µL) and incubation continued under dark conditions (5 min). The 0.1% NED (Sigma-Aldrich, USA) solution (50 µL) was sequentially added and incubated again (dark condition, 30 min). The absorbance was immediately measured at 540 nm. The inhibition of NO radical formation was calculated by the equation below:
A0 is expressed as an absorbance of a negative control, and A is expressed as the absorbance of a tested sample after the reaction.
4.4. In Vitro Cytotoxic Activities
4.4.1. Cytotoxicity on Breast Cancer Cells
The hormone-dependent T47D cells (ATCC: HTB-133) were cultured in RPMI-1640 (HyClone Laboratories, Inc., Logan, UT, USA) with L-glutamine (2 mM; Sigma-Aldrich, USA) and supplemented with penicillin-streptomycin (100 U/mL; Sigma-Aldrich, USA), insulin (0.2 U/mL; Sigma-Aldrich, USA), glucose (4.5 g/L; Sigma-Aldrich, USA), and fetal bovine serum (10%; JR Scientific, Inc., USA). The cells were added to each well at a density of 0.5 × 104 to 2 × 104 cells and grown for 24 h (37 °C, 5% CO2, 95% humidity). DMEM (HyClone Laboratories, USA) was used to culture hormone-independent MDA-MB-231 cells (ATCC: HTB-26), containing 100 U/mL penicillin-streptomycin and 10% fetal bovine serum. The culture conditions were similar to T47D cell culture; each well contained a range of 0.5 × 104 and 2 × 104 cells and the temperature was set at 37 °C, with 5% CO2, and 95% humidity. Doxorubicin was used as a standard drug. The cytotoxicity of polyphenols, BM, and PL-MEs was assessed by MTT assay. Doxorubicin (Sigma-Aldrich, USA), DMSO, and tested samples (Que, Kae, RA, BM, and PL-MEs) were dissolved in DMEM. The solutions were added to the well filled with T47D or MDA-MB-231. The treatment incubation was allowed for 48 h. The MTT (Sigma-Aldrich, USA) solution (100 µL, in serum-free cell culture medium) was added into the wells, and the incubation continued at 37 °C (95% humidity, 5% CO2). After that, the solution was replaced with DMSO (100 µL). The absorbance was measured at 550 nm with a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The experiments were carried out in triplicate. The cytotoxicity against breast cancer cell lines was reported as an IC50 value in μg/mL.
4.4.2. Cytotoxicity on Human Keratinocyte
The human epidermal keratinocytes (HaCaT) cells were cultured in DMEM supplemented with fetal bovine serum (10%), penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37 °C with 5% CO2. The cells were seeded into a 96-well plate (104 cells/well) and grown for 16 h. Polyphenols, BM, and PL-MEs were added and incubated with the cells for 24 h. Cisplatin was used as a standard drug. The cell viability was determined by MTT assay. The culture media was discarded and changed with MTT solution (0.5 mg MTT in 1 mL of complete medium). This mixture was incubated at 37 °C for 3 h. Next, the solution was replaced with DMSO (100 µL). The absorbance was measured at 570 nm using a microplate reader (Varioskan™ LUX multimode microplate reader, Thermo Scientific™). The experiments were conducted in triplicate. The percentage of inhibition was determined.
4.5. Statistical Analysis
One-way analysis of variance by Duncan’s Multiple Range Test (p < 0.05) was accomplished with IBM SPSS Statistics version 29 to determine whether there was a significant difference in the mean between groups.
5. Conclusions
This study addressed the different bioactivities of common dietary polyphenols and how a microemulsion carrier may improve their bioactivities. The optimized microemulsion carrier was made from 1% oleic acid, 3% ethanol, 10% polysorbate 20, and 86% ultrapure water. After loading pure polyphenols into a microemulsion, the size and PDI of all formulations were maintained under 100 nm and 0.5, respectively, even after more than two months of stability tests. These characteristics confirmed the homogeneity and stability of the PL-ME formulations. QM, KM, and RM had a high polyphenol encapsulation efficiency, which was higher than 90%. All polyphenol-loaded microemulsion formulations exhibited non-spherical droplet morphologies. This type of morphology is commonly observed in oil-in-water microemulsion systems. Among all tested dietary polyphenols and their microemulsion formulations, RM performed comparably well or even better than standard compounds and pure rosmarinic acid as an antioxidant that inhibited DPPH and nitric oxide radicals. Incorporation of polyphenols into the optimized microemulsion carrier not only drastically inhibited the proliferation of both hormone-dependent and hormone-independent breast cancer cells, but also the normal dermal cells. Further studies are recommended to assess the potential involvement of polysorbate 20 and oleic acid in the toxicity of the microemulsion carrier system. Nevertheless, the results of this study demonstrate the potential use of common dietary polyphenols (especially RA), and how microemulsion carriers protect the antioxidant activity of oxidation-prone polyphenols, which may be valuable in developing antioxidant supplements for breast cancer prevention. For the future direction of this study, it would be interesting to explore additional modifications of the microemulsion formula, such as the utilization of water-soluble polyphenolate sodium salts, to possibly improve the solubility of lipophilic polyphenols without excessive surfactants [65].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92020025/s1, Figure S1: Gradual change of blank microemulsion (BM) and PL-ME formulations (QM, KM, and RM) at different pH values and times; Figure S2: Dietary polyphenols exhibit safer inhibitory activity on normal dermal cells (HaCaT) proliferation than cisplatin; Figure S3: Inhibitory effect of PL-MEs on proliferation of normal dermal cells (HaCaT); Figure S4: Appearance of quercetin (Q)- and rosmarinic acid (R)-loaded microemulsions based on experiment in Table S3; Table S1: First screening of component and optimization of microemulsion carrier without polyphenols; Table S2: Second screening of components and further optimization of FA3 without polyphenols to reduce EtOH concentration; Table S3: Incorporating dietary polyphenols (Que and RA) into selected microemulsion carrier candidates (microemulsion carrier B).
Author Contributions
G.P.C.: Conceptualization, Methodology, Investigation, Data curation, Analysis and Writing—original draft preparation; D.L.: Methodology, Investigation and Writing—review and editing; J.C.: Methodology, Investigation and Writing—review and editing; C.A.: Methodology, Investigation and Writing—review and editing; C.S.: Supervision and Writing—review and editing; A.P.: Supervision, Conceptualization, Methodology, Validation, Analysis and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is partly supported by Grants for the Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (Grant no. DNS 66_004_53_002_3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Acknowledgments
The authors thank Pakamas Intachote, Suchada Sengsai, and Busakorn Saimanee for the cytotoxicity tests at Chulabhorn Research Institute.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sedeta, E.T.; Jobre, B.; Avezbakiyev, B. Breast cancer: Global patterns of incidence, mortality, and trends. J. Clin. Oncol. 2023, 41, 10528. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, A.B. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011, 50, 187–193. [Google Scholar] [CrossRef]
- Jiwa, M.; Long, A.; Shaw, T.; Pagey, G.; Halkett, G.; Pillai, V.; Meng, X. The management of acute adverse effects of breast cancer treatment in general practice: A video-vignette study. J. Med. Internet Res. 2014, 16, e204. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Bellanger, M.; Barry, K.; Rana, J.; Regnaux, J.-P. Cost-Effectiveness of Lifestyle-Related Interventions for the Primary Prevention of Breast Cancer: A Rapid Review. Front. Med. 2020, 6, 325. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C.; et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. 2020, 38, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.Y.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Yadegarynia, S.; Pham, A.; Ng, A.; Nguyen, D.; Lialiutska, T.; Bortolazzo, A.; Sivryuk, V.; Bremer, M.; White, J.B. Profiling flavonoid cytotoxicity in human breast cancer cell lines: Determination of structure-function relationships. Nat. Prod. Commun. 2012, 7, 1295–1304. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.G.; Chan, S.L.F.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A. Microemulsions: A novel approach to enhanced oil recovery: A review. J. Petrol. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Yang, F.; He, Q.; Dai, X.; Zhang, X.; Song, D. The potential role of nanomedicine in the treatment of breast cancer to overcome the obstacles of current therapies. Front. Pharmacol. 2023, 14, 1143102. [Google Scholar] [CrossRef]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Biruss, B.; Dietl, R.; Valenta, C. The influence of selected steroid hormones on the physicochemical behaviour of DPPC liposomes. Chem. Phys. Lipids 2007, 148, 84–90. [Google Scholar] [CrossRef]
- Mori Cortés, N.; Califano, A.N.; Lorenzo, G. Physical and chemical stability under environmental stress of microemulsions formulated with fish oil. Food Res. Inter. 2019, 119, 283–290. [Google Scholar] [CrossRef]
- Feng, J.-L.; Wang, Z.-W.; Zhang, J.; Wang, Z.-N.; Liu, F. Study on food-grade vitamin E microemulsions based on nonionic emulsifiers. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 1–6. [Google Scholar] [CrossRef]
- Mandal, S. Curcumin, a promising anti-cancer therapeutic: It’s bioactivity and development of drug delivery vehicles. Int. J. Drug Res. Technol. 2016, 6, 43–57. [Google Scholar]
- Luo, Z.; Murray, B.S.; Yusoff, A.; Morgan, M.R.A.; Povey, M.J.W.; Day, A.J. Particle-Stabilizing Effects of Flavonoids at the Oil−Water Interface. J. Agric. Food Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef]
- Yuliani, S.; Muchtadi, T.R.; Syakir, M. Changes in characteristics of nanoemulsion of cinnamon oil and their relationships with instability mechanisms during storage. J. Food Process. Preserv. 2018, 42, e13745. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [PubMed]
- Chansiri, G.; Lyons, R.T.; Patel, M.V.; Hem, S.L. Effect of surface charge on the stability of oil/water emulsions during steam sterilization. J. Pharm. Sci. 1999, 88, 454–458. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat Doost, A.; Dewettinck, K.; Devlieghere, F.; Van der Meeren, P. Influence of non-ionic emulsifier type on the stability of cinnamaldehyde nanoemulsions: A comparison of polysorbate 80 and hydrophobically modified inulin. Food Chem. 2018, 258, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; McClements, D.J. Food-grade microemulsions, nanoemulsions and emulsions: Fabrication from sucrose monopalmitate & lemon oil. Food Hydrocolloids 2011, 25, 1413–1423. [Google Scholar] [CrossRef]
- Rolland, M.; Truong, N.P.; Parkatzidis, K.; Pilkington, E.H.; Torzynski, A.L.; Style, R.W.; Dufresne, E.R.; Anastasaki, A. Shape-Controlled Nanoparticles from a Low-Energy Nanoemulsion. JACS Au 2021, 1, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Musakhanian, J.; Rodier, J.-D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Hatzistamatiou, K.; Lykidou, S.; Barmpalexis, P.; Nikolaidis, N. Preparation and Investigation of the SPF and Antioxidant Properties of O/W and W/O Emulsions Containing Vitamins A, C and E for Cosmetic Applications. Cosmetics 2023, 10, 76. [Google Scholar] [CrossRef]
- Segger, D.; Aßmus, U.; Brock, M.; Erasmy, J.; Finkel, P.; Fitzner, A.; Heuss, H.; Kortemeier, U.; Munke, S.; Rheinländer, T.; et al. Multicenter study on measurement of the natural pH of the skin surface. Int. J. Cos. Sci. 2008, 30, 75. [Google Scholar] [CrossRef]
- Üstündağ Okur, N.; Çağlar, E.Ş.; Arpa, M.D.; Karasulu, H.Y. Preparation and evaluation of novel microemulsion-based hydrogels for dermal delivery of benzocaine. Pharm. Dev. Technol. 2017, 22, 500–510. [Google Scholar] [CrossRef]
- Park, C.; Zuo, J.; Somayaji, V.; Lee, B.-J.; Löbenberg, R. Development of a novel cannabinoid-loaded microemulsion towards an improved stability and transdermal delivery. Int. J. Pharm. 2021, 604, 120766. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.A.; Donsì, F.; Pinheiro, A.C.; Ferrari, G.; Vicente, A.A. Development and Characterization of Lipid-Based Nanosystems: Effect of Interfacial Composition on Nanoemulsion Behavior. Food Bioprocess Technol. 2020, 13, 67–87. [Google Scholar] [CrossRef]
- Huang, S.-s.; Zheng, R.-l. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wei, Z.; Teichmann, A.T.; Wieland, F.H.; Wang, A.; Lei, X.; Zhu, Y.; Yin, J.; Fan, T.; Zhou, L.; et al. Development of a novel nitric oxide (NO) production inhibitor with potential therapeutic effect on chronic inflammation. Eur. J. Med. Chem. 2020, 193, 112216. [Google Scholar] [CrossRef]
- Donsì, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion- based delivery systems. Procedia Food Sci. 2011, 1, 1666–1671. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Guo, R.X.; Fu, X.; Chen, J.; Zhou, L.; Chen, G. Preparation and Characterization of Microemulsions of Myricetin for Improving Its Antiproliferative and Antioxidative Activities and Oral Bioavailability. J. Agric. Food Chem. 2016, 64, 6286–6294. [Google Scholar] [CrossRef]
- Amuti, A.; Wang, X.; Zan, M.; Lv, S.; Wang, Z. Formulation and characterization of curcumin-loaded microemulsions: Evaluation of antioxidant stability and in vitro release. J. Mol. Liq. 2021, 336, 116881. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Mi, M.; Ling, W.; Zhu, J.; Zhang, Q.; Wei, N.; Zhou, Y.; Tang, Y.; Yu, X.; Zhang, T.; et al. Structurally related anticancer activity of flavonoids: Involvement of reactive oxygen species generation. J. Food Biochem. 2010, 34, 1–14. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.-L.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Li, Y.; Le Maux, S.; Xiao, H.; McClements, D.J. Emulsion-Based Delivery Systems for Tributyrin, a Potential Colon Cancer Preventative Agent. J. Agric. Food Chem. 2009, 57, 9243–9249. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, S.; Lee, H.; Yoon, J.; Lee, E.K. Oleic acid-embedded nanoliposome as a selective tumoricidal agent. Colloids Surf. B: Biointerfaces 2016, 146, 585–589. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin™) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef]
- Kotmakchiev, M.; Kantarcı, G.; Çetintaş, V.B.; Ertan, G. Cytotoxicity of a Novel Oil/Water Microemulsion System Loaded with Mitomycin-C in In Vitro Lung Cancer Models. Drug Dev. Res. 2012, 73, 185–195. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Alkhayyal, N.S. Cytotoxicity of Gemcitabine-Loaded-Microemulsions in Breast and Colon Cancer Cells. Trop. J. Pharm. Res. 2014, 13, 217–224. [Google Scholar] [CrossRef]
- Mandal, S.D.; Mandal, S.; Patel, J. Brain targeting efficiency of Curcumin loaded mucoadhesive microemulsion through intranasal route. J. Pharm. Investig. 2016, 46, 179–188. [Google Scholar] [CrossRef]
- Roohinejad, S.; Oey, I.; Wen, J.; Lee, S.J.; Everett, D.W.; Burritt, D.J. Formulation of oil-in-water β-carotene microemulsions: Effect of oil type and fatty acid chain length. Food Chem. 2015, 174, 270–278. [Google Scholar] [CrossRef]
- Son, H.-Y.; Lee, M.-S.; Chang, E.; Kim, S.-Y.; Kang, B.; Ko, H.; Kim, I.-H.; Zhong, Q.; Jo, Y.-H.; Kim, C.-T.; et al. Formulation and Characterization of Quercetin-loaded Oil in Water Nanoemulsion and Evaluation of Hypocholesterolemic Activity in Rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Intanai, N. Formulation of Nanoemulsion Containing Ellagic Acid from Pomegranate Rind Extract for Skin Delivery. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2011. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Liana, D.; Eurtivong, C.; Phanumartwiwath, A. Boesenbergia rotunda and Its Pinostrobin for Atopic Dermatitis: Dual 5-Lipoxygenase and Cyclooxygenase-2 Inhibitor and Its Mechanistic Study through Steady-State Kinetics and Molecular Modeling. Antioxidants 2024, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, N.; Häckl, K.; Touraud, D.; Kunz, W. Investigation of the salting-in/-out, hydrotropic and surface-active behavior of plant-based hormone and phenolic acid salts. J. Colloid Interface Sci. 2023, 641, 631–642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).