Abstract
Coccoloba uvifera is a Mayan medicinal plant, and these leaves are used as antidiarrheal and diuretic agents. In the present work, we develop in-vitro, ex-vivo, in-vivo, and in-silico strategies to evaluate several aqueous extracts of C. uvifera leaves. In vitro tests showed that decoction extract (CuDe) presented the best yield and chlorophyll, phenol, and flavonoid content; however, CuDe showed low antioxidant activity (DPPH model). All aqueous extracts exert spasmolytic and vasorelaxant activity in a concentration-dependent manner (ex vivo), and in vivo tests showed that CuDe exerts the best antiperistaltic and diuretic effects. The in-silico analysis suggests that C. uvifera triterpenes act as a ligand of GPCR, and β-sitosterol could act as an antagonist of muscarinic acetylcholine receptor subtype 3 (m3AChR). In the context of aqueous extracts of C. uvifera, β-sitosterol and their heterosides were identified by FTIR and 1H-NMR spectroscopy. The concerted binding of β-sitosterol and other triterpenes within the m3AChR binding site may be relevant for the induction of relaxant effects at the gastrointestinal smooth muscle level. In this context, C. uvifera is a high-value plant species that requires analytical and pharmacological studies to confirm traditional medicinal use.
1. Introduction
As noted in the literature, there has been an increase in information related to the involvement of chemical entities in the pharmacological effects induced by medicinal plants [1]. Currently, many medicinal plants are used in cardiovascular, respiratory, gastrointestinal, and urinary diseases, among others [2]. The therapeutic potential of medicinal plants increases with regional idiosyncrasy, as well as with a shortage of allopathic medicines [3]. In a health system, herbal preparations represent a relevant strategy for therapeutic diseases. In addition, it is the historical strategy used for the identification of new chemical entities with pharmacological applications.
Historically, the study of natural products has been interested in the isolation of chemical entities, physicochemical characterization [4], and their pharmacological evaluation. Technological development has allowed for the application of bio-guided strategies and the identification of numerous chemical entities with therapeutic applications [5]. The evolution in the study of natural products is directed toward the use of mathematical and statistical methods in the chemical context [6], allowing for the application of computational approaches in the discovery of drugs from natural products [7]. Nowadays, more and more research simultaneously uses computational models, chemical or cellular experiments, and well-characterized and controlled animal models [8].
Coccoloba uvifera L. (Polygonaceae), also called “beachgrape”, is a tree native to the tropical and subtropical coasts of the Pacific and Gulf of Mexico. Teas and decoctions from aerial parts of C. uvifera have been used in the treatment of diarrhea [9] and dysentery [10,11]. Antimicrobial [12], antifungal, anti-hyperglycemic, anti-inflammatory, antioxidant [11,13], choline- and butyryl-cholinesterase inhibition, and photo-protective and cytotoxic/anti-proliferative in LNCaP cell line [14,15,16] activities have been reported to be inhabited by the extracts of the aerial parts. Hydroalcoholic extracts are reported as healing and not irritating to humans [16]. Moreover, phytochemical studies have allowed for the identification of several families of secondary metabolites like alkaloids, saponins, flavonoids, polyphenols, triterpenes, and sterols [12], amongst others. Flavonoids (myricetin 3-O-rhamnoside, myricetin 3-O-glucoside, quercetin 3-O-rhamnoside, quercetin 3-O-arabinoside [11], sterols (β-sitosterol), diterpenes (royleanone), triterpenes (lupeol, α-amyrin), β-amyrin [17], phenolic acids (gallic acid), anthraquinones (emodin, chrysophanol, physcion, rhein), and isochromene) were identified or isolated in C. uvifera leaves [18].
Despite extensive studies being conducted, few of them report on the functional properties at the gastrointestinal smooth muscle level. In this context, C. uvifera is a high-value plant species that requires pharmacological and phytochemical studies to confirm traditional medicinal use. Then, the present work focuses on the phytochemical and pharmacological evaluation (in silico, in vitro, ex vivo, and in vivo) of several aqueous extracts from the leaves of C. uvifera.
2. Materials and Methods
2.1. Plant Material
Leaves of C. uvifera (LCu) were collected in the urban coastal area of San Francisco de Campeche, Campeche, Mexico, in April 2021. The specimens collected were identified and deposited in UAC-Herbarium (V: P-7570).
2.2. Preparation of the Extracts
After drying at room temperature (seven days), LCu were crushed and grounded to storage in sealed plastic bags (Ziploc®). All aqueous extraction methods, a fixed mass of C. uvifera, and a volume of distilled water (ratio: 1/28; g/mL) were employed.
For maceration (CuMa), LCu was kept in contact with water for 24 h at room temperature. Percolation (CuPe): A column (i.d. 2 cm; 25 mL) was packed by LCu, and separation was started by eluting distilled water (24 °C) at 1 mL/min flux (±25 min). Infusion (CuIn): Water at 100 °C was transferred to the LCu flask, homogenized, and removed from the heat source; the extraction process was carried out until the extract reached room temperature (±35 min). Decoction (CuDe): The temperature of the LCu extraction system was raised from room temperature to boiling point (±97 °C) and kept for 15 min, and then it was removed from the heat source. Digestion (CuDi): The temperature of the LCu extraction system was raised to 45 °C and kept for 15 min. The extraction process for CuDe and CuDi was carried out until reaching 23 °C. Finally, all the extracts were filtered and dried using forced air at 60 °C and stored at 5 °C for further analysis.
2.3. Spectroscopic Analysis
In total, 5 mg of CuMa, CuPe, CuIn, CuDe, and CuDi and 195 mg of KBr were mixed in an agate mortar, and FTIR analyses were carried out. For this, pellets were loaded in Thermo Nicolet Nexus 670: 500–4000 cm−1 at 4 cm−1 of resolution. 1H-NMR (600 MHz, DMSO-d6) analyses were carried out using the Varian-Agilent AR Premium Compact spectrometer (Santa Clara, CA, USA). The spectra 1H were acquired with 64 and 50,000 scans, and the spectral width was 16 and 250 ppm, respectively.
2.4. Ex Vivo Evaluation
All experimental procedures and protocols with animals were developed according to NOM-062-ZOO-1999 [19], and they comply with the international guidelines on the care and use of laboratory animals (12 h light/dark cycle; 25 °C; food and water ad libitum). Adult male rats, Wistar strain (±275 g), were used after a three-day adaptation period. After abdominal dissection, the ileum and thoracic aorta were obtained free of connective tissue and fat. All tissues were maintained in a Ringer–Krebs–Henseleit solution (RKHS).
For spasmolytic evaluation [20], segments of the ileum (±0.5 cm) were used; tissues were stabilized at one gram of tension for 30 min. The activity exerted by aqueous extracts of C. uvifera (0.03 → 300 µg/mL) and papaverine (0.001 → 3 µg/mL) solutions was compared to the basal state of the spasmodic activity of the ileum. For vasorelaxant evaluation [21], aortic rings (±0.3 cm) were stabilized at three grams of tension for 30 min. Then, all the aorta ring tissues were sensitized with noradrenaline (NA, 1 × 10−7 M) for 15 min, washed with RKHS, and re-sensitized with NA after 30 min. The presence of endothelium was corroborated by the response to carbachol (1 × 10−6 M). The vasorelaxant activity induced by aqueous extracts of C. uvifera (0.1 → 300 µg/mL) and carbachol (0.00056 → 2 µg/mL) solutions was compared with the maximal contraction induced by NA. Acqknowledge software (MP150, Biopac Instruments, Co., Goleta, CA, USA) was used, and six independent experiments were performed (n = 6) for each extract.
2.5. In Vivo Evaluation
Male rats were divided into the following experimental groups: decoction (CuDe; 13.7 mg/mL); digestion (CuDi; 2.24 mg/mL); infusion (CuIn; 3.5 mg/mL); maceration (CuMa; 11.14 mg/mL); percolation (CuPer; 1.79 mg/mL); furosemide; and vehicle (H2O). All test solutions were administered orally in an aqueous solution ad libitum. Finally, in metabolic cages (Tecniplast®, West Chester, PA, USA), water and food feasting, as well as solid and liquid excreta production, were recorded for 8 h. All experimental groups were subjected to four independent experiments with three replicates each (n = 12).
2.6. In Vitro Tests
The chlorophyll content [22] in CuIn, CuMa, CuPer, CuDe, and CuDi was estimated by measuring absorbance at λ = 645 and 663 nm, as well as the use of ChlTOT (μg/mL) 20.2 (A645) + 8.02 (A663) formula. Neutral and acidified (HCl 0.1 N; t = 2 h) aqueous extracts of C. uvifera (10 mg/mL) were evaluated using the Lieberman–Burchard test; the change in color to green was considered positive [23].
For simple phenols [24], 0.4 mL from GA (1.04–8.2 μg/mL) or extract stock solution (10 μg/mL) and 0.4 mL of Folin–Ciocalteu reagent were transferred to 1 mL of distilled water and incubated for 30 min in darkness. The reaction was stopped with Na2CO3 10% (1.6 mL), and the absorbance was recorded at 765 nm. The content of simple phenols was expressed as equivalents (mg/g) of gallic acid (GA). For flavonoids [25], 0.3 mL of quercetin (1–10 μg/mL) or extract stock solution (10 μg/mL) was added to 0.9 mL of MeOH, 0.15 mL of AlCl3 10% solution, 0.15 mL of CH3CO2K 1 M, and 1.8 mL of distilled water. All samples were incubated for 30 min at room temperature in the dark, and the absorbance was recorded at 415 nm. The content of flavonoids in aqueous extracts was expressed as equivalents (mg/g) of quercetin.
For antioxidant activity [26], 0.2 mL of C. uvifera extracts (100 µg/mL), C. sinensis (50 µg/mL) extract, or quercetin (10 μg/mL) solution was added to 1.8 mL of DPPH 0.2 M. After mixing, all the samples were incubated at room temperature in the dark for 30 min. Then, absorbance was recorded at 517 nm, and antioxidant activity was calculated. The percentage of the discoloration (%) of DPPH was calculated using: . All samples were subjected to three independent experiments with three replicates each.
2.7. In Silico Approach
Petra/osiris/molinspiration analysis (POM) is one approach that has been used in Medicinal Chemistry to identify the pharmacophore site that affects the pharmacological activity. In this sense, POM analysis predicts biological activities of chemical entities through steric/electrostatic properties with ligand–receptor interaction [27], is validated with ±7000 drug molecules available in databases, and has been used in the evaluation of natural products with applications in hypertension [28].
On the other hand, to determine the interaction with m3AChR, molecular docking studies with m3AChR were performed. In this sense, the active site on m3AChR was validated using scopolamine (muscarinic receptor antagonist and natural product isolated from Solanaceae species) as the native ligand (PDB: 5ZHP). RMSD was set to less than 2.5 Å to determine the best docking position between m3AChR and ligands using Autodock Vina [29]. The validation was performed with 1000 poses, each in ten replicates, selecting the lowest energy value. Protein visualization and overlap were carried out using Pymol 3.1 (Schrödinger, San Diego; http://www.pymol.org/).
The docking of each ligand (β-sitosterol, lupeol, α-amyrin, β-amyrin, and royleanone) was simulated using the program AutoDock Vina, which has been used to estimate the conformation of protein–ligand complexes and significantly improves the average accuracy of the binding mode predictions. All calculations for protein-fixed ligand-flexible docking were analyzed using the Lamarckian Genetic Algorithm (LGA) method. The docking site on m3AChR was defined by establishing a grid box using Pymol 3.1. The grid box size for the coordinates x, y, and z was 25 Å, with a grid spacing of 0.375 Å, centered on x = −2.265, y = 7.164, and z = 17.568 Å. The best conformation was chosen based on the lowest binding energy after the docking search was completed. Autodock Vina was set for 1000 modes, and in terms of exhaustiveness (exhaustiveness of the global search, roughly proportional to time) for each ligand structure, and for each run, the best pose was saved. The average binding energy for the best poses was used as the final binding energy value; this process was repeated ten times [29].
2.8. Data Analysis
Data were stored in a database and processed through Origin Microcal 8.0. The results are expressed as the mean ± standard error of the mean. Finally, an analysis of variance was performed followed by Tukey’s test; values of p < 0.05 were considered significant.
3. Results
3.1. Phytochemical In Vitro Test
The use of different extraction methods in the C. uvifera leaves allowed for recording variations in the extraction yield as well as other phytochemical parameters. Table 1 shows the extraction yields, chlorophyll, phenols, flavonoid content, and antioxidant activity for different C. uvifera leaf extracts. Colorimetric analysis through the Lieberman–Burchard test indicates the presence of sterols; likewise, the color intensity increased in the aqueous extracts subjected to acid hydrolysis.

Table 1.
Yield and chlorophyll, phenols, and flavonoid content in different aqueous extracts derived from leaves of C. uvifera.
The chlorophyll, simple phenols, and flavonoid content improve with an increase in temperature (CuDe > CuMa; p < 0.05) and time (CuMa > CuPer; p < 0.05) of extraction. In the DPPH model, all extracts were evaluated at 100 mg/mL, the methanol extract of C. sinensis was evaluated at 50 mg/mL, and quercetin was evaluated at 10 mg/mL. The antioxidant activity induced by CuPer (Emax: 64.3 ± 1.1%) was significantly greater than CuMa, CuDi, CuIn, and CuDe; however, it was lower than C. sinensis (Emax: 53.28 ± 0.2%) and quercetin (Emax: 91.62 ± 0.13%). The antioxidant activity exerted by C. uvifera decreases with increasing temperature and extraction time (Table 1).
3.2. Spectroscopy Analysis
The IR spectrum of aqueous extracts of C. uvifera is illustrated in Figure 1. The FTIR spectrum of the extracts possesses distinctive bands at 3255.25 ± 9.45 (-OH stretch), 2930.5 ± 1.7 1 (methyl groups), 1622 ± 2.1 (unconjugated olefinic -C=C-), 1437.1 ± 2.1 (cyclic methylenes), 1364.6 ± 3.2 (gem-dimethyl), 1220.3 ± 2.1 (OH secondary), and ±1070 cm−1 (C-O-C vibrations), as well as bands at 829, 776, and 609 cm−1. These bands could be related to β-sitosterol [30] and β-sitosterol glucoside; in fact, bands at 1641 (-C=C-), 1443 (cyclic -CH2), and 1055 cm−1 (C-O-C) showed a clear displacement of absorbances [31], so these bands could be used for quantitative purposes.
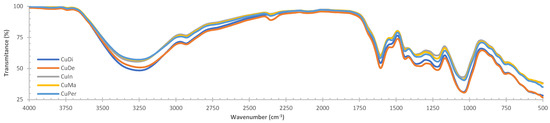
Figure 1.
Fourier transform infrared (FTIR) spectroscopy spectra of aqueous extracts of the aerial parts of C. uvifera leaves (dried solid mass, KBr).
1H-NMR experiments allow us to strengthen the identification of β-sitosterol and β-sitosterol glucoside (Figure 2) in the aqueous extracts of C. uvifera leaves. As can be seen in Figure 3, overlapping signals at δ = 0.76 → 1.25 ppm were assigned to methyl hydrogens at H-19 (s, 3H), H-21 (d, 3H), H-29 (t, 3H), H-26 (d, 3H), H-27 (d, 3H), and H-18 (s, 3H). The signals at δ = 0.95, 3.1, and 5.3 ppm correspond to Me-18 (t), H-3 (1H, m), and H-6 (1H, br d), respectively. In the same way, the presence of heterosides was corroborated to the signal at δ = 4.21 ppm (H-1′, d) [32].
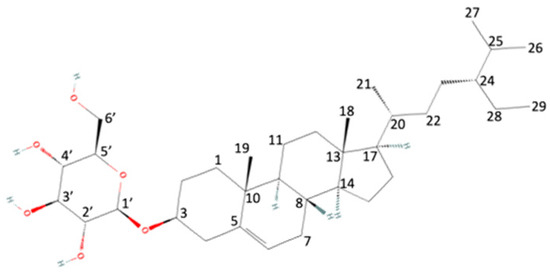
Figure 2.
Two-dimensional structure of β-sitosterol glucoside. Red bonds indicate the electronegativity induced by oxygen atoms.
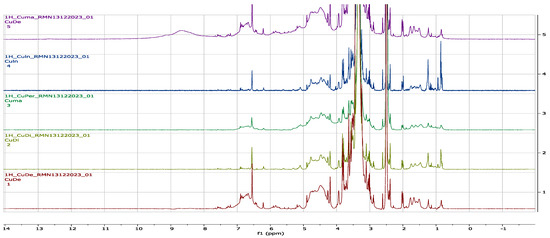
Figure 3.
Representative H-NMR profiles (region 0 to 14 ppm) of the aqueous extracts from C. uvifera leaves obtained by different extraction methods.
3.3. Ex Vivo and In Vivo Evaluation
CuInf, CuMa, CuDe, and CuDi extracts induce partial spasmolytic and vasorelaxant (endothelium +) effects in a concentration-dependent manner; decreased vasorelaxant activity is observed in CuPer. The potency and efficacy of spasmolytic and vasorelaxant effects were lower than papaverine (Figure 4A) and carbachol (Figure 4B, Table 2).
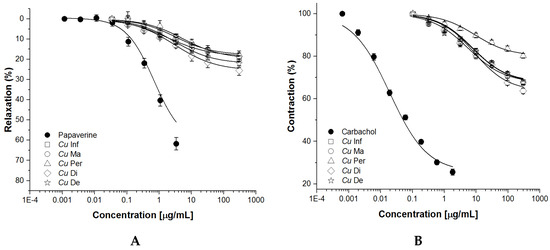
Figure 4.
Spasmolytic (A) and vasorelaxant (B) effects induced by C. uvifera extracts (CuInf, CuMa, CuPer, CuDe, and CuDi) (0.3 → 300 μg/mL) in rat-isolated ileum strips and aortic rings (E+) precontracted with NE (1 × 10−7 M). Results are expressed as the mean ± SEM of six experiments (p < 0.05 vs. papaverine or carbachol, respectively).

Table 2.
Effects induced by aqueous extracts derived from leaves of C. uvifera.
For the in vivo experimental model, the extraction method of C. uvifera leaves influences the oral consumption of liquids (CuDe: 14.8 ± 0.6 mL; CuDi: 15.9 ± 0.9 mL; CuInf: 16.8 ± 1.1 mL; CuMa: 20.6 ± 1.3 mL; CuPer: 17.3 ± 1.0 mL), with respect to the vehicle (14.9 ± 1.2 mL) and furosemide (17.1 ± 0.4 mL) groups (Table 2).
CuMa and CuPer were the best-accepted extracts and reduced solid excreta (−55.2 ± 9.3% and −24.3 ± 7.9%, respectively) to the vehicle (3.4 ± 0.3 g; 100 ± 8.8%; p < 0.05) and furosemide (−23.5 ± 7.9%) groups. Additionally, furosemide (17.1 ± 0.4 mL; ≈9.6%) and CuDe extract (18.9 ± 0.5 mL; ≈21.2%) induce an increase in urine volume to the vehicle group (15.6 ± 1.0 mL; p < 0.05; Figure 5); C. uvifera exerts a potentially concentration-dependent diuretic effect. Finally, eating habits were maintained, and food consumption did not change.
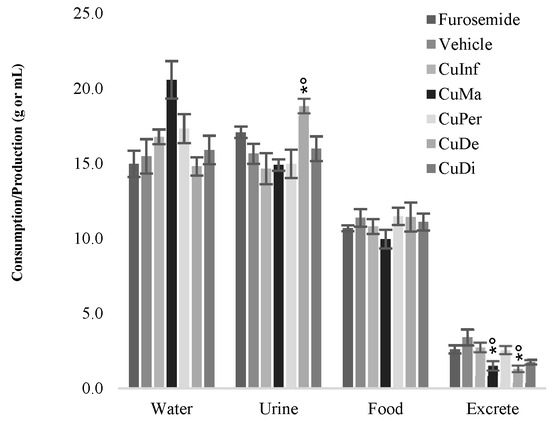
Figure 5.
Consumption of water, food, as well as urine and feces production induced by CuInf, CuMa, CuPer, CuDe, and CuD, furosemide, and vehicle groups. Results are expressed as the mean ± SEM of four experiments (n = 12; p < 0.05 vs. * furosemide; vs. ○ vehicle).
3.4. In Silico Approaches
Fourteen metabolites have been described in C. uvifera leaves, and each one has different theoretical physicochemical properties that determine the extraction in aqueous systems (Table 3).

Table 3.
Physicochemical properties by Osiris calculations of chemical entities identified and/or isolated from leaves of C. uvifera.
Molinspiration is a virtual tool that allows for the calculation of molecular properties that influence the bioavailability of chemical entities that are pharmaceutical candidates [33]. Sterols and pentacyclic triterpenes registered low TPSA values, which suggests low aqueous solubility, unlike flavonoid glycosides. Royleanone, gallic acid, and anthraquinones (emodin, chrysophanol, physcion, rhein) registered cLogP values appropriate for absorption at the membrane level and did not break Lipinski’s rule, so they could cross the enterocyte membrane, among others. The bioavailability of metabolites with one or more violations of the Lipinski rule could be justified using active transport mechanisms.
β-sitosterol, lupeol, α-amyrin, and β-amyrin described in C. uvifera leaves show the best biological potential (Table 4) by acting as a nuclear receptors ligand (NRL), enzyme inhibitors (Eis), or a ligand of the G protein-coupled receptor (GPCR), and, to a lesser extent, as protease inhibitors (PIs) and ion channel modulators (ICMs). The aqueous nature of traditional preparations and their oral use allow us to infer that the induction of biological effects will be primarily in the gastrointestinal tract (stomach, duodenum, ileum, jejunum, and colon). In the heat map, the green color is applied to metabolites with potential biological activities and low toxicity (Table 4). Emodin, gallic acid, chrysophanol, physcion, rhein, and royleanone present in the leaves of C. uvifera are secondary metabolites with toxicological potential.

Table 4.
Molecular properties of chemical entities identified in leaves of C. uvifera.
3.5. Molecular Docking m3AChR
m3AChR participates in physiological and pathophysiological conditions of gastrointestinal motility [34]. To identify interactions of diterpenes (royleanone) and triterpenes (β-sitosterol, lupeol, α- amyrin, β-amyrin) from C. uvifera with m3AChR, we carried out molecular docking simulation between terpenoids and m3AChR. First, the active site of the m3AChR chain was validated with a co-crystallized native ligand: scopolamine (a muscarinic antagonist). Comparison of the poses obtained by the AutoDock Vina program against those of the crystallized protein yielded a root mean square deviation (RMSD) = 2.48 Å, indicating an appropriate optimization score. These values are small and support binding at the simulation site with the original orientation of the co-crystallized molecule (Figure 6A,B).
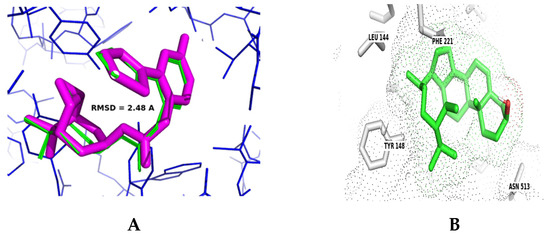
Figure 6.
Ligand binding site of m3AChR protein with co-crystalized native scopolamine (green) and scopolamine, as posed by the Autodock Vina program (pink; (A)). Molecular docking simulation showing the interaction of β-sitosterol with active site residues of m3AChR (grey; (B)).
β-sitosterol, lupeol, α-amyrin, β-amyrin, and royleanone exhibited docking poses with high binding affinities (in terms of coupling energy). The binding energies and amino acid interactions for each triterpene with m3AChR are given in Table 5. The binding interactions of β-sitosterol (Figure 6) with the active site of m3AChR are stabilized through hydrophobic interactions. From docking results, binding energies for m3AChR with β-sitosterol and royleanone are ±10 and 16.6% less than scopolamine.

Table 5.
Binding affinity for the molecular coupling in m3AChR with scopolamine and triterpenes described in C. uvifera leaves.
The Ki value for the binding of β-sitosterol with m3AChR was lower than scopolamine (Ki = 0.21 vs. 0.03 μM; ±10 times less); royleanone and β-amyrin registered ±16 and ±1700 times less affinity to m3AChR, respectively. Docking analysis showed that β-sitosterol with hydrophobic interactions has a higher binding affinity for m3AChR concerning β-amyrin and royleanone. The alkyl chain in C17 of β-sitosterol forms alkyl–alkyl interactions with Leu144, Tyr148, Leu225, and Tyr529, and pi–alkyl and pi–sigma interactions were observed with Phe221 and Trp525. H-bonds in β-amyrin (Asn526) seem not to be relevant in the interaction with Acho-M3r, pi–sigma interactions were observed with Trp525, and alkyl interactions were prevalent with ring A. All details of the bonding are given in Table 5.
4. Discussion
Aqueous preparations are typically made from polar solvents and use continuous and discontinuous processes of extraction [35]; additionally, temperature and time exert variations in the chemical content of the aqueous extracts. In this context, several aqueous extracts from dry leaves of C. uvifera were obtained and tested; CuDe favors the best extraction yield, but the content of chlorophylls, phenols, and flavonoids, however, exerts low antioxidant activity. CuMa demonstrates excellent yield; however, the aglycone extraction capacity is limited. In this sense, the yield of CuDe and CuMa could be related to the extraction of heterosides among other components of polar nature.
Time, temperature, polarity solvent, and pH are involved in the extraction procedure to obtain raw material [35,36]. In CuDe, the increased temperature favors the solubility of metabolites as well as their diffusion [37]; however, it is reported that pH, temperature, and oxidizing environments modify the stability of aglycones and heterosides of flavonoid [38], temperatures above 50 °C decrease the content of polyphenols due to thermal degradation [39], phenolic alcohols and carboxylic acids could be related to the formation of artifacts [40], and aromatic alcohols are prone to the oxidation and formation of H2O2, quinones, and semiquinones [41], facts that suggest the loss of antioxidant activity. CuPer induces increased antioxidant activity and is a low thermal stress process.
The partial spasmolytic and vasorelaxant effects induced by C. uvifera are exerted in a concentration-dependent manner through actions at the smooth muscle level; on the other hand, temperature and time extraction reduce the effects at the smooth muscle level. In the context of aqueous extracts of C. uvifera, pharmacological bases are established for the search for new drugs or their use in the practice of clinical and traditional medicine [42]. It is highlighted that flavonoids [43], terpenoids [44], and stilbenoids [45], among other metabolites, are identified as smooth muscle relaxants.
The organoleptic properties of aqueous extracts are related to the extraction method; in the in vivo model, ad libitum administration of the aqueous extracts of C. uvifera leaves modify oral consumption and consequently the dosage, a situation that differentially affects intestinal transit and diuresis. Emodin, chrysophanol, physcion, and rhein are reported to exert laxative effects through a potential interaction with c-kit, 5-HT4, and AQP3 [46]; however, aqueous extracts of C. uvifera reduce stool output. The absence of laxative effects in the experimental groups can be related to the low abundance of anthraquinones. More than 0.5% of anthraquinones in dry material are recommended to be considered a laxative medication [47]. Quantitative studies are required to corroborate this assertion.
To recognize the metabolite(s) involved in the effects induced by C. uvifera, POM analysis was developed. In this context, the main transport mechanism of aglycones is simple diffusion. The biological activity may be related to enzymatic inhibition and interaction with G protein-coupled receptors distributed in the gastrointestinal tract [48,49,50,51,52,53]. Muscarinic receptors play an important role in the control of gastrointestinal and vascular smooth muscle [54]. m3AChR is coupled to the G protein (Gq) and mediates an increase in intracellular calcium (Ca2+) that typically causes the constriction of smooth muscle; additionally, in vascular endothelial cells, it increases the production of nitric oxide (NO) and causes relaxation and vasodilation [55]. Triterpenes such as ursolic acid [21], oleanolic acid, erythrodiol [56], and others exert relaxant effects on smooth muscle tissues.
Phytochemical reports [17] as well as chemical and spectroscopic analyses suggest the presence of β-sitosterol in C. uvifera leaves. Aglycone and β-sitosterol heterosides have been reported in various plant species [57,58], and the presence of heterosides favors solubility in aqueous extraction systems (e.g., CuIn, CuMa, CuPer, CuDe, or CuDi); the acid or enzymatic hydrolysis [59] of β-sitosterol heterosides could improve the release of β-sitosterol, as well as the spasmolytic effect. The docking of scopolamine used as the native ligand, with the modeled m3AChR protein, was simulated using the AutoDock Vina. To explore the binding affinity and the molecular basis of the interactions with m3AChR, docking analysis of di- and triterpenes generates negative values for free energy, suggesting a high affinity for the binding pocket. β-sitosterol has a higher binding affinity for m3AChR based on lower binding energies that contribute to a stable protein–ligan complex and thus induce a tone reduction in smooth muscle. The spasmolytic effects induced by aqueous extracts of C. uvifera could be exerted through actions with m3AChR and a reduction in the peristalsis of the gastrointestinal tract [48]. Figure 7 explains the molecular mechanism of β-sitosterol on gastrointestinal smooth muscle relaxation.
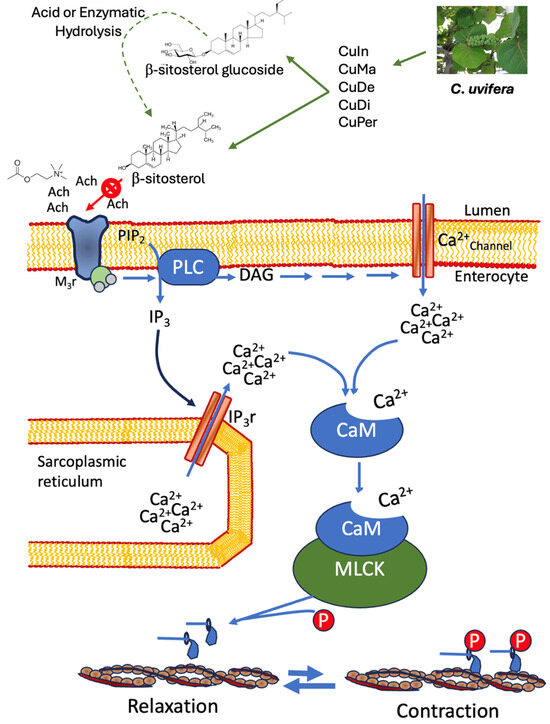
Figure 7.
Interaction of β-sitosterol on gastrointestinal smooth muscle to induce smooth muscle relaxation. Ach: acetylcholine; M3r: muscarinic M3 receptor; PLC: phospholipase C; PIP2: phosphatidylinositol bisphosphate; IP3: inositol triphosphate; DAG: diacylglycerol; IP3r: inositol triphosphate receptor; CaM: calmodulin; MLCK: Myosin light-chain kinase. The arrow indicates the signal transduction flux for the induction of smooth muscle contraction/relaxation.
Finally, temperature favors the diuretic effect. Flavonoid heterosides hydrolysis could be related to vasorelaxant and diuretic effects. The bioavailability of flavonoid heterosides or their aglycones (myricetin and quercetin) could be related to the diuresis exerted by C. uvifera (CuDe) concerning the vehicle (±26.8%) and furosemide (±10.5%) groups. Myricetin 3-O-rhamnoside (myricitrin) exerts diuresis, natriuresis, and kaliuretic effects in a dose-dependent manner [60]; on the other hand, Quercetin-3-O-β-D-glucopyranoside is related to diuretic activity in a concentration-dependent manner [61]. Myricetin exerts a wide variety of biological effects, including ACE inhibition and the release of nitric oxide or prostacyclin at the endothelium level [62]. Quercetin exerts a vasodilation effect by stimulating the vascular CaV1.2 channel current [63]. This result suggests a potential diuretic effect of aqueous extracts from C. uvifera leaves. However, it is required to determine whether the effects are dose-dependent.
5. Conclusions
The temperature and extraction time modify the content of the pigments, simple phenols, and flavonoids present in C. uvifera extracts and negatively affect the antioxidant activity; however, these factors favor the extraction of metabolites with actions at the smooth muscle level.
C. uvifera exerts a spasmolytic and vasorelaxant effect in a concentration-dependent manner, suggesting interactions with specific molecular targets on the smooth muscle. POM analysis indicates that metabolites interact with gastrointestinal enzymes and GPCRs. Molecular docking indicates that β-sitosterol is an antagonist of m3AChR and affects the gastrointestinal level function; the presence of β-sitosterol and their heterosides was by FTIR and 1H-NMR. In the context of the antiperistaltic effects, it is necessary to determine the effective dosage of CuDe extract and β-sitosterol, among other participating metabolites.
Finally, the flavonoid heterosides and their aglycones (myricetin and quercetin) from C. uvifera leaves are related to diuretic effects.
Author Contributions
Conceptualization, F.J.A.-C., D.R.A.-K. and E.H.-N.; methodology, F.J.A.-C., D.R.A.-K. and E.H.-N.; software, F.J.A.-C. and E.H.-N.; validation, D.L.G.-G., G.A. and F.J.A.-C.; formal analysis, G.A., D.R.A.-K., E.H.-N. and F.J.A.-C.; investigation, J.L.A.-G., E.J.G.-A., P.Z.-C. and D.L.G.-G.; resources, E.J.G.-A., P.Z.-C., D.L.G.-G. and F.J.A.-C.; data curation, D.R.A.-K., D.L.G.-G., G.A. and F.J.A.-C.; writing—original draft preparation, F.J.A.-C., D.R.A.-K. and E.H.-N.; writing—review and editing, F.J.A.-C., J.L.A.-G., E.J.G.-A., P.Z.-C., D.L.G.-G., D.R.A.-K., G.A. and E.H.-N.; visualization, D.R.A.-K. and F.J.A.-C.; supervision, F.J.A.-C. and E.H.-N.; project administration, F.J.A.-C.; funding acquisition, F.J.A.-C. and E.H.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
General Directorate of Postgraduate Studies and Research, Autonomous University of Campeche. Code: 035/UAC/2023.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated and analyzed in this work are mentioned in this document.
Acknowledgments
All authors would like to thank the “Faculty of Chemical Biological Sciences” from “Autonomous University of Campeche” for the facilities provided in the use of laboratories, equipment, and installations to develop this work. We thank Iliana G. Osorio Horta and Mónica Anahi Guillen Poot for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Juárez-Rosete, C.R.; Aguilar-Castillo, J.A.; Juárez-Rosete, M.E.; Bugarin-Montoya, R.; Juarez-Lopez, P.; Cruz-Crespo, E. Hierbas aromaticas y medicinales en Mexico: Tradicion e innovacion. Rev. Bio Cienc. 2013, 2, 119–129. [Google Scholar]
- La Torre-Cuadros, M.D.L.A.; Islebe, G.A. Traditional ecological knowledge and use of vegetation in southeastern Mexico: A case study from Solferino, Quintana Roo. Biodivers. Conserv. 2003, 12, 2455–2476. [Google Scholar] [CrossRef]
- Hernández, A.D.; Durán, I.S. Shortage of medicines in the health institutions in ZMG in Mexico. J. Int. Manag. Stud. 2015, 15, 27–32. [Google Scholar] [CrossRef]
- Salam, A.M.; Lyles, J.T.; Quave, C.L. Methods in the extraction and chemical analysis of medicinal plants. In Book Methods and Techniques in Ethnobiology and Ethnoecology, 2nd ed.; Albuquerque, U., de Lucena, R., Cruz da Cunha, L., Alves, R., Eds.; Springer Protocols Handbooks; Humana Press: New York, NY, USA, 2019; pp. 257–283. [Google Scholar]
- Sánchez-Aguirre, O.A.; Asseleih, L.M.C.; Medina, A.S.; González, G.S. Aplicación de Métodos Quimiométricos en la Investigación de Productos Naturales. In Miscelánea Científica de México: Biología y Química I; Solano-Sosa, C.E., Sánchez-Morales, M.A., Vázquez-García, G.V., Martínez-García, A., Ramos Guerrero, E.L., Eds.; Temacilli Editorial: Mexico City, Mexico, 2020; pp. 211–220. [Google Scholar]
- Hibbert, D.B. IUPAC project: A glossary of concepts and terms in chemometrics. Anal. Chim. Acta 2009, 642, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L. Computational approaches for the discovery and development of pharmacologically active natural products. Biomolecules 2021, 11, 630. [Google Scholar] [CrossRef]
- Mukherjee, D.; Royce, S.G.; Sarkar, S.; Thorley, A.; Schwander, S.; Ryan, M.P.; Porter, A.P.; Chung, K.F.; Tetley, T.D.; Zhang, J.; et al. Modeling in vitro cellular responses to silver nanoparticles. J. Toxicol. 2014, 2014, 852890. [Google Scholar] [CrossRef]
- Coe, F.G. Ethnomedicine of the Rama of southeastern Nicaragua. J. Ethnobiol. 2008, 28, 1–38. [Google Scholar] [CrossRef]
- Vázquez-Yanes, C.A.I.; Batis-Muñoz, A.I.; Alcocer-Silva, M.I.; Sanchez, D. Potentially Valuable Trees and Shrubs for Ecological Restoration and Reforestation; Technical Report J084 Project; CONABIO-Instituto de Ecología, UNAM: México City, Mexico, 1990. [Google Scholar]
- Abdel-Hakim, F.; Gad, H.A.; Radwan, R.A.; Ayoub, N.; El-Shazly, M. Biological and phytochemical review on the genus Coccoloba (Polygonaceae). Arch. Pharm. Sci. Ain Shams Univ. 2019, 3, 180–194. [Google Scholar] [CrossRef]
- Gámez-Farías, Y.V. Evaluación Fitoquímica y Actividad Antibacteriana de Extractos de Coccoloba uvifera Jacq. (Polygonaceae) de la Localidad de El Peñón. Bachelor’s Degree, Universidad de Oriente, Cumaná, Venezuela, 2012. [Google Scholar]
- Povi, L.; Batomayena, B.; Hodé, T.A.; Kwashie, E.G.; Kodjo, A.; Messanvi, G. Phytochemical screening, antioxidant, and hypoglycemic activity of Coccoloba uvifera leaves and Waltheria indica roots extracts. Int. J. Pharm. Pharm. Sci. 2015, 7, 279–283. [Google Scholar]
- Segura-Campos, M.R.; Ruiz-Ruiz, J.; Chel-Guerrero, L.; Betancourt-Ancona, D. Coccoloba uvifera (L.) (Polygonaceae) fruit: Phytochemical screening and potential antioxidant activity. J. Chem. 2015, 2015, 534954. [Google Scholar] [CrossRef]
- Bailey, C.; Christian, K.R.; Pradhan, S.; Nair, M.G.; Christian, O.E. Anti-inflammatory, and antioxidant activities of Coccoloba uvifera (Seagrapes). Curr. Top. Phytochem. 2011, 10, 55–60. [Google Scholar]
- Piloto-Alfonso, A.M.; García-Simón, G.; Valdivieso-García, G.A. Estudio farmaco-toxicológico del extracto fluido de uva Caleta al 50%. Arch. Toxicol. 2001, 1, 126–131. [Google Scholar]
- Ramos-Hernández, J.A.; Calderón-Santoyo, M.; Navarro-Ocaña, A.; Barros-Castillo, J.C.; Ragazzo-Sanchez, J.A. Use of emerging technologies in the extraction of lupeol, α-amyrin and β-amyrin from sea grape (Coccoloba uvifera L.). J. Food Sci. Technol. 2018, 55, 2377–2383. [Google Scholar] [CrossRef]
- Malathi, S.; Masilamani, P.; Balasubramanian, V.; Rao, R.B.; Brindha, P. Constituents of Coccoloba uvifera leaves. Fitoterapia 1995, 66, 277. [Google Scholar]
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA): Ciudad de México, Distrito Federal, Mexico, 2001.
- Estrada-Soto, S.E.; González-Maldonado, D.; Castillo-España, P.; Aguirre-Crespo, F.J.; Sanchez-Salgado, J.C. Spasmolytic effect of Mentha pulegium L. involves ionic flux regulation in rat ileum strips. J. Smooth Muscle Res. 2010, 46, 107–117. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.J.; Navarrete-Vazquez, J.G.; Estrada-Soto, S.E. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, K.; Banu, N. Extraction, and estimation of chlorophyll from medicinal plants. Int. J. Sci. Res. 2015, 4, 209–212. [Google Scholar]
- Burke, R.W.; Diamondstone, B.I.; Velapoldi, R.A.; Menis, O. Mechanisms of the Liebermann-Burchard and Zak Color Reactions for Cholesterol. Clin. Chem. 1974, 20, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Puangpronpitag, D.; Areejitranusorn, P.; Boonsiri, P.; Suttajit, M.; Yongvanit, P. Antioxidant activities of polyphenolic compounds isolated from Antidesma thwaitesianum Müll. Arg. seeds and marcs. J. Food Sci. 2008, 73, C648–C653. [Google Scholar]
- Bernard, D.; Isaac Kwabena, A.; Daniel Osei, O.; Achel Daniel, G.; Achoribo Elom, S.; Sandra, A. The effect of different drying methods on the phytochemicals and radical scavenging activity of Ceylon Cinnamon (Cinnamomum zeylanicum) plant parts. Eur. J. Med. Plants 2014, 4, 1324–1335. [Google Scholar]
- Galvez, M.A.C. Evaluation of DPPH free radical scavenging activity and phytochemical screening of selected folkloric medicinal plants in Tinoc, Ifugao, Cordillera Administrative Region, Philippines. Int. J. Sci. Res. Publ. 2015, 5, 440–445. [Google Scholar]
- Mabkhot, Y.N.; Alatibi, F.; El-Sayed, N.N.E.; Al-Showiman, S.; Kheder, N.A.; Wadood, A.; Rauf, A.; Bawazeer, S.; Hadda, T.B. Antimicrobial activity of some novel armed thiophene derivatives and petra/osiris/molinspiration (POM) analyses. Molecules 2016, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Hakkou, Z.; Maciuk, A.; Leblais, V.; Bouanini, N.E.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Rauf, A.; Hadda, T.B.; et al. Antihypertensive and vasodilator effects of methanolic extract of Inula viscosa: Biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed. Pharmacother. 2017, 93, 62–69. [Google Scholar] [CrossRef]
- Cob-Calan, N.N.; Chi-Uluac, L.A.; Ortiz-Chi, F.; Cerqueda-García, D.; Navarrete-Vázquez, J.G.; Ruiz-Sánchez, E.; Hernández-Núñez, E. Molecular Docking and dynamics simulation of protein β-Tubulin and antifungal cyclic lipopeptides. Molecules 2019, 24, 3387. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Amna, T.; Al-Musayeib, N.; Zabin, S.A.; Hassan, M.S.; Khil, M.S. Encapsulation of β-Sitosterol in polyurethane by sol-gel electrospinning. Appl. Biochem. Biotechnol. 2017, 182, 624–634. [Google Scholar] [CrossRef] [PubMed]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; González-Cortazar, M.; Arenas-Ocampo, M.L.; Jiménez-Aparicio, A.R. identification and quantification of β-Sitosterol β-d-glucoside of an ethanolic extract obtained by microwave-assisted extraction from Agave angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef] [PubMed]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Bagrel, D.; Abdelsattar, E.A. Chemical and Biological Investigation of Ochrosia elliptica in Egypt. Rec. Nat. Prod. 2017, 11, 552–557. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Igarashi-Hisayoshi, Y.; Ihara, E.; Bai, X.; Higashi, C.; Ikeda, H.; Tanaka, Y.; Hirano, M.; Ogino, H.; Chinen, T.; Taguchi, Y.; et al. Determination of Region-Specific Roles of the M3 Muscarinic Acetylcholine Receptor in Gastrointestinal Motility. Dig. Dis. Sci. 2023, 68, 439–450. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurized solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Nagaich, U.; Gulati, N.; Sharma, V.K.; Khosa, R.L.; Partapur, M.U. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: A recent review. J. Adv. Pharm. Educ. Res. 2012, 2, 32–67. [Google Scholar]
- Garzón, G.A. Anthocyanins as natural colorants and bioactive compounds: A review. Acta Biolo. Colomb. 2008, 13, 27–36. [Google Scholar]
- Rajbhar, K.; Dawda, H.; Usha, M. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Maltese, F.; Van Der Koov, F.; Verpoorte, R. Solvent-derived artifacts in natural products chemistry. Nat. Prod. Commun. 2009, 4, 447–454. [Google Scholar] [PubMed]
- Wong, S.K.; Sytnyk, W.; Wan, J.K.S. Electron spin resonance study of the self-disproportionation of some semiquinone radicals in solution. Can. J. Chem. 1972, 50, 3052–3057. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.J.; Salgado, J.C.S. ¿Qué sabe Ud. acerca de... la curva dosis-respuesta? Rev. Mex. Cienc. Farm. 2010, 41, 57–59. [Google Scholar]
- Barbosa da Rocha, M.; Menezes-Souza, F.V.; Dos Santos, E.C.; Pizza, C.; Goulart-Santana, A.E.; Moretti-Marcal, R. Antispasmodic effect of 4’-methylepigallocatechin on guinea pig ileum. Fitoterapia 2012, 83, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Lima, T.; Mendoca-Mota, M.; Barbosa-Filho, J.M.; Dos Santos, M.R.V.; De Sousa, D.P. Structural relationships and vasorelaxant activity of monoterpenes. DARU J. Pharm. Sci. 2012, 20, 23. [Google Scholar] [CrossRef]
- Estrada-Soto, S.E.; Lopez-Guerrero, J.J.; Villalobos-Molina, R.; Mata, R. Endothelium-independent relaxation of aorta rings by two stilbenoids from the orchids Scaphyglottis livida . Fitoterapia 2006, 77, 236–239. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β-glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A.N. Anthraquinones: An Overview. Stud. Nat. Prod. Chem. 2018, 58, 313–338. [Google Scholar]
- Ruoff, H.J.; Fladung, B.; Demol, P.; Weihrauch, T.R. Gastrointestinal receptors, and drugs in motility disorders. Digestion 1991, 48, 1–17. [Google Scholar] [CrossRef]
- Lyford, G.L.; Farrugia, G. Ion channels in gastrointestinal smooth muscle and interstitial cells of Cajal. Curr. Opin. Pharmacol. 2003, 3, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Verkman, A.S. Aquaporin water channels in gastrointestinal physiology. J. Physiol. 1999, 517, 317–326. [Google Scholar] [CrossRef]
- Daniel, H.; Zietek, T. Taste and move glucose and peptide transporters in the gastrointestinal tract. Exp. Physiol. 2015, 100, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Steffansen, B.; Nielsen, C.U.; Brodin, B.; Eriksson, A.H.; Andersen, R.; Frokjaer, S. Intestinal solute carriers: An overview of trends and strategies for improving oral drug absorption. Eur. J. Pharm. Sci. 2004, 21, 3–16. [Google Scholar] [CrossRef]
- Degirolamo, C.; Sabbà, C.; Moschetta, A. Intestinal nuclear receptors in HDL cholesterol metabolism. J. Lipid. Res. 2015, 56, 1262–1270. [Google Scholar] [CrossRef]
- Flores-Soto, M.E.; Segura-Torres, J.E. Structure, and function of the acetylcholine of muscarinical and nicotinical type. Rev. Mex. Neuroci. 2005, 6, 315–326. [Google Scholar]
- Caulfield, M.P. Muscarinic receptors—Characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar]
- Rodríguez-Rodríguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutiérrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Hossain, M.S. Scopoletin and β-sitosterol glucoside from roots of Ipomoea digitata . J. Pharmacogn. Phytochem. 2015, 4, 5–7. [Google Scholar]
- Maldonado, E.; Amador, S.; Juárez-Jaimes, V. Secondary metabolites from Asclepias otarioides . J. Mex. Chem. Soc. 2015, 59, 50–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Zhang, Y.; Zhang, M. Degradation kinetics of jujuboside A by rat intestinal flora and identification of the metabolites by HPLC MS/MS. Int. J. Food Prop. 2014, 17, 1841–1849. [Google Scholar] [CrossRef]
- Tenfen, A.; Mariano, L.N.B.; Boeing, T.; Cechinel-Zanchett, C.C.; Mota da Silva, L.; de Andrade, S.F.; de Souza, P.; Cechinel-Filho, V. Effects of myricetin-3-O-α-rhamnoside (myricitrin) treatment on urinary parameters of Wistar rats. J. Pharm. Pharmacol. 2019, 71, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Bracci, A.; Amat, A.G.; Maione, F.; Cicala, C.; Mascolo, N.; De Feo, V. Diuretic activity of Lophophytum leandri . Nat. Prod. Cammun. 2012, 7, 1934578X1200700112. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Trezza, A.; Spiga, O.; Mugnai, P.; Saponara, S.; Sgaragli, G.; Fusi, F. Functional, electrophysiology, and molecular dynamics analysis of quercetin-induced contraction of rat vascular musculature. Eur. J. Pharmacol. 2022, 918, 174778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).