Abstract
Korean mistletoe (Viscum album var. coloratum) has been traditionally used as a remedy for cancer, diabetes, and hypertension. This study investigated the immuno-modulatory effects of Korean mistletoe water extract, specifically on MDA-MB-231 cells, a highly metastatic breast cancer cell line, when co-cultured with THP-1 human macrophage cells. When compared to MDA-MB-231 cells cultured alone, the co-culture of MDA-MB-231/THP-1 cells treated with mistletoe extract showed a significant reduction in IL-6 secretion. Additionally, these co-cultures exhibited elevated levels of IL-4, TGF-β, and IFN-y. These results suggest that water extracts from mistletoe have the potential to induce mitochondria-targeted apoptosis in MDA-MB 231 cells stimulated by THP macrophages. Regarding apoptosis, in MDA-MB-231 cells co-cultured with THP-1 macrophages, mistletoe water extract treatment triggered a significant increase in Bax/Bcl-2 ratio, caspase-3 activation, and PARP inactivation. In addition, there was a significant increase in E-cadherin and a decrease in N-cadherin. Treatment of Korean mistletoe also led to significant reductions in both MMP-2 and -9. Furthermore, inhibition of cell migration in MDA-MB-231/THP-1 co-cultured cells was observed. In summary, this study highlights the potential of Korean mistletoe as a prospective drug for the treatment of triple-negative breast cancer, particularly through its ability to regulate human immunity.
1. Introduction
European mistletoe (Loranthaceae) grows parasitically on trees, including oaks and apples, across Europe and Asia [1]. This semi-parasite has been used for generations as a medicinal plant in Germany and Switzerland to treat hypertension, arterial sclerosis, and cancer [2,3]. Several studies are proving its pharmacological effects. Various active components isolated from mistletoe have been demonstrated to possess anti-cancer effects in extensive detail [4,5]. The active ingredients responsible for these effects are lectin (a glycoprotein), viscotoxin (a protein component), and oleanolic acid (a triterpenoid). Scientific evidence suggests that these components not only possess anti-cancer properties but also play a vital role in enhancing the immune system’s activity [2,6,7,8,9]. For example, among the pharmacological components of mistletoe, polysaccharides, oligosaccharides, amines, and alkaloids have a lesser direct killing effect on cancer cells than lectin, but they can activate macrophages to induce cell-mediated immunity [10]. In particular, a variant of European mistletoe, Korean mistletoe (Viscum album L. var. coloratum), has been reported to have superior immune cell stimulation activity compared to European mistletoe [11]. Korean mistletoe is a domestically native plant distinct from European mistletoe, and it has been used as a medicine for back pain, high blood pressure, and toothache in private and oriental medicine [12,13]. Recently, researchers have actively investigated the anti-cancer activity of Korean mistletoe extract. Most studies on chemotherapy with mistletoe use mistletoe water extract [14]. The anti-cancer activity of mistletoe extract is produced not only by the direct cytotoxic effects of its constituents but also by inducing tumor-specific cell-mediated immune enhancement. This occurs due to the activation of immune cells (such as macrophages) against tumor cells [15].
Clinically diverse phenotypes characterize breast cancer, with different subtypes classified into progesterone receptor (PR), estrogen receptor (ER), and human epithelial growth factor receptor 2 (HER2) based on immunohistochemical staining expression [16]. Triple-negative breast cancer, a subtype in which PR, ER, and HER2 are not expressed, represents 10–20% of all breast cancer patients [17,18,19]. Therapeutic effects are noticeably lower in triple-negative breast cancers compared to other hormone receptors or treatments targeting HER2-positive subtypes. Consequently, treatment for triple-negative breast cancer still relies heavily on non-specific measures such as surgery, radiation therapy, and chemotherapy, despite their associated side effects [19,20]. Furthermore, triple-negative breast cancer aggressively metastasizes to major organs such as the bone, liver, lung, and brain throughout the course of the tumor. Doctors identify metastasis as a significant obstacle in effectively treating triple-negative breast cancer, which consequently results in a clinically poor prognosis [21,22,23,24,25,26].
Breast cancer encompasses an intricate microenvironment within the tumor, involving blood vessels, immune cells, fibroblasts, cytokines, and extracellular matrices. These components interact with each other, playing significant roles at every stage of metastasis [27]. Macrophages, integral to innate immunity, function differently within the tumor microenvironment compared to general macrophages. Specifically, in breast cancer microenvironments, they transform into tumor-associated macrophages (TAMs) upon activation [28]. TAMs constitute around 50% of the total cell population in this microenvironment, making them the most abundant component. They secrete stimulatory or inhibitory signaling molecules that manipulate growth during solid tumor progression [29,30,31].
The human immune response and inflammation lead to the secretion of inflammatory mediators from cells to protect against external stimuli like infections or tissue damage. However, this process can also promote chronic cancer cell death by inhibiting inflammatory mediators like interleukin (IL)-4, IL-6, interferon (IFN)-γ, and transforming growth factor (TGF)-β [13,32]. Tumor cells secrete matrix metalloproteases (MMPs) and proteolytic enzymes, along with inflammatory cytokines, that degrade the extracellular matrix forming the cellular scaffold and modulate the intra-tumoral environment [33,34].
Moreover, an inflamed microenvironment leads to the persistent activation of signal transducer and transcription (STAT) proteins within cells, triggering further inflammation along with metastasis and neovascular synthesis in cancerous cells, exacerbating the tumor [35]. Under normal conditions, STAT proteins play crucial roles in cell development, differentiation, and survival. However, excessive activation of these same proteins often underlies cancer development. An illustrative case of this phenomenon is the active pathway of STAT3, extensively studied due to its overexpression across various cancer cell types [36]. In an inflammatory microenvironment, tumor cells interact with IL-6 via IL-6 receptors, leading to their conversion into phospho-STAT3 (p-STAT3), an activated form [37]. P-STAT3 infiltrates the nucleus of tumor cells, upregulating metastasis and neovascularization-related genes [38]. While advancements in early diagnosis technology and anti-cancer drugs have led to a decrease in cancer mortality rates [39], cancer cell metastasis remains the primary cause of death in cancer patients [40]. Thus, the development of anti-cancer drugs inhibiting STAT3 activation is anticipated to offer therapeutic benefits by curbing cancer cell metastasis and new blood vessel synthesis.
In addition, the activation of Bcl-2 family proteins such as Bax and Bcl-2 triggers the direct apoptosis of tumor cells [41]. These activated Bax protein oligomers bind to the mitochondrial outer membrane, inducing mitochondrial outer membrane permeabilization (MOMP) [42,43]. This process allows apoptosome leakage from the mitochondria, subsequently activating cysteine-aspartic proteases (caspases) [44]. Among these, caspase-3 acts as an effector caspase, inducing apoptosis by deactivating poly ADP-ribose polymerase (PARP) in the nucleus of tumor cells, thereby hindering DNA repair [45]. Apoptosis is genetically regulated by infection and DNA damage, setting it apart from necrosis. In the study of anti-cancer drugs, inducing apoptosis is crucial; unlike necrosis, it avoids triggering additional inflammatory reactions. Consequently, only tumor cells are selectively eliminated without causing harm to normal tissues [46].
Furthermore, cancer cell metastasis is a sequential process, progressing from primary tumors to the development of new tumors in distant organs. Epithelial–mesenchymal transition (EMT) is a theory closely associated with the early stages of this metastasis process [47]. The initiation phase occurs when MMP-2 and MMP-9 are secreted by the primary tumor, enabling the infiltration of local tissues and blood vessels [48]. Consequently, these infiltrated cancer cells gradually undergo a phenotypic change from epithelial to mesenchymal cells, characterized by a decrease in the expression of the cell adhesion molecule E-cadherin and an increase in the expression of N-cadherin, which weakens cell adhesion [49,50]. EMT transforms solid tumor cells into mesenchymal cells, endowing these newly transformed cells with enhanced mobility. This mobility allows detached cancerous epithelial cells to enter circulatory systems like blood vessels and metastasize to other organs. A notable feature of EMT is the conversion of cadherins, in particular an increase in N-cadherin accompanied by a loss of E-cadherin [51].
Recent studies have actively pursued immuno-cancer research, with a focus on investigating anti-cancer activity by meticulously examining and enhancing tumor microenvironments. However, no research has reported on how mistletoe water extract activates macrophages to improve the tumor microenvironment. Therefore, in this experiment, we aimed to explore the potential of Korean mistletoe extract as an immune enhancer. We sought to compare and investigate whether activated macrophages induce apoptosis more effectively in triple-negative breast cancer cells while concurrently inhibiting EMT and neovascular synthesis.
2. Materials and Methods
2.1. Reagents and Antibodies
The antibodies used in this study were obtained from multiple providers: Cell Signaling Technology (CST, Danvers, MA, USA), Thermo Fisher Scientific (Waltham, MA, USA), and BD Biosciences (Franklin Lakes, NJ, USA). The biotin anti-human IL-6 detection antibody used for the enzyme-linked immunosorbent assay (ELISA) was procured from BD Biosciences. For Western blot experiments, primary antibodies were supplied by CST, including anti-mouse Bcl-2 (1:1000, Cat#15071), anti-rabbit Bax (1:1000, Cat#5023), anti-rabbit caspase-3 (1:1000, Cat#9664), anti-rabbit cleaved caspase-3 (1:1000, Cat#9664), anti-rabbit PARP (poly ADP-ribose polymerase, Cat#9532) (1:1000), anti-rabbit cleaved PARP (1:1000, Cat#5625), anti-mouse STAT3 (Tyr705, 1:1000, Cat#9138), anti-mouse p-STAT3 (1:1000, Cat#9145), anti-rabbit MMP-2 (1:1000, Cat#40994), anti-rabbit MMP-9 (1:1000, Cat#13667), anti-rabbit E-cadherin (1:1000, Cat#3195), and anti-rabbit N-cadherin (1:1000, Cat#13116). Cleaved caspase-3 (1:1000, Cat#700182) and β-actin antibodies (Cat#MA1-744) were obtained from Thermo Fisher Scientific. For Western blot experiments, secondary antibodies including anti-mouse IgG and horseradish peroxidase (HRP)-linked antibodies (Cat#7056) as well as anti-rabbit IgG and HRP-linked antibodies (Cat#7074), were procured from CST. In the immunofluorescence experiment, Alexa Fluor™ 488 goat anti-rabbit IgG (H+L) served as the secondary antibody and was acquired from Thermo Fisher Scientific.
2.2. Manufacturing Mistletoe Water Extract
Korean mistletoe was harvested from oak trees in Kangwon-do, Korea. The botanical verification was conducted by Professor Jon-Suk Lee from Seoul Women’s University, Korea, and a voucher specimen (VCA101) was deposited at the College of Pharmacy, Sunchon National University, Korea. Initially, 100 g of crushed samples containing dried mistletoe leaves, stems, and branches (EV-MC6000, Everyhome Co., Busan, Republic of Korea) were combined with distilled water in a proportion of four times the weight of the dried sample. This mixture was gently agitated for a day within a shaking incubator operating at 120 rpm and maintained at a temperature of 4 °C. Subsequently, the resulting supernatant was separated via filtration using cotton material. The remaining residue underwent two additional extraction cycles using the same quantity of distilled water, followed by shaking. The obtained supernatant was then subjected to centrifugation at 4 °C for 20 min at 4500 rpm utilizing a centrifuge (VS-550, Vision Co., Daejeon, Republic of Korea), and then filtered using filter paper (No. 2, Advantec Toyo Roshi Kaisha, Ltd., Tokyo, Japan). The filtered supernatant was freeze-dried and stored at −20 °C until it was ready for use.
2.3. Cell Culture
Human breast cancer cells (MDA-MB-231) and human mononuclear cells (THP-1) were procured from the Korean Cell Line Bank (Seoul, Republic of Korea). Both cell lines were cultivated in a RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco Co., Grand Island, NY, USA), 2.05 mM L-glutamine, and 1% antibiotics (100 U/mL penicillin-100 μg/mL streptomycin). The cells were maintained in a controlled environment at 37 °C in a 5% CO2 incubator (Sanyo, Osaka, Japan).
2.4. Cell Viability
The MTT (3-(4,5-dimethylthiazol-2-yl) assay was employed to evaluate the effect of mistletoe extract on the viability of MDA-MB-231 cells and THP-1 cells. The MTT reagent used for this assay was procured from Duchefa Biochemie (Haarlem, Netherlands). For the MDA-MB-231 cells, a concentration of 1 × 104 cells/well was added to 96-well plates (SPL Life Sciences Co., Ltd., Pocheon-si, Republic of Korea) and subsequently cultured at 37 °C for 24 h within a humidified 5% CO2 incubator (Sanyo) without mistletoe extract. After removing the supernatant, mistletoe water extract was introduced, and the cells were further cultured for either 24 h or 48 h. In the case of THP-1 cells, differentiation into macrophages was achieved by treating the cells with phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, St. Louis, MO, USA). The differentiated macrophages were then distributed into a 100 mm cell culture dish and incubated at 37 °C for 48 h within a humidified 5% CO2 incubator (Sanyo). Afterward, the differentiated macrophages were washed with phosphate-buffered saline (PBS, VWR Life Science, Radnor, PA, USA), followed by an additional 48 h incubation with the same medium. The cells were subsequently seeded into a 96-well plate at a concentration of 1 × 104 cells/well and exposed to various concentrations (0.1–500 µg/mL) of mistletoe extract. To measure cell viability, 50 μL of MTT solution (5 mg/mL) prepared in PBS was added to each well and allowed to incubate at 37 °C for 4 h. After the supernatant was removed and the formazan crystals were dissolved using with 200 μL of dimethyl sulfoxide (DMSO), absorbance was assessed at 570 nm using a microplate reader (Sunrise Technologies, Männedorf, Zürich, Switzerland).
2.5. MDA-MB-231/THP-1 Co-Culture Model
The co-culture of MDA-MB-231 cells and THP-1 macrophages was performed using a Transwell system (Corning, NY, USA), utilizing both 6-well and 24-well plates. THP-1 cells, previously differentiated into macrophages, were introduced into the upper chamber of the Transwell insert, which had a pore size of 0.4 μm. On the other hand, MDA-MB-231 cells were added to the lower chamber of the Transwell system. The cells added to their respective compartments were cultured and allowed to attach for a period of 24 h at 37 °C within a humidified 5% CO2 incubator (Sanyo). Following the attachment phase, mistletoe water extract at various concentrations was introduced, and the co-culture was continued for an additional 24 and 48 h. Subsequently, the MDA-MB-231 cells that were attached to the lower chamber were recovered, and the supernatants were collected for use in the subsequent experimental procedures. A schematic representation of the co-culture of MDA-MB-231 and M0 macrophages is provided in Figure 1.
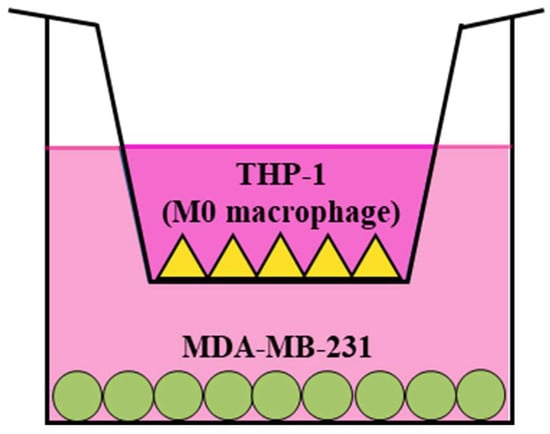
Figure 1.
MDA-MB-231/THP-1 M0 macrophage co-culture model using Transwell system. THP-1 monocytes were differentiated with PMA (10 ng/mL) for 48 h before being plated in the upper insert. MDA-MB-231 cells were plated in the bottom chamber. Subsequently, both cell types were incubated in the presence or absence of mistletoe water extracts.
2.6. Wound Healing Assay
MDA-MB-231 cells were plated in a 24-well plate at a concentration of 2 × 105 cells/mL. They were then allowed to culture for 24 h, reaching a cell density of approximately 90%. Following this incubation period, a gap of a certain size was created by gently scratching the central portion of the cell monolayer on the plate using a sterile 200 μL pipette tip. The scratched monolayer was subsequently washed twice with phosphate-buffered saline (PBS). After the creation of the scratch, various concentrations of mistletoe water extract were added to the wells, and the cells were further cultured for 24 and 48 h. During this time, the extent of cell movement into the scratched area was observed and documented using an optical microscope (Nikon, Tokyo, Japan). The resulting gaps caused by the migration of cells were then quantified using Image J software (version 1.53e, National Institutes of Health, Bethesda, MD, USA).
2.7. Migration Assay
To assess the migration of MDA-MB-231 cells, a 24-Transwell plate with an 8.0 μm pore size was employed. Initially, M0 macrophages or RPMI with 10% FBS were introduced into the Transwell chamber and incubated for 24 h at 37 °C in a 5% CO2 incubator (Sanyo). The mistletoe water extract was diluted with serum-free RPMI, and 100 μL of the diluted extract was added to the Transwell insert. MDA-MB-231 cells were suspended in the serum-free RPMI 1640 medium and placed in the Transwell insert, where they were incubated for either 24 or 48 h. For the visualization of cells that migrated through the filter and reached the bottom surface of the insert, Giemsa staining was employed. Briefly, the residual medium within the insert was removed, and the insert was washed twice with PBS (pH 7.4). The cells were fixed at room temperature for 2 min using 3.7% formaldehyde. After two washes with PBS to remove the formaldehyde, 100% methanol was added and incubated for 20 min at room temperature. Following this, the insert was washed twice with PBS, and a 0.4% Giemsa solution (Sigma-Aldrich) was added. The insert was further incubated for 15 min at room temperature. After removing excess reagents with PBS, the cells on the top surface of the insert were gently wiped away using a cotton swab. The insert was then dried, and an optical microscope (Nikon) was used to count the number of cells that had migrated.
2.8. ELISA
The quantification of secreted IL-6 from MDA-MB-231 cells and THP-1 M0 macrophages was performed using an ELISA kit (BD Biosciences), following the provided instructions. Initially, 100 μL of the capture antibody diluted in a coating buffer (0.1 M sodium carbonate, pH 9.0) was added to each well of a 96-well EIA/RIA plate (Corning), and the plate was incubated at 4 °C for 24 h. Subsequently, the plate was washed three times with a washing buffer containing PBS and 0.05% Tween-20. Then, a blocking buffer consisting of PBS with 1% bovine serum albumin (BSA) was added to the wells and allowed to incubate at room temperature for 1 h. After another three washes, the cell supernatant and the standard solutions were added to the wells and incubated at room temperature for 2 h. After an additional five washes with the washing buffer, the detection antibody was added to the wells, and the plate was incubated in darkness for 1 h. Following this, equal volumes of 3,3′,5,5′-Tetramethylbiphenyl-4,4′-diamine (TMB) Solution (0.4 g/L, Thermo Scientific, Cat#34021) and Peroxide Solution (0.02% hydrogen peroxide in citric acid buffer, Thermo Scientific, Cat#34021) were mixed, and 100 μL was added to each well; the plate was once again placed in darkness for an incubation of 30 min. After this incubation, 100 μL of 2 M sulfuric acid was introduced into the wells to stop the reaction, and the absorbance was measured at a wavelength of 450 nm.
2.9. Cytokine Array
The qualitative analysis of human inflammatory cytokines was carried out following the provided instructions using the Multi-analyte ELISArray kit (MEH-003A, QIAGEN, Hilden, Germany). In this assay, each well of the ELISArray plate was loaded with the assay buffer and 50 μL of the cell supernatant. The plate was then allowed to incubate at room temperature for a period of 2 h. Following the incubation, the plate was subjected to a series of washing steps, performed three times using a washing buffer to remove any residual contents from the wells. Subsequently, 100 μL of the detection antibody was added to each well, and the plate was once again incubated at room temperature for 1 h. After this incubation, the plate was washed three times to ensure proper removal of excess detection antibody. Next, 100 μL of avidin-HRP conjugate antibody was introduced to the wells, and the plate was incubated in darkness for 30 min. Following this incubation, the plate underwent four additional washing steps. Subsequently, 100 μL of the developing solution was added to each well, and the plate was placed in darkness for an additional incubation of 15 min. To stop the reaction, an equal volume of stop solution was added to each well. The absorbance of the samples was measured at a wavelength of 450 nm using a microplate reader (Sunrise Technologies).
2.10. Western Blotting
Cells were washed twice with PBS. To each well, 180 μL of radio-immuno preservation assay (RIPA) buffer (Thermo Scientific) and 1.8 μL of protease and phosphatase inhibitor cocktail (×100) (Thermo Scientific) were added. Centrifugation was performed at 4 °C for 20 min at 14,000 rpm. The total protein content was determined using the bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific) following the manufacturer’s protocol. The sample was mixed with sample loading dye (×5) (Chembio, Medford, NY, USA) and heated at 100 °C for 5 min. The mixture was loaded into the wells of a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) setup. The protein was transferred from the gel to a polyvinylidene fluoride (PVDF) membrane filter (Merck Millipore Ltd., Middlesex, MA, USA). The membrane was washed with tris-buffered saline (TBS) containing 50 mM tris-Cl and 150 mM NaCl (pH 7.5) for 5 min. The membrane was blocked with a solution of 5% (w/v) bovine serum albumin (BSA) (VWR Life Science) at room temperature. The membrane was washed three times with TBS-T (TBS with 0.1% Tween-20), and 10 μL of the primary antibody was diluted in 10 mL of blocking buffer, added to the membrane, and incubated at 4 °C for 24 h. The membrane was washed three times with TBS-T. The membrane was exposed to a horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h. The membrane was washed three times with TBS-T and the expressed protein was visualized using the Western Bright ECL kit (Advansta Inc., San Jose, CA, USA).
2.11. Immunofluorescence
MDA-MB-231 cells were attached to a 24-well plate containing a coverslip (12 mm, SPL Life Sciences), and various concentrations of mistletoe water extract were added and incubated for 48 h at 37 °C in a humidified 5% CO2 incubator (Sanyo). After washing three times with PBS, 500 μL of 4% formaldehyde (pH 7.4) was added and incubated at 37 °C for 10 min to fix the cells. After washing three times, 0.1% Triton X-100 was added and incubated for 15 min at room temperature. After washing three times, 500 μL of 2% (w/v) BSA (VWR Life Science) was added and incubated at room temperature for 1 h. The primary antibody was added and incubated at 4 °C for 24 h. After washing three times, the secondary antibody was added and incubated for 45 min at room temperature. After washing three times, DNA was stained with 4,6-diamidino-2-phenylindole (DAPI) for 20 min in the dark and observed with a fluorescence microscope (Nikon).
2.12. Statistical Analysis
All experiments were conducted in triplicate, and the results are presented as means ± standard deviation (S.D.). Statistical analysis of the data was performed using GraphPad software (version 7.00). Significant differences between groups were determined using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests for post hoc analysis. Statistical significance was defined as p < 0.05, p < 0.01, and p < 0.001, indicating the level of significance for the observed differences between groups.
3. Results
3.1. Cell Viability
Different concentrations of mistletoe extract were introduced to the MDA-MB-231 human triple-negative breast cancer cell line and THP-1 human mononuclear cells that had been differentiated into macrophages. Subsequently, the cells were incubated for either 24 h or 48 h, and their viability was assessed using the MTT assay (Figure 2). Through this analysis, we determined that THP-1 macrophages exhibited a survival rate of over 80% when treated with concentrations of 2 μg/mL or lower for both the 24 and 48 h groups. As a result, we selected two specific doses for further experimentation.
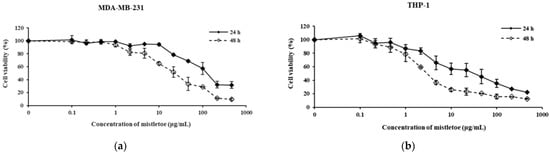
Figure 2.
Cytotoxic effect of mistletoe water extracts in (a) MDA-MB-231 and (b) THP-1 cells for 24 and 48 h. Both cell lines were exposed to various concentrations of mistletoe water extracts. Cell viability was assessed using the MTT assay. Untreated control cell viability was set at 100%. Results represent the means ± standard deviation (S.D.) of triplicate experiments.
3.2. Wound Healing and Migration Assay
For the wound healing assay, we first cut an arbitrary wound into the plate, treated it with mistletoe water extract, then monitored cell movement towards the wounded area over time (Figure 3). As a result, 61.1 ± 3.4% and 74.8 ± 3.8% of the wound area were moved in the MDA-MB-231 cells treated with mistletoe at 0.1 μg/mL concentrations for 24 and 48 h, respectively, whereas the MDA-MB-231/THP-1 co-culture group was significantly inhibited by 38.2 ± 3.4% and 57.9 ± 4.4% at 24 and 48 h, respectively. In addition, 57.6 ± 3.3% and 68.9 ± 2.9% of the wound area were moved in the MDA-MB-231 group treated with 2 μg/mL mistletoe for 24 and 48 h, respectively, whereas the MDA-MB-231/THP-1 co-culture group was significantly inhibited by 36.7 ± 1.6% and 45.9 ± 4.8% at 24 and 48 h, respectively (Figure 3d). Using the Transwell migration assay method, the MDA-MB-231 or MDA-MB-231/THP-1 groups were added into the insert membranes with pore sizes that cells can penetrate, and the number of breast cancer cells moving through the membranes under mistletoe extract treatment was observed (Figure 4). As a result, when mistletoe at a concentration of 0.1 μg/mL was treated for 48 h, 71.5 ± 5.3% of cells migrated through the membrane in the MDA-MB-231 group compared to the control, whereas 60.0 ± 3.2% of cells migrated through the membrane in the MDA-MB-231/THP-1 co-culture group compared to the control. When 2 μg/mL mistletoe was added for 48 h, 64.4 ± 2.8% of cells migrated through the membrane in the MDA-MB-231 group compared to the control, whereas 48.3 ± 2.4% of cells migrated through the membrane in the MDA-MB-231/THP-1 co-culture group.
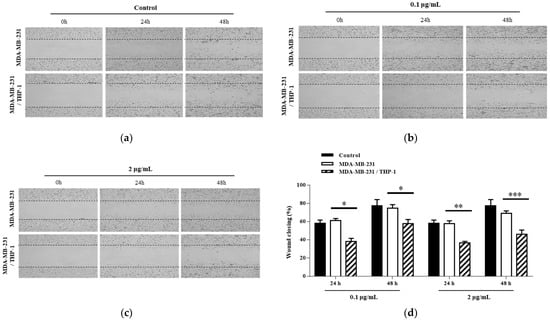
Figure 3.
Effects of Korean mistletoe water extracts on wound healing assay in MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells. Following the creation of scratches on both cell groups, various concentrations of Korean mistletoe water extracts (a) 0, (b) 0.1, and (c) 2 μg/mL were applied immediately. Representative images from the wound healing assay were captured at 0, 24, and 48 h post-treatment. The closure of the wound area was observed under a light microscope (×40). Additionally, (d) a quantitative bar graph of the closed wound area was generated using an image analysis software (Image J). The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, ** p < 0.01, * p < 0.001).
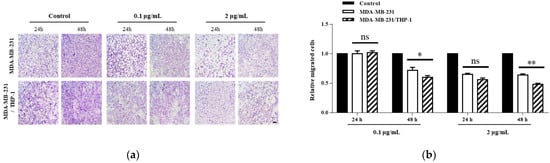
Figure 4.
Effect of Korean mistletoe water extracts on Transwell migration assay in (a) MDA-MB-231 and (b) MDA-MB-231/THP-1 co-cultured cells. Mistletoe water extracts were applied at various concentrations (0, 0.1, and 2 μg/mL) for 24 and 48 h. Migrated cells were observed using a light microscope (magnification, ×100; scale bar, 100 μm). The number of migrated cells was quantified using Image J software. Scale bars: 20 μm. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (** p < 0.01, * p < 0.001, ns: not significant).
3.3. Secretion of Inflammatory Cytokines
A quantitative and qualitative study was conducted by ELISA and cytokines array to investigate if Korean mistletoe water extract may modulate the release of inflammation-related cytokines produced by triple-negative breast cancer cells by activating macrophages. Initially, MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells underwent treatment with or without 100 ng/mL lipopolysaccharides (LPS); this process served to induce the expression of inflammatory cytokines in MDA-MD-231 cells. Following treatment with 0.1 and 2 μg/mL concentrations of Korean mistletoe water extracts for a period of 48 h, MDA-MB-231 cells, THP-1 macrophages, and MDA-MB-231/THP-1 co-cultured cells underwent testing to quantify IL-6 expression through ELISA. Consequently, when cells were not stimulated with LPS, MDA-MB-231 cells secreted 1501.5 ± 110.6 pg/mL and MDA-MB231/THP-1 co-cultured cells secreted 1418.5 ± 80.4 pg/mL of IL-6. However, after exposure to 100 ng/mL LPS, MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells showed an elevated IL-6 expression of to1987.6 ± 65.7 pg/mL and 1847.9 ± 120.5 pg/mL, respectively. There was no significant difference in IL-6 expression between the MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells. Following this, when 0.1 μg/mL mistletoe water extract was administered to MDA-MB-231 cells, we confirmed an IL-6 secretion level of 2053.1 ± 77.9 pg/mL, whereas MBA-MB-231/THP-1 co-cultured cells showed an IL-6 secretion level of 1503.2 ± 89.8 pg/mL. Additionally, treatment of MDA-MB-231 cells with 2 μg/mL mistletoe water extract resulted in an IL-6 secretion of 1993.1 ± 71.6 pg/mL. However, in MDA-MB-231/THP-1 co-cultured cells, the expression of IL-6 was significantly inhibited to 1447.6 ± 74.5 pg/mL (Figure 5). Moreover, through qualitative analysis aimed at determining the regulation of cytokine expression, it was observed that there was an increase in the expression levels of IL-4, TGF-β, and IFN-γ in MDA-MB-231/THP -1 co-cultured cells compared to the MDA-MB-231 group (Figure 6).
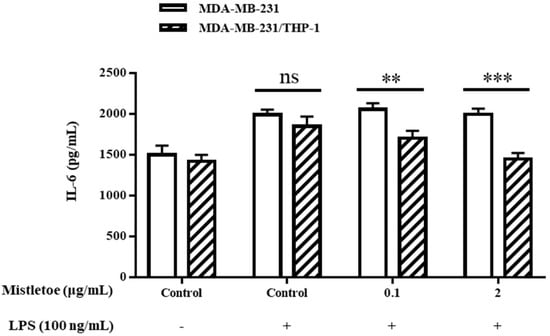
Figure 5.
Effects of mistletoe water extracts on pro-inflammatory cytokine (IL-6) production in LPS-stimulated MDA-MB-231 and MDA-MDA-MB-231/THP-1 co-cultured cells. Cells were treated with or without various concentrations of mistletoe water extracts (0, 0.1, and 2 μg/mL) and LPS (100 ng/mL) for 48 h. Cell supernatant was collected and assessed using an ELISA kit. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, ** p < 0.01, ns: not significant).
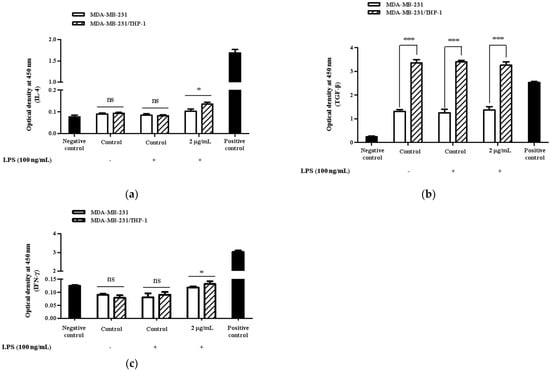
Figure 6.
Effects of Korean mistletoe water extracts on (a) IL-4, (b) TGF-β, and (c) IFN-γ secretion in MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells. Both cell groups were treated with or without mistletoe water extracts (0 or 2 μg/mL) and LPS (100 ng/mL) for 48 h. Cell supernatants were then collected and qualitative relative profiling of cytokine levels was measured using the Multi-Analyte ELISArray kit according to the manufacturer’s protocol. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, * p < 0.001, ns: not significant).
3.4. Expression of Apoptosis-Related Proteins
The apoptosis-related protein expressions were assessed in triple-negative breast cancer cells upon administration of Korean mistletoe extract. Mistletoe water extract was administered to MDA-MB-231 cells and MDA-MB-231 cells co-cultured with THP-1 cells for a duration of 48 h, followed by conducting Western blot and immunofluorescence assays. Western blot analysis measured the ratio of Bax and Bcl-2 proteins, which are proteins that induce MOMP in mitochondrial outer membranes. When treated with 0.1 μg/mL mistletoe extract, MBA-MB-231 cells and MBA-MB-231/THP-1 co-cultured cells showed no significant difference in Bax/Bcl-2 protein ratio, compared to the controls. However, when treated with 2 μg/mL mistletoe extract, the MDA-MB-231/THP-1 co-culture group showed a protein ratio of 2.1 ± 0.1 times (Figure 7c). To determine if MOMP induced the activation of caspase-3 protein to cleaved caspase-3, we measured the ratio of cleaved caspase-3 and caspase-3 proteins via Western blot. Upon treatment with 0.1 μg/mL Korean mistletoe water extract, no significant differences were shown in either group when compared to the control. However, when treated with 2 μg/mL mistletoe extract, MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells showed a cleaved caspase-3/caspase-3 protein ratio of 1.6 ± 0.1 and 2.7 ± 0.1 times, respectively (Figure 7d). Therefore, the ratio of cleaved caspase-3/caspase-3 protein was significantly increased in MDA-MB-231 cells co-cultured with THP-1 cells compared to single-cultured MDA-MB-231 cells. We also assessed the influence of cleaved caspase-3 on PARP protein deactivation through its transformation into cleaved PARP. As a result, when treated with 0.1 μg/mL mistletoe extract, the expression of cleaved PARP protein was 0.9 ± 0.1 times and 1.2 ± 0.1 times that of the control in MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells, respectively. In addition, when treated with 2 μg/mL mistletoe extract, the expression of cleaved PARP protein was 0.9 ± 0.2 times and 1.4 ± 0.2 times that of the control in MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells, respectively (Figure 7e). The immunofluorescence staining confirmed the expression level of apoptosis proteins Bax, cleaved caspase-3, and cleaved PARP; interestingly, the protein expression of Bax and cleaved-caspase-3 increased dose-dependently in MDA-MB-231/THP-1 co-cultured cells (Figure 8a,b). However, there were no significant differences in cleaved PARP expression in both MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells, compared to the controls (Figure 8c).
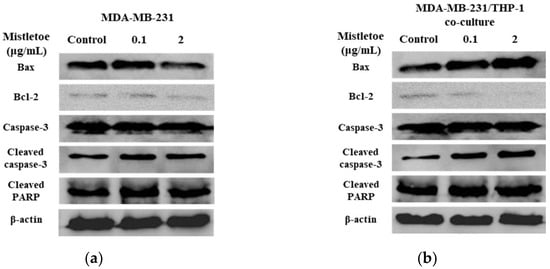
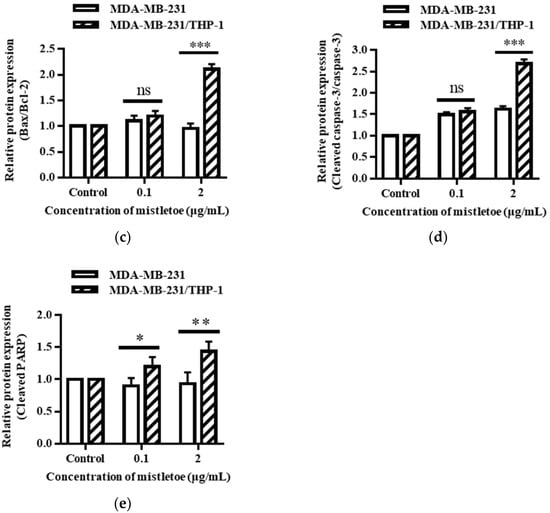
Figure 7.
Effects of Korean mistletoe water extracts on apoptosis-related protein expression in (a) MDA-MDA-231 and (b) MDA-MB-231/THP-1 co-cultured cells using Western blot analysis. Both cell groups were treated with mistletoe water extracts (0, 0.1 and 2 μg/mL) for 48 h, and their relative protein expression levels (c) Bax/Bcl-2, (d) cleaved caspase-3/caspase-3, and (e) cleaved PARP were quantified and presented using a bar graph. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, ** p < 0.01, * p < 0.001, ns: not significant).
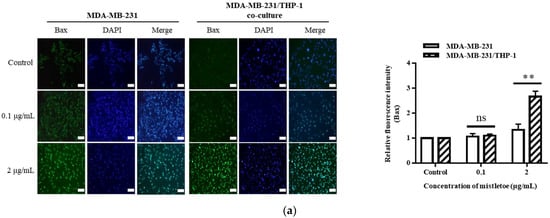
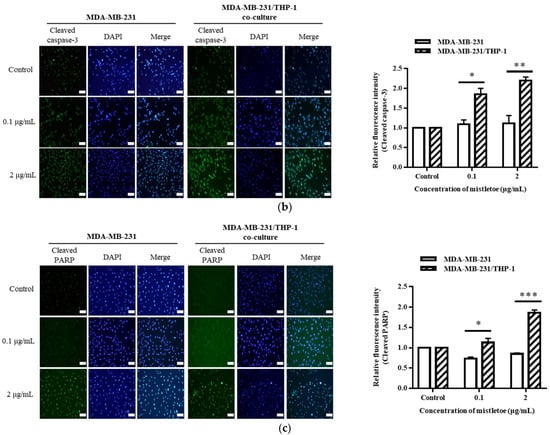
Figure 8.
Representative images and quantitative bar graph of pro-apoptosis proteins using immunofluorescence staining. MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells were treated with various concentrations of Korean mistletoe water extracts (0, 0.1, and 2 μg/mL) for 48 h. The expressed proteins (a) Bax, (b) cleaved caspase-3, and (c) cleaved PARP were labeled with Alexa Fluor™ 488 (green), while nuclear DNA was counterstained with DAPI (blue) then observed using fluorescence microscope (magnification ×200). Fluorescence intensity was quantified using Image J software. Scale bars: 20 μm. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, ** p < 0.01, * p < 0.001, ns: not significant).
3.5. Inhibition of STAT3 Activation
Unregulated STAT3 protein activation is frequently seen in cancer cells, and it is involved in cell proliferation and differentiation, which leads to tumor malignancy [52]. Therefore, to confirm whether Korean mistletoe extract can inhibit STAT3 protein activation in triple-negative breast cancer cells in the breast cancer microenvironment, Western blot assay was performed by treating MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells for 48 h with 0.1 and 2 μg/mL of Korean mistletoe extract (Figure 9a,b). The ratio of the expression of the total STAT3 protein and the phosphorylated and activated p-STAT3 protein was calculated and compared. When 0.1 μg/mL mistletoe was treated, p-STAT3/STAT3 protein was expressed 0.9 ± 0.1 times and 1.13 ± 0.1 times more than the control in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, and no significant difference was observed between the two groups. However, when 2 μg/mL mistletoe was treated, p-STAT3/STAT3 protein was expressed 1.2 ± 0.1 times and 0.7 ± 0.1 times in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference in inhibiting the activation of the STAT3 protein (Figure 9c).
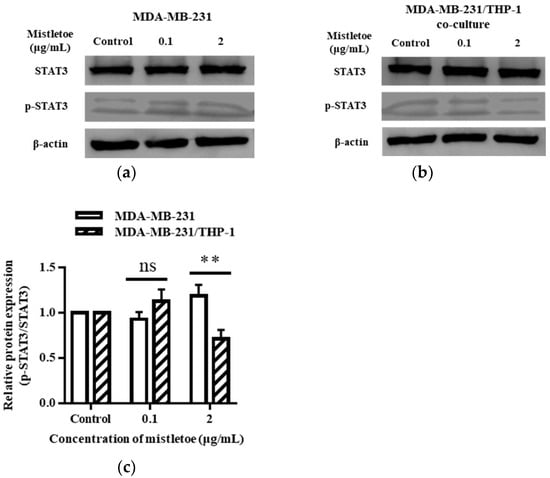
Figure 9.
Inhibitory effects of Korean mistletoe water extract on STAT3 activation in (a) MDA-MB-231 and (b) MDA-MB-231/THP-1 co-cultured cells. After treatment with Korean mistletoe water extracts at concentrations of 0, 0.1, and 2 μg/mL for 48 h, Western blot analysis was conducted. (c) The quantification bar graph represents the ratio of p-STAT3/STAT3 protein expression. Each bar represents the mean ± standard deviation (S.D.) of experiments performed in triplicates. The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (** p < 0.01, ns: not significant).
3.6. Inhibition of MMP-2 and -9 Expression
MMP-2 and MMP-9 are enzymes released by the metastatic cancer cell line MDA-MB-231 that tear down the extracellular matrix, a barrier surrounding the cell that allows tumor cells to migrate to neighboring organs [53]. We confirmed the suppression of MMP-2 or MMP-9 expression through Western blot and immunofluorescence assays after treating MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells for 48 h with 0.1 and 2 μg/mL of mistletoe extract (Figure 10a,b). As a result, when MDA-MB-231 and MDA-MB-231/THP-1 co-culture groups were treated with 0.1 g/mL mistletoe extract, no significant difference in MMP-2 protein expression was found. However, when 2 μg/mL mistletoe was treated, MMP-1 protein was expressed 0.9 ± 0.1 times and 0.4 ± 0.2 times less in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference in inhibiting the MMP-1 protein expression (Figure 10a). Similarly, when MDA-MB-231 and MDA-MB-231/THP-1 co-culture groups were treated with 0.1 g/mL mistletoe extract, no significant difference in MMP-9 protein expression was found. On the other hand, when 2 μg/mL mistletoe was treated, MMP-1 protein was expressed 0.7 ± 0.1 times and 0.4 ± 0.1 times less in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference in inhibiting the MMP-9 protein expression (Figure 10b). In addition, immunofluorescence revealed a significant inhibition of expression in the MBA-MB-231/THP-1 co-culture group upon treatment with 2 μg/mL mistletoe water extract (Figure 11).
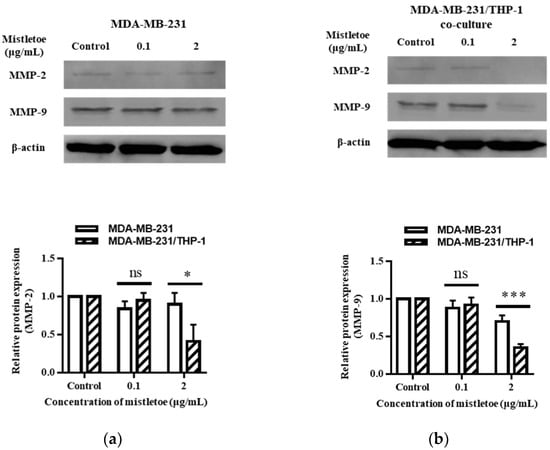
Figure 10.
Inhibitory effects of mistletoe water extract on MMP-2 and MMP-9 expression in (a) MDA-MB-231 and (b) MDA-MB-231/THP-1 co-cultured cells. Both groups were treated with mistletoe water extracts (0, 0.1, and 2 μg/mL) for 48 h, and their relative protein expression levels were quantified and presented using a bar graph. Each bar represents the mean ± standard deviation (S.D.) of experiments performed in triplicates. The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, * p < 0.001, ns: not significant).
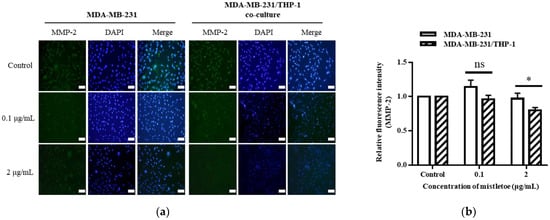
Figure 11.
Representative images from immunofluorescence staining of (a) MMP-2 and (b) their quantitative bar graph. MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells were treated with various concentrations of Korean mistletoe water extracts (0, 0.1, and 2 μg/mL) for 48 h. The expressed proteins were labeled with Alexa Fluor™ 488 (green), while nuclear DNA was counterstained with DAPI (blue) then observed using a fluorescence microscope (magnification ×200). Fluorescence intensity was quantified using Image J software. Scale bars: 20 μm. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (* p < 0.001, ns: not significant).
3.7. Effect on EMT Marker Expression
Through the regulation of related proteins, epithelial–mesenchymal transition (EMT) converts epithelial cells into an invasive and metastatic state. E-cadherin and N-cadherin are recognized as the markers for this transformation, where E-cadherin is a suppressor and N-cadherin an activator [54]. To evaluate whether mistletoe could inhibit the metastasis of breast cancer cells by modulating the expression of cadherin protein, 0.1 and 2 μg/mL of mistletoe extract was added to MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells and incubated for 48 h. As a result, E-cadherin, an EMT-inhibiting protein, was expressed 1.7 ± 0.2 times more than the control in MDA-MB-231/THP-1 co-cultured cell when treated with 0.1 μg/mL mistletoe extract; however, there was no significant difference in MDA-MB-231 cells. When 2 μg/mL of mistletoe was treated, E-cadherin protein was expressed 1.4 ± 0.1 times and 2.5 ± 0.3 times more than the control in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference between the two groups (Figure 12).
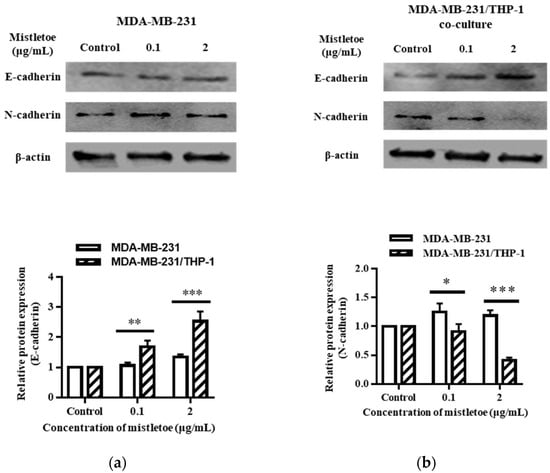
Figure 12.
Effects of Korean mistletoe water extracts on regulating EMT markers in (a) MDA-MB-231 and (b) MDA-MB-231/THP-1 co-cultured cells using Western blot analysis. Both cell groups were treated with mistletoe water extracts (0, 0.1, and 2 μg/mL) for 48 h, and their relative protein expression levels were quantified and presented using a bar graph. The results are represented as the mean ± standard deviation (S.D.). The p-value indicates significant differences between MDA-MB-231 and MDA-MB-231/THP-1 co-cultured cells (*** p < 0.001, ** p < 0.01, * p < 0.001).
In addition, the expression of N-cadherin, a protein that induces EMT, was confirmed via Western blot. When 0.1 μg/mL of mistletoe was treated, N-cadherin protein was expressed 1.3 ± 0.2 times and 0.9 ± 0.1 times more compared to the control in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference between the two groups. In the case of 2 μg/mL mistletoe treatment, N-cadherin protein was expressed 1.2 ± 0.1 times and 0.4 ± 0.1 times more compared to the control in the MDA-MB-231 and MDA-MB-231/THP-1 co-culture group, respectively, showing a significant difference between the two groups (Figure 12).
4. Discussion
Breast cancer is a major problem for women worldwide, accounting for around one-quarter of all female cancer cases [39,55,56]. Breast cancer treatment often entails targeting particular receptors such as ER, PR, and HER2, inducing pharmacological reactions that result in cell death. Developing medications that successfully attack triple-negative breast cancer, on the other hand, provides a distinct difficulty. This subtype, which affects around 10% to 20% of patients, lacks the typical receptors ER, PR, and HER2 [16]. The only therapy presently approved by the Food and Medication Administration (FDA) in the United States is a medication that inhibits the expression of programmed death-ligand 1 (PD-L1) in triple-negative breast cancer [48]. To make things worse, triple-negative breast cancer is notorious for its high recurrence rates and disease spread to distant organs, both of which contribute considerably to death rates [40]. To prevent any further complications, the primary treatment for triple-negative breast cancer involves surgical surgery, radiation therapy, and non-specific chemotherapy [20]; however, these chemotherapeutic procedures—utilizing highly cytotoxic drugs—are associated with a wide range of side effects. These adverse reactions in turn diminish the quality of life among patients suffering from triple-negative breast cancer [18]. Therefore, discovering medications that precisely suppress triple-negative breast cancer without causing adverse effects is emerging.
Mistletoe, a medicinal plant widely distributed throughout Europe and Asia, serves as a complementary agent for anti-cancer drugs that increase apoptosis in cancer cells, decrease cancer mortality, and lessen drug adverse effects [1,3]. Recent studies have shown that mistletoe water extract promotes cell-mediated immunity to tumor cells by protecting monocytes DNA and stimulating immune cells such as macrophages as well as direct cytotoxicity to cancer cells [57]. The bone marrow produces monocytes, which then differentiate into macrophages; these macrophages undergo further differentiation, dependent on various environmental factors, which enable them to perform dual functions within the tumor microenvironment. In general, macrophages use cell-mediated immune responses to kill cancer cells, mediate phagocytosis, and cause vascular damage and necrosis of tumors; however, in a malignly established tumor microenvironment, cancer cells survive, proliferate angiogenesis, and their immune avoidance contributes to cancer progression and metastasis [58]. In recent immuno-cancer studies using macrophages, methods of manipulating macrophages to enhance anti-cancer activity or blocking the access of macrophages to tumors have been mainly proposed. This study confirms that mistletoe water extract holds potential as an immuno-cancer drug for treating triple-negative breast cancer by manipulating the function of macrophages.
When we treated mistletoe extract on MDA-MB-231 cells, a triple-negative breast cancer cell, for 48 h, the 50% inhibitory concentration (IC50) was 20 μg/mL. According to the plant screening system of the National Cancer Institute (NCI) in the United States, the crude extract of plants is considered to be cytotoxic in vitro when IC50 is less than 20 μg/mL after 24–72 h culture [59]. Our results are similar to those of Goda et al., who conducted prior studies on the cytotoxicity of mistletoe extracts in MDA-MB-231 cells [60]. Therefore, we considered mistletoe water extract as a potential cytotoxic drug for triple-negative breast cancer. Furthermore, when mistletoe extracts were treated on THP-1 cells (human mononuclear cell lines), our findings revealed a survival rate of more than 80% at dosages less than 2 μg/mL. Mishra et al. confirmed that the mistletoe water extract for THP-1 cells showed a survival rate of about 60% at 2 g/mL [61], which differed slightly from our findings. However, this might be because Mishra et al. employed Viscum articulatum and did not differentiate THP-1 cells into macrophages, which differs from our work.
Cancer cells secrete inflammatory mediators; these are a significant factor in tumor progression [32]. For instance, in the case of triple-negative breast cancer-secreting cytokines such as IL-4, IL-6, IFN-, and TGF-β, it is these that cause chronic inflammation within this specific microenvironment. The over-expression of such inflammatory cytokines results in accelerated metastasis, alongside neovascularization and cancer cell infiltration [62]. The inflammatory cytokine, IL-6, which plays a pivotal role in cancer progression through the activation of the JAK2/STAT3 signaling pathway [48], has been found to associate STAT3-activated cancer cells with survival and differentiation, metastasis, and infiltration, as well as an increase in EMT [18]. This study’s results revealed that when THP-1 cells (differentiated into macrophages) and MDA-MB-231/THP-1 co-cultured cells were treated with 0.1 and 2 μg/mL Korean mistletoe water extract, there was a significant reduction in IL-6 secretion compared to that seen in MDA-MB-231 cells. Additionally, we studied the JAK2/STAT3 signaling pathway using Western blotting. When we treated the MDA-MB-231/THP-1 co-cultured cells with mistletoe extract at a concentration of 2 μg/mL, it significantly reduced p-STAT3 protein expression. Choi et al. reported through their studies on complex plant extracts that they inhibit STAT3 activation within MDA-MB-231 cells; such inhibition further suppresses IL-6 production in cancer cells [17]. Based on this report, we can assume that Korean mistletoe extract actively reduces the secretion of IL-6 in MDA-MB231 cells by inhibiting the activation of STAT3 between tumor cells and macrophages. Faggioli et al. [63] observed an increase in IL-6 expression within MDA-MB231 cells via nuclear factor kappa (NF-kB)-light-chain enhancer pathway in parallel studies [63], and Suarez-Crevo et al. [64] suggested that the increase in p38, which is a mitogen-activated protein kinase (MAPK) signaling subgroup, and extracellular signal-regulated kinases (ERK1/2) mediated IL-6 expression in MDA-MB-231 cells [64]. However, since this study cannot confirm whether mistletoe water extract affects IL-6 expression in MDA-MB-231 cells by targeting molecules other than STAT3 in MDA-MB-231 cells, it is necessary to study other signaling proteins closely related to inflammatory cytokines. In addition, expressions of IL-4, TGF-β, and IFN-γ were qualitatively analyzed using the Multi-Analyte ELISArray kit (Qiagen) to find out what immune regulation Korean mistletoe extract is involved in between MDA-MB-231 and macrophages. As a result, the expression of the anti-inflammatory cytokines IL-4 and TGF-β increased in the MDA-MB-231/THP-1 co-culture group compared to the MBA-MB-231 cell culture group. It was reported that TGF-β reacts with TGF-β receptor 1 to activate the formation of reactive oxygen specifications (ROS) in cells through the TGF-β/Smad pathway, which activates the suppressor of mothers again (Smad) protein, resulting in the activation of mitochondria, cytochrome c, caspase-9, and finally caspase-3 [65,66]. Since ELISArray confirmed that TGF-β increased expression in the MDA-MB-231/THP-1 co-culture group compared to the MDA-MB-231 cell culture group, we expected that apoptosis targeting mitochondria in triple-negative breast cancer cells would increase in the co-culture group. Also, it was reported that IFN-γ is involved when M0 macrophages are differentiated into M1 macrophages [67]. Based on these facts, we can assume that Korean mistletoe extract can differentiate M0 macrophages into M1 via initiating IFN-γ expression. However, a follow-up study is needed.
Aside from direct apoptosis, the mechanism of tumor cell death produced by the component of mistletoe water extract is linked to immune cell activation, namely natural killer cells (NK cells), lymphocytes, and macrophages [67,68,69]. Clinical investigations have indicated that immune cells triggered by mistletoe water extracts react directly or indirectly to tumor cells, inhibiting tumor cell proliferation and improving patient survival rates [12,70]. Therefore, we investigated whether the activation of human macrophage THP-1 by Korean mistletoe water extract could induce apoptosis in MDA-MB-231 cells through cell-mediated immunity. MDA-MB-231 cells and the MDA-MB-231/THP-1 co-culture group were each exposed to Korean mistletoe water extracts at concentrations of 0.1 and 2 μg/mL for a duration of 48 h. It was observed that in the MDA-MB-231/THP-1 co-culture group at a concentration of 2 μg/mL, there was a significant augmentation in the Bax/Bcl-2 protein expression ratio compared to the MDA-MB-231 cell culture group, indicating a significant increase in the initiation of apoptosis.
Additionally, we hypothesized that mistletoe could release apoptosomes into the mitochondria’s cytoplasm, through Bcl-2 family regulation, which leads to caspase-3 activation. Typically, caspase-3 exists in the cytoplasm in an inactive form, and when cleaved by caspase-9 or other proteases, it is activated when the active site is exposed [71]. To compare and analyze the degree of activation of caspase-3 in this study, the ratio of cleaved caspase-3 was confirmed. When 2 μg/mL mistletoe extract was treated, the expression of cleaved caspase-3 increased significantly in the MDA-MB-231/THP-1 co-culture group. From this result, it can be said that mistletoe can regulate Bax and Bcl-2 proteins leading to an increase in MOMP, resulting in caspase-3 activation.
Apoptosis is caused when cleaved caspase-3 cuts and inactivates PARP, which aids in the repair of damaged DNA in tumor cells’ nuclei. Therefore, increasing cleaved PARP induces apoptosis in tumor cells [72].
In this study, cleaved PARP expression increased in both the 0.1 and 2 μg/mL mistletoe-treated MDA-MB-231/THP co-culture group. However, the expression of cleaved caspase-3 increased only in the 2 μg/mL mistletoe-treated MDA-MB-231/THP-1 co-culture group. There was a correlation between the increase in cleaved caspase-3 and cleaved PARP, but it was not proportional. This could be explained by a previous study indicating that cleaved caspase-3 enters the nucleus through active transport in the nuclear pore complex rather than through simple diffusion. Furthermore, active nuclear transport of cleaved caspase-3 is dependent on morphological changes in the nucleus caused by various apoptotic triggers and transport proteins that carry out active nuclear transport [73]. Also, Cui et al. reported that the cutting of PARP during apoptosis was performed by calpain, a calcium-dependent protease, rather than caspase-3, by inducing p53 genes and caspase-9 in breast cancer cell lines [74]. Based on the findings, it is possible to explain why there was an increase in cleaved PARP expression even though there was no significant rise in cleaved caspase-3 when mistletoe water extract at 0.1 g/mL was treated. The mechanism by which mistletoe extract induces apoptosis in MDA-MB-231 cells is likely to cleave PARP and trigger apoptosis through pathways other than caspase-3.
At each stage of metastasis, breast cancer epithelial cells are polarized into endothelial cell phenotypes through the progress of EMT, increasing mobility, and a loss of E-cadherin and an increase in N-cadherin are observed at the molecular level [75,76]. In addition, MMPs are involved in the process of cancer cells breaking down and invading the extracellular matrix. Among various types of MMPs, MMP-2 and MMP-9, with the activity of breaking down gelatin, are secreted to the cell surface and locally decompose the extracellular matrix that binds to the cell membrane, making the cell motile [77]. Because the findings imply that the mobility of cancer cells is connected to the level of MMP expression, investigations on cancer metastasis inhibition targeting MMPs are being conducted [78,79,80]. Therefore, in this study, when mistletoe water extract was treated on MDA-MB-231 cells and MDA-MB-231/THP-1 co-cultured cells, the difference in mobility inhibition between the two groups was compared via wound healing and Transwell migration assay. Also, to validate the suppression of metastasis of triple-negative breast cancer cells, the regulation of E-cadherin and N-cadherin, and the inhibition of MMP-2 and MMP-9 expression, were confirmed. Firstly, the MDA-MB-231 cell culture group and the MDA-MB-231/THP-1 co-culture group were attached to a plate or membrane insert, and then the mobility inhibition of MDA-MB-231 cells was observed after they were treated with mistletoe extract. It was confirmed that the co-culture group significantly inhibited the movement to the wound area compared to the MDA-MB-231 cell culture group. The mobility of MDA-MB-231 attached to the insert membrane was inhibited by mistletoe extract, dose-dependently.
To compare the inhibition of metastasis between the two groups due to the regulation of cell movement-related protein expression at the molecular level, an EMT marker that regulates the mobility of cancer cells within the tumor microenvironment was observed through Western blot analysis. First, when the expression of E-cadherin, an EMT inhibitor, was observed, there was a significant increase in expression in the MDA-MB-231/THP-1 co-culture group compared to the MDA-MB-231 cell culture group. In addition, expression of the EMT product, N-cadherin, was considerably reduced in the co-culture group compared to the single culture group. Therefore, it was confirmed that mistletoe water extract can inhibit the EMT of MDA-MB-231 cells at the molecular level by activating THP-1 macrophages. Furthermore, the comparison of Western blot results between the two groups revealed a significant inhibition in the expression of MMP-2 and MMP-9. Following 48 h of treatment with mistletoe water extract at a dosage of 2 μg/mL, both MMP-2 and MMP-9 were considerably suppressed in the MDA-MB-231/THP-1 co-culture group. Therefore, it was confirmed at the molecular level that mistletoe water extract can inhibit metastasis by inhibiting the stage of local infiltration of tissue by activating THP-1 macrophages and inhibiting the expression of MMP-2 and MMP-9 in MDA-MB-231 cells through cell-mediated immunity. The observed effects on EMT markers and MMPs provide valuable insights into the potential mechanisms underlying mistletoe water extract’s anti-metastatic properties. These findings align with previous research indicating the anti-cancer properties of mistletoe extracts and highlight its potential as a therapeutic agent in the field of metastatic breast cancer [81].
According to the findings of this research, Korean mistletoe water extract inhibits triple-negative breast cancer cells by enhancing the human immune system via activating macrophages. In particular, we could confirm apoptosis which induced MOMP in triple-negative breast cancer cells through cell-mediated immunity of activated macrophages. In addition, we looked into the STAT3 pathway and also the regulation of cancer cell mobility by controlling numerous inflammation-related cytokines. However, further studies are needed to determine whether mistletoe can influence the immunity of breast cancer subtypes other than triple-negative breast cancer cells. We also need to determine how macrophages were activated and which component in mistletoe extract contributes to this activation. Lastly, the subject of whether mistletoe influences other immune cells in the human body, such as natural killer (NK) cells, remains unanswered, thus further study is needed.
Author Contributions
Conceptualization: C.-E.H. and S.-Y.L.; funding acquisition: S.-Y.L.; investigation: W.-T.L., C.-E.H. and S.-Y.L.; methodology: W.-T.L. and C.-E.H.; resources: W.-T.L. and C.-E.H.; supervision: S.-Y.L.; writing—original draft: W.-T.L.; writing—review and editing: S.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orhan, D.D.; Küpeli, E.; Yesilada, E.; Ergun, F. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z. Naturforsch. C, J. Biosci. 2006, 61, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kuttan, G.; Vasudevan, D.M.; Kuttan, R. Effect of a preparation from Viscum album on tumor development in vitro and in mice. J. Ethnopharmacol. 1990, 29, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Review article: Influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef]
- Onay-Uçar, E.; Karagöz, A.; Arda, N. Antioxidant activity of Viscum album ssp. album. Fitoterapia 2006, 77, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Attar, R.; Tabassum, S.; Fayyaz, S.; Ahmad, M.S.; Nogueira, D.R.; Yaylim, I.; Timirci-Kahraman, O.; Kucukhuseyin, O.; Cacina, C.; Farooqi, A.A.; et al. Natural products are the future of anticancer therapy: Preclinical and clinical advancements of Viscum album phytometabolites. Cell. Mol. Biol. (Noisy-le-grand) 2015, 61, 62–68. [Google Scholar]
- Park, J.H.; Hyun, C.K.; Shin, H.K. Cytotoxic effects of the components in heat-treated mistletoe (Viscum album). Cancer Lett. 1999, 139, 207–213. [Google Scholar] [CrossRef]
- Zarkovic, N.; Vukovic, T.; Loncaric, I.; Miletic, M.; Zarkovic, K.; Borovic, S.; Cipak, A.; Sabolovic, S.; Konitzer, M.; Mang, S. An overview on anticancer activities of the Viscum album extract Isorel. Cancer Biother. Radiopharm. 2001, 16, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Enesel, M.B.; Acalovschi, I.; Grosu, V.; Sbarcea, A.; Rusu, C.; Dobre, A.; Weiss, T.; Zarkovic, N. Perioperative application of the Viscum album extract Isorel in digestive tract cancer patients. Anticancer Res. 2005, 25, 4583–4590. [Google Scholar]
- Jurin, M.; Zarkovic, N.; Borovic, S.; Kissel, D. Viscum album L. preparation Isorel modifies the immune response in normal and in tumour-bearing mice. Anti-Cancer Drugs 1997, 8 (Suppl. S1), S27–S31. [Google Scholar] [CrossRef]
- Mueller, E.A.; Anderer, F.A. AViscum album oligosaccharide activating human natural cytotoxicity is an interferon γ inducer. Cancer Immunol. Immunother. 1990, 32, 221–227. [Google Scholar] [CrossRef]
- Kim, J.C.; Yoon, T.J.; Song, T.; Kim, Y.H.; An, H.S.; Kim, J.B. ucosal Immunoadjuvant Activity of Korean Mistletoe Lectin-C. Korean J. Food Sci. Technol. 2011, 43, 72–76. [Google Scholar] [CrossRef]
- Lee, J.L.; Jeon, Y.H.; Yang, H.S.; Lee, K.B.; Song, K.S.; Kang, T.B.; Kim, J.B.; Yoo, Y.C. The immunostimulatory activity of the water-extract of Korean mistletoe fruit to activate murine peritoneal macrophages. Kor. J. Pharmacogn. 2010, 41, 122–129. [Google Scholar]
- Lee, S.-j.; Lee, M.K.; Choi, G.-P.; Yu, C.Y.; Roh, S.-K.; Kim, J.-D.; Lee, H.Y.; Lee, J.-H. Growth enhancement and cytotoxicity of Korean mistletoe fractions on human cell lines. Korean J. Med. Crop Sci. 2003, 11, 62–70. [Google Scholar]
- Melo, M.N.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; Castro, J.L.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J.; Yoo, Y.C.; Kang, T.B.; Song, S.K.; Lee, K.B.; Her, E.; Song, K.S.; Kim, J.B. Antitumor activity of the Korean mistletoe lectin is attributed to activation of macrophages and NK cells. Arch. Pharm. Res. 2003, 26, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- Choi, Y.K.; Cho, S.G.; Woo, S.M.; Yun, Y.J.; Park, S.; Shin, Y.C.; Ko, S.G. Herbal extract SH003 suppresses tumor growth and metastasis of MDA-MB-231 breast cancer cells by inhibiting STAT3-IL-6 signaling. Mediators Inflamm. 2014, 2014, 492173. [Google Scholar] [CrossRef]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells Via Targeting the STAT3 Signaling Pathway. Iran J. Pharm. Res. 2020, 19, 122–133. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L.; Chen, S. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010, 70, 3594–3605. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Bäuerle, T.; Adwan, H.; Kiessling, F.; Hilbig, H.; Armbruster, F.P.; Berger, M.R. Characterization of a rat model with site-specific bone metastasis induced by MDA-MB-231 breast cancer cells and its application to the effects of an antibody against bone sialoprotein. Int. J. Cancer 2005, 115, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Kanaya, N.; Phung, S.; Liu, Z.; Chen, S. Whole blueberry powder modulates the growth and metastasis of MDA-MB-231 triple negative breast tumors in nude mice. J. Nutr. 2011, 141, 1805–1812. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lin, W.L.; Chen, N.F.; Chuang, S.K.; Tseng, T.H. Demethylwedelolactone derivatives inhibit invasive growth in vitro and lung metastasis of MDA-MB-231 breast cancer cells in nude mice. Eur. J. Med. Chem. 2012, 56, 361–367. [Google Scholar] [CrossRef]
- An, J.; Wang, L.; Zhao, Y.; Hao, Q.; Zhang, Y.; Zhang, J.; Yang, C.; Liu, L.; Wang, W.; Fang, D.; et al. Effects of FSTL1 on cell proliferation in breast cancer cell line MDA-MB-231 and its brain metastatic variant MDA-MB-231-BR. Oncol. Rep. 2017, 38, 3001–3010. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Saadi, W.; Lin, F.; Minh-Canh Nguyen, C.; Li Jeon, N. Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis. Exp. Cell Res. 2004, 300, 180–189. [Google Scholar] [CrossRef]
- Place, A.E.; Jin Huh, S.; Polyak, K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef]
- Lu, X.; Yang, R.; Zhang, L.; Xi, Y.; Zhao, J.; Wang, F.; Zhang, H.; Li, Z. Macrophage Colony-stimulating Factor Mediates the Recruitment of Macrophages in Triple negative Breast Cancer. Int. J. Biol. Sci. 2019, 15, 2859–2871. [Google Scholar] [CrossRef]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann. N. Y. Acad. Sci. 2021, 1499, 18–41. [Google Scholar] [CrossRef]
- Park, H.J.; Chi, G.Y.; Choi, Y.H.; Park, S.H. The root bark of Morus alba L. regulates tumor-associated macrophages by blocking recruitment and M2 polarization of macrophages. Phytother. Res. 2020, 34, 3333–3344. [Google Scholar] [CrossRef]
- Thabet, N.A.; El-Guendy, N.; Mohamed, M.M.; Shouman, S.A. Suppression of macrophages- Induced inflammation via targeting RAS and PAR-4 signaling in breast cancer cell lines. Toxicol. Appl. Pharmacol. 2019, 385, 114773. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, C. Berberine Inhibits MDA-MB-231 Cells by Attenuating Their Inflammatory Responses. Biomed Res. Int. 2020, 2020, 3617514. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Piva, F.; Massari, F.; Ciccarese, C.; Burattini, L.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M.; Santini, D.; et al. Role of STAT3 pathway in genitourinary tumors. Future Sci. OA 2015, 1, Fso15. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.J.; Darvin, P.; Kang, D.Y.; Sp, N.; Joung, Y.H.; Park, J.H.; Kim, S.J.; Yang, Y.M. Silibinin downregulates MMP2 expression via Jak2/STAT3 pathway and inhibits the migration and invasive potential in MDA-MB-231 cells. Oncol. Rep. 2017, 37, 3270–3278. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005, 10 (Suppl. S3), 20–29. [Google Scholar] [CrossRef]
- Beberok, A.; Wrześniok, D.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDA-MB-231 cells via the p53/Bax/Bcl-2 signaling pathway. Int. J. Oncol. 2018, 52, 1727–1737. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Westphal, D.; Dewson, G.; Ma, S.; Hockings, C.; Fairlie, W.D.; Lee, E.F.; Yao, S.; Robin, A.Y.; Smith, B.J.; et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 2013, 152, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Gillies, L.A.; Kuwana, T. Apoptosis regulation at the mitochondrial outer membrane. J. Cell. Biochem. 2014, 115, 632–640. [Google Scholar] [CrossRef]
- Lindholm, D.; Eriksson, O.; Korhonen, L. Mitochondrial proteins in neuronal degeneration. Biochem. Biophys. Res. Commun. 2004, 321, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Baek, J.Y.; Kim, K.D.; Choi, Y.H.; Lee, J.D. Induction of apoptosis by pachymic acid in T24 human bladder cancer cells. J. Life Sci. (Calicut) 2015, 1, 93–100. [Google Scholar] [CrossRef]
- McConkey, D.J. Biochemical determinants of apoptosis and necrosis. Toxicol. Lett. 1998, 99, 157–168. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Nordin, F.J.; Pearanpan, L.; Chan, K.M.; Kumolosasi, E.; Yong, Y.K.; Shaari, K.; Rajab, N.F. Immunomodulatory potential of Clinacanthus nutans extracts in the co-culture of triple-negative breast cancer cells, MDA-MB-231, and THP-1 macrophages. PLoS ONE 2021, 16, e0256012. [Google Scholar] [CrossRef]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia annua L Inhibit Adhesion and EMT of Highly Metastatic Breast Cancer Cells MDA-MB-231. Phytother. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef]
- Olmeda, D.; Moreno-Bueno, G.; Flores, J.M.; Fabra, A.; Portillo, F.; Cano, A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 2007, 67, 11721–11731. [Google Scholar] [CrossRef]
- Ameri, K.; Luong, R.; Zhang, H.; Powell, A.A.; Montgomery, K.D.; Espinosa, I.; Bouley, D.M.; Harris, A.L.; Jeffrey, S.S. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br. J. Cancer 2010, 102, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in cancer metastasis and translational advances. Biomed Res. Int. 2013, 2013, 421821. [Google Scholar] [CrossRef]
- Parsons, S.L.; Watson, S.A.; Brown, P.D.; Collins, H.M.; Steele, R.J. Matrix metalloproteinases. Br. J. Surg. 1997, 84, 160–166. [Google Scholar] [PubMed]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, P.; Xu, L.X. Iron promotes breast cancer cell migration via IL-6/JAK2/STAT3 signaling pathways in a paracrine or autocrine IL-6-rich inflammatory environment. J. Inorg. Biochem. 2020, 210, 111159. [Google Scholar] [CrossRef]
- Büssing, A.; Raak, C.; Ostermann, T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): A meta-analysis. Evid. Based Complement. Alternat. Med. 2012, 2012, 219402. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Amini, N.; Supriyanto, E.; Abdul Majid, F.A.; Kumar Jaganathan, S.; Jamil, S.; Hamzehalipour Almaki, J.; Nasiri, R. Antioxidant Activity and ROS-Dependent Apoptotic Effect of Scurrula ferruginea (Jack) Danser Methanol Extract in Human Breast Cancer Cell MDA-MB-231. PLoS ONE 2016, 11, e0158942. [Google Scholar] [CrossRef]
- Goda, M.S.; Elhady, S.S.; Nafie, M.S.; Bogari, H.A.; Malatani, R.T.; Hareeri, R.H.; Badr, J.M.; Donia, M.S. Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents. Metabolites 2022, 12, 921. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, S.; Sharma, R.S.; Singh, S.; Sardesai, M.M.; Sharma, S.; Mishra, V. Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J. Ethnopharmacol. 2018, 219, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, T.; Kudome, Y.; Kishimoto, Y.; Saita, E.; Tanaka, M.; Taguchi, C.; Hirakawa, S.; Mitani, N.; Kondo, K.; Iida, K. Aronia berry extract inhibits TNF-α-induced vascular endothelial inflammation through the regulation of STAT3. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, L.; Costanzo, C.; Merola, M.; Bianchini, E.; Furia, A.; Carsana, A.; Palmieri, M. Nuclear factor kappa B (NF-kappa B), nuclear factor interleukin-6 (NFIL-6 or C/EBP beta) and nuclear factor interleukin-6 beta (NFIL6-beta or C/EBP delta) are not sufficient to activate the endogenous interleukin-6 gene in the human breast carcinoma cell line MCF-7. Comparative analysis with MDA-MB-231 cells, an interleukin-6-expressing human breast carcinoma cell line. Eur. J. Biochem. 1996, 239, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Cuervo, C.; Harris, K.W.; Kallman, L.; Väänänen, H.K.; Selander, K.S. Tumor necrosis factor-alpha induces interleukin-6 production via extracellular-regulated kinase 1 activation in breast cancer cells. Breast Cancer Res. Treat. 2003, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, M.; Yamada, M.; Miake, Y.; Yanagisawa, T. Transforming growth factor β inducible apoptotic cascade in epithelial cells during rat molar tooth eruptions. Anat. Sci. Int. 2010, 85, 92–101. [Google Scholar] [CrossRef]
- Tewari, D.; Priya, A.; Bishayee, A.; Bishayee, A. Targeting transforming growth factor-β signalling for cancer prevention and intervention: Recent advances in developing small molecules of natural origin. Clin. Transl. Med. 2022, 12, e795. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- Coeugniet, E.G.; Elek, E. Immunomodulation with Viscum album and Echinacea purpurea extracts. Onkologie 1987, 10, 27–33. [Google Scholar] [CrossRef]
- Elluru, S.; Duong Van Huyen, J.P.; Delignat, S.; Prost, F.; Bayry, J.; Kazatchkine, M.D.; Kaveri, S.V. Molecular mechanisms underlying the immunomodulatory effects of mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung 2006, 56, 461–466. [Google Scholar] [CrossRef]
- Heiny, B.M.; Beuth, J. Mistletoe extract standardized for the galactoside-specific lectin (ML-1) induces beta-endorphin release and immunopotentiation in breast cancer patients. Anticancer Res. 1994, 14, 1339–1342. [Google Scholar]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, E.-J.; Lim, D.; Kim, J.-S.; Lim, S.; Shin, H.-K.; Park, J.-H. Inhibitory Effect of the Hexane Extract of Saussurea lappa on the Growth of LNCaP Human Prostate Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2008, 37, 8–15. [Google Scholar] [CrossRef][Green Version]
- Kamada, S.; Kikkawa, U.; Tsujimoto, Y.; Hunter, T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J. Biol. Chem. 2005, 280, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yu, J.H.; Wu, J.N.; Tashiro, S.; Onodera, S.; Minami, M.; Ikejima, T. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol. Sin. 2007, 28, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Garcia, A.; Xu, S.; Powell, D.R.; Vertino, P.M.; Singh, S.; Marcus, A.I. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS ONE 2013, 8, e75069. [Google Scholar] [CrossRef] [PubMed]
- VanSaun, M.N.; Matrisian, L.M. Matrix metalloproteinases and cellular motility in development and disease. Birth Defects Res. C Embryo Today 2006, 78, 69–79. [Google Scholar] [CrossRef]
- Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef]
- Itoh, T.; Tanioka, M.; Matsuda, H.; Nishimoto, H.; Yoshioka, T.; Suzuki, R.; Uehira, M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metastasis 1999, 17, 177–181. [Google Scholar] [CrossRef]
- Shay, G.; Lynch, C.C.; Fingleton, B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015, 44–46, 200–206. [Google Scholar] [CrossRef]
- Kienle, G.S.; Kiene, H. Complementary cancer therapy: A systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur. J. Med. Res. 2007, 12, 103–119. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).