1. Introduction
Remdesivir (REM) is a prodrug of a monophosphoramidate nucleoside that is designed to easily pass the cell membrane and efficiently deliver its active metabolite [
1]. Upon entering in the target cells, through intracellular metabolic activation, remdesivir monophosphate is rapidly converted into its active triphosphate form due to its ability to bypass the first inefficient and rate-limiting phosphorylation step [
2]. The active triphosphate form can be incorporated into nascent viral RNA chains and interfere with RNA-dependent-RNA polymerase (RdRp), leading to the premature termination of viral RNA transcription and, finally, inhibition of the viral replication [
3,
4,
5]. Recently, studies demonstrated the effectiveness of remdesivir (Veklury
®, Gilead Science Inc., Foster City, CA, USA) as a more evident and promising drug for COVID-19 therapy [
6,
7]. It was the first medicament approved for the treatment of the disease. According to Clinical Drug Information [
8], remdesivir is a broad-spectrum antiviral substance with pronounced in vitro and in vivo activity against a wide spectrum of RNA viruses, including coronaviruses [
9]. Moreover, the drug completed phase III of a clinical trial for the treatment of Ebola virus infection and the pharmacokinetics and safety for the human cells or tissues have relatively complete data [
10].
The low water solubility of REM (0.028 mg/mL at room temperature [
11]) restricts the direct intravenous infusion. Therefore, the application of surfactants, polymers, or co-solvents is necessary to ensure the formation of stable and clear aqueous solutions suitable for intravenous administration. In the product Veklury
® (Gilead Science Inc.), the cyclodextrin derivate (sulfobutylether-beta-cyclodextrin sodium, SBECD) is used to improve the water solubility of REM.
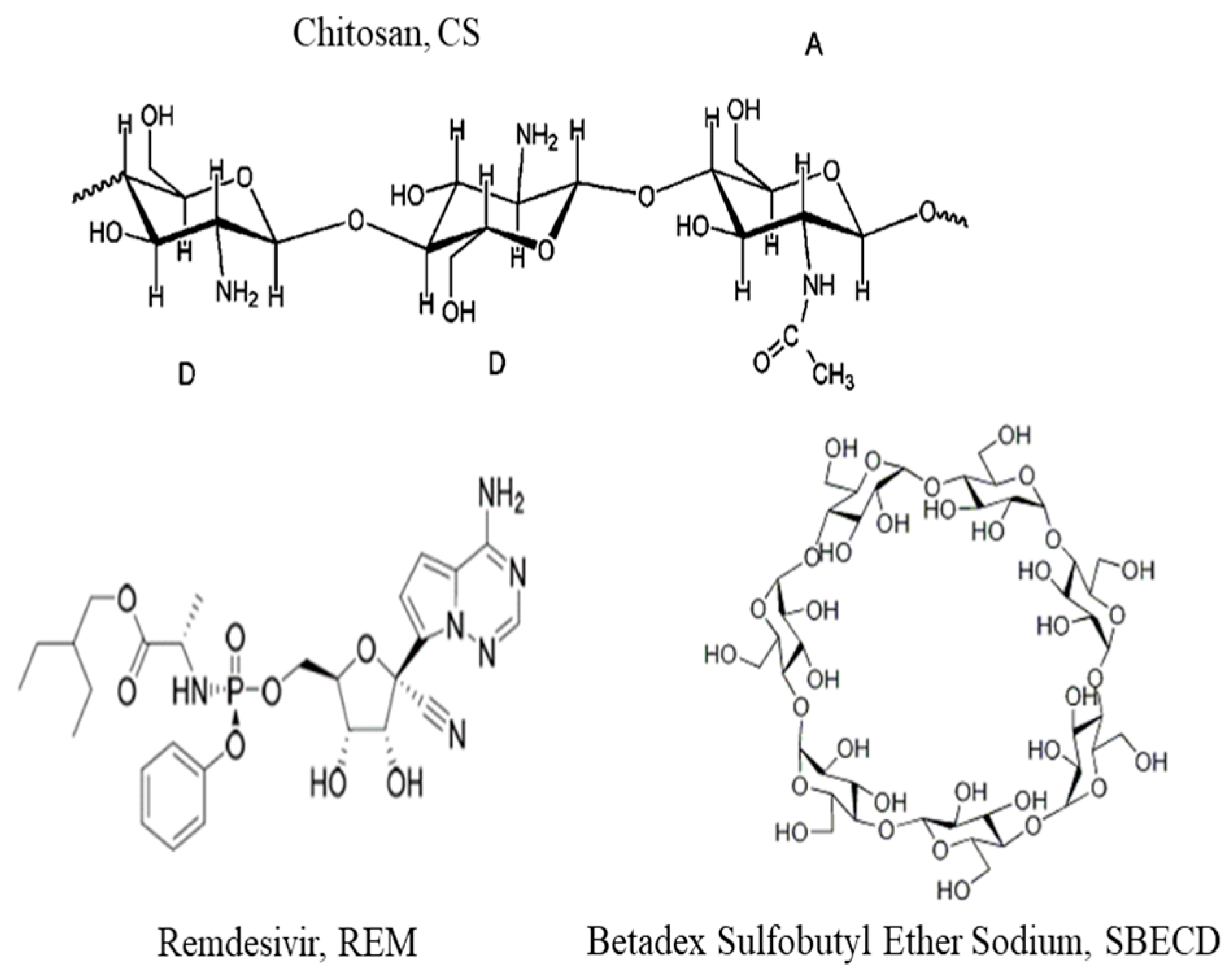
SBECD is highly soluble in water compounds that show typical behavior for cyclodextrin derivates hydrophobic/hydrophilic. The physicochemical properties of SBECD provide ideal conditions for the solubilization of hydrophobic drugs. The chiral molecule is composed of 7 α-D glucopyranose units, derivative variably substituted on 2-, 3-, and 6-positions of β-cyclodextrin. The molecule is suitable for molecular inclusion and one molecule of the active compound can be trapped in the cavity of the host molecule of SBECD [
12,
13,
14]. However, it was reported that the direct entry of large amounts of SBECD into the bloodstream was limited for patients with renal insufficiently, and the drug was also not able to exert a fully therapeutic effect in the lung because of poor accumulation after intravenous administration and low expression of the enzymes necessary for activation [
15]. Moreover, due to the high liver metabolism and low oral bioavailability, REM cannot be administered orally [
16]. Therefore, the inhalation administration of REM can be an appropriate strategy to overcome these limitations [
17]. Recently, studies reported the encapsulation of pure remdesivir in liposomes [
17], lipid nanocarriers [
18], polymer-drug conjugates [
19], or hyper-branched dendritic nanocarriers [
20].
The present study addressed the development of a procedure for the formation and characterization of stable chitosan-based oil-core capsules for encapsulation and target delivery of Veklury
®. Veklury
® was chosen instead of pure remdesivir because of its high water solubility. The cores of the capsules were oil droplets stabilized by thick lecithin-chitosan membrane resulting from the strong electrosteric interactions between chitosan monomers and hydrophilic/hydrophobic domains of lecithin [
21,
22]. The drug was loaded into the chitosan layer due to the electrostatic attraction interaction between the positively charged chitosan monomers and negatively charged SBECD-REM complexes (the model investigation of the interaction of the produced carriers with a lipid layer, as a model of a biological membrane, was a subject of the next study [
23]).
A major component of the produced capsules is chitosan. It is a well-studied cationic polysaccharide with many applications in the area of drug and gene delivery nanomaterials, etc., including for the pulmonary system. Based on its structure, the polymer is capable of participating in electrostatic, hydrophobic, and hydrogen types of bonding. Moreover, the variations in the molecular structure of chitosan through control on the degree of acetylation (DA) or molecular weight (Mw) may entail different charge distribution, physicochemical properties of molecules, and biological activity. In order to evaluate the effect of the properties of chitosan on the properties of the produced capsules, chitosan samples with different physicochemical characteristics were used in the investigation.
To achieve a target delivery of the capsules, an aptamer with high binding affinity to viral spike glycoproteins of human coronavirus HCoV-O43 is involved in the formulations. The spike glycoproteins protruding from the viral surface play an important role in the infection process from the virus to the host cell. Since the function of the receptor-binding domain (RBD) of the protein is indispensable, the surface structure of the protein is particularly important and responsible for determining the range of the host, the specificity of the virus, and the mortality rate. This makes RBD of the spike glycoprotein a key target for diagnosis, treatment, and vaccination.
2. Materials and Methods
2.1. Materials
Chitosans (Sigma Aldrich, Taufkirchen, Germany) with low (50–190 kDa) CS-L and medium (190–310 kDa) CS-M molecular weight and degree of deacetylation (DDA) 75–85% were chosen for this study (product numbers 448869 and 448877). The stock polymer solutions were prepared with a concentration of 1 mg/mL in hydrochloric acid (HCl) and were filtered through a 0.45 µm filter (Minisart®, Sartorius, Gottingen, Germany) to remove the possible aggregates.
The stock solution of Veklury® (Gilead Science Inc. Ireland UC, Ireland) with a concentration of 150 mg/mL was prepared in double distilled water. The concentration of remdesivir in stock solution was estimated at 8.3 × 10−3 M.
Betadex sulfobutyl ether sodium (SBECD) was purchased from Sigma Aldrich (product number PHR2923).
For the formation of the core of the capsules, soybean lecithin, PC 40% (Avanti, Germany, 341602G), was used. Miglyol 812 N
® was kindly provided by Sasol Germany GmbH, Witten, Germany. The molecular structures of used compounds are presented in
Figure 1.
The aptamer selection was performed by using a web server called PRIdictor (Protein–RNA Interaction predictor), which can predict mutual binding sites in oligonucleotide sequence in RNA molecules and protein at the nucleotide- and residue-level resolutions from their sequences [
23]. PRIdictor was used as a web-based application [
24]. The selected oligonucleotide sequence of aptamer (5′-AAA CAU UGC AC-3′) was synthesized from Biomers (Ulm, Germany). According to the product information, the molecular weight was 3457 g/mol. The sample was dissolved in double distilled water and the aptamer concentration in the stock solution was 60.76 μM.
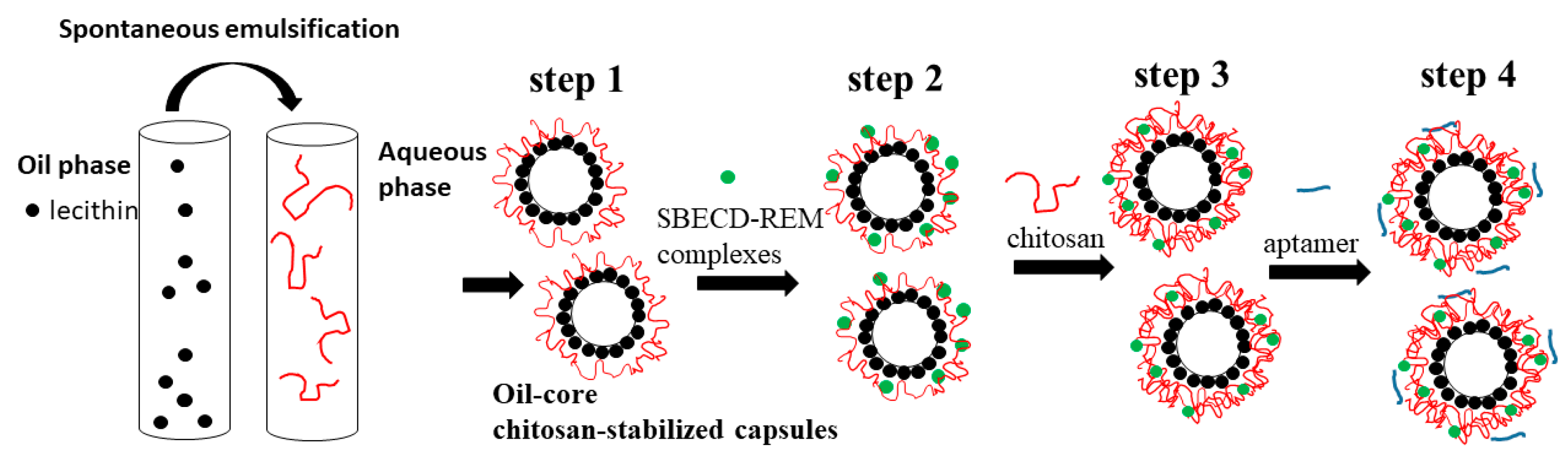
2.2. Preparation of the Chitosan-Stabilized Oil-Core Capsules and Remdesivir Encapsulation
The subsequent steps in the experimental procedure used for the formation of the capsules are presented in
Figure 2.
Step 1. The cores of the capsules were oil droplets stabilized by lecithin/chitosan membrane produced according to the procedure originally described by Calvo et al. [
25]. Briefly, the oil phase was prepared from 0.5 mL of a 40 mg/mL ethanolic lecithin solution mixed with 0.125 mL Miglyol 812 N
® and 9.5 mL ethanol, and the aqueous phase was chitosan solution (20 mL, 0.5 mg/mL). After mixing, the organic solvent was evaporated at 40 °C. The produced droplets were positively charged because of the deposition of a thick chitosan layer on their surface. For comparison, following the same procedure, an oil-in-water nanoemulsion was prepared by replacing the chitosan solution with a solution of hydrochloric acid (pH 4.04). In order to prevent a possible aggregation in the subsequent steps, the stock dispersion was diluted 1:19 with a solution of HCl (pH~4.04). The dispersion was sonicated for 15 min before the deposition of the drug (the estimated concentration was 7.25 × 10
10 droplets/mL) (
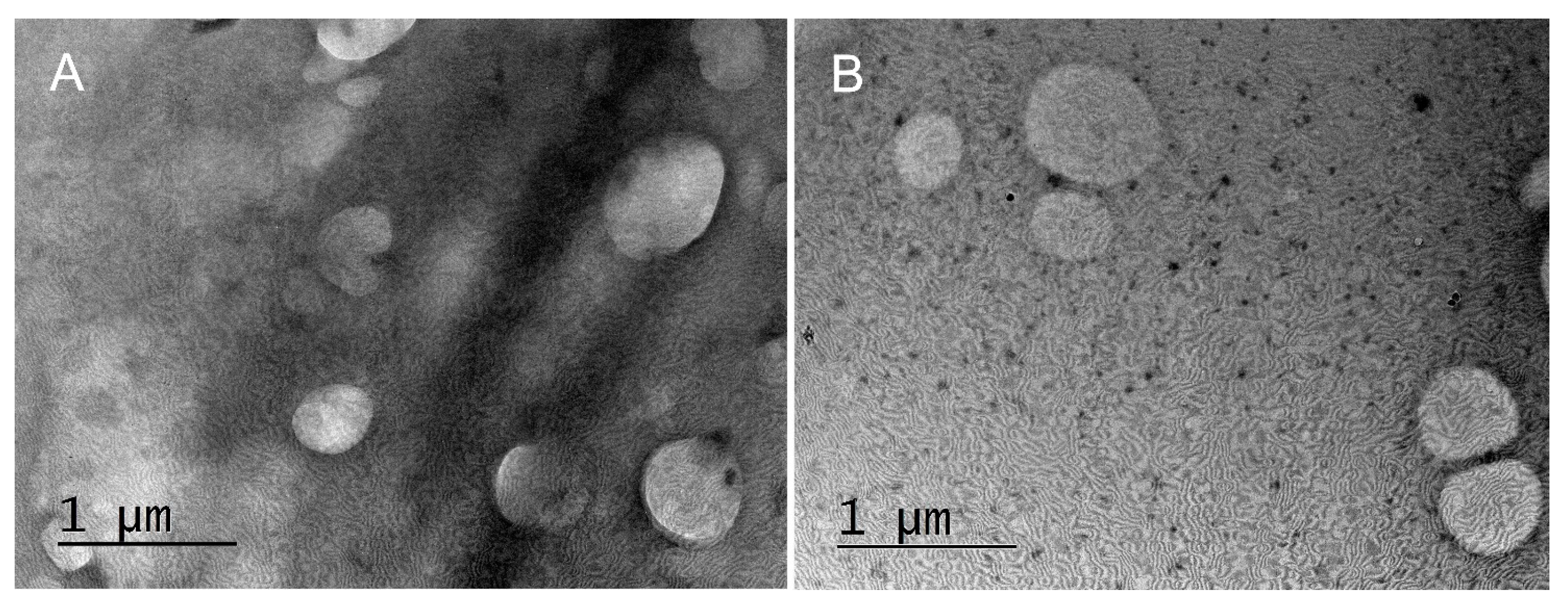
Figure 3).
Step 2. The negatively charged drug was impregnated into the chitosan layer of the produced structures. The diluted dispersion of oil-core capsules (20 mL) was added to a high enough concentration of the drug (0.9 mg/mL, Veklury
®) and stirred for 20 min at room temperature (24 °C). The estimated final concentration of REM in dispersion was 50 μM (the amount of the drug was high enough to ensure the formation of stable structures and high encapsulation efficiency at almost constant experimental conditions of the dispersion (ionic strength and pH) (
Table 1)). The excess non-adsorbed drug was removed by centrifugation (15,000 rpm, 21,382 g, at 15 °C for 60 min) using a laboratory centrifuge (PW-352R, Poland). The extracted cream was diluted to the initial volume with a solution of HCl (pH~4.04) and sonicated in an ultrasonic bath for 10 min.
Step 3. In order to improve the stability of the produced structures, a chitosan layer was adsorbed on the capsules’ surface. The polymer concentration (0.2 mg/mL) was high enough to ensure an overcompensation of the surface charge and re-stabilization of the dispersion. (The suitable concentrations of chitosan and SBECD-REM used in the procedure were determined also by additional investigations.)
In the subsequent step (step 4), the aptamer molecules (5′-AAA CAU UGC AC-3′) were adsorbed on the surface (5 μM). The dispersion (1836 µL) and aptamer solution (164 µL) were mixed using Vortex for a minute and the produced dispersion was then placed to rest without stirring for 20 min at room temperature (24 °C).
2.3. Determination of the Amount of Encapsulated Remdesivir
The concentration of REM loaded into the structures was determined by the difference between the initial concentration of the compound added to the dispersion and the concentration in the supernatant after centrifugation (after step 2,
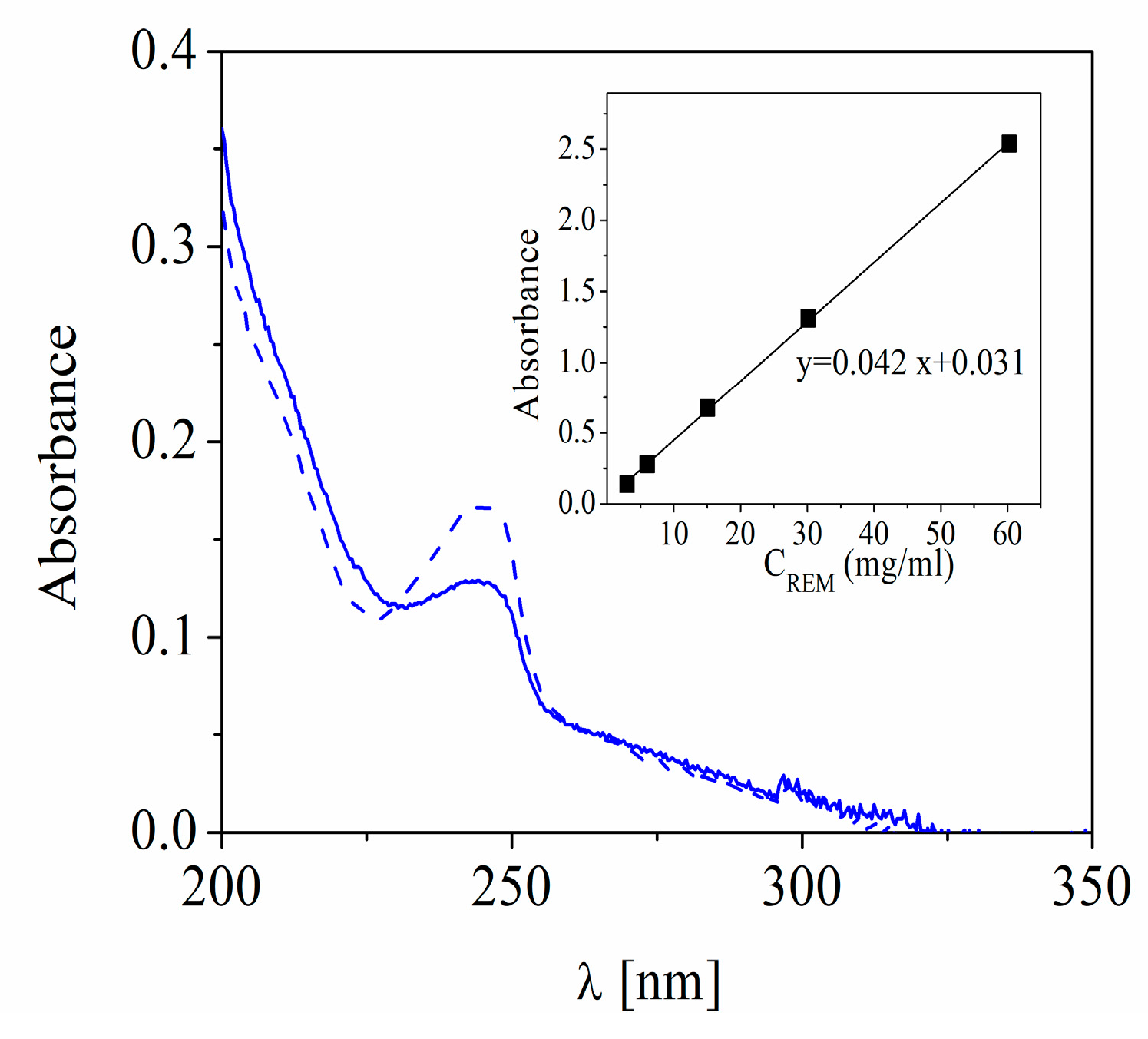
Figure 1) by monitoring with T60 UV-Visible Spectrophotometer (PG Instruments Limited, UK).
The drug was detected at a wavelength of 245 nm corresponding to the maximum absorbance peak of SBECD-REM complexes and the amount of free drug in solution was calculated by using an appropriate calibration curve. The encapsulation efficiency (EE%) was estimated by using
where C
total is the initial concentration of REM added to the dispersion (0.03 mg/mL) and C
free is the calculated concentration of the compound in the supernatant.
The drug loading capacity (in %) was calculated with the following the equation
where m
encapsulated is the amount of drug successfully loaded into the capsules (in mg/mL) and m
capsules is the amount of capsules (0.24 mg/mL).
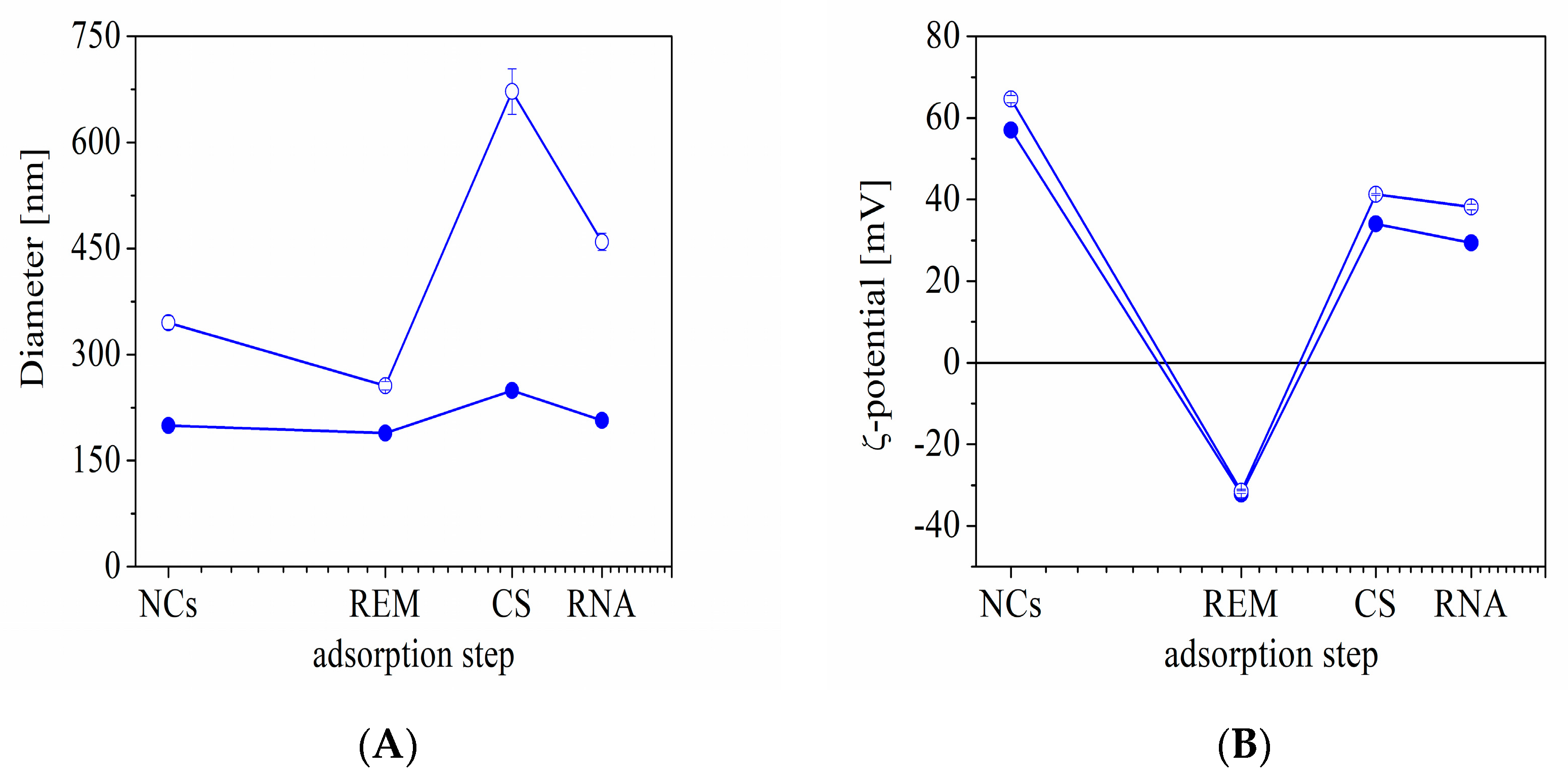
2.4. Determination of the Surface Charge and Size of the Capsules
The surface charge and hydrodynamic diameter of the produced structures were evaluated after each deposition step by using dynamic light scattering with non-invasive backscattering (DLS-NIBS, measuring angle 173°). The hydrodynamic thickness after each deposition step was determined by comparing the capsule size before and after adsorption. For estimation of the film thickness, it was assumed that the regular polymer adsorption was achieved on the surface and the spherical shape of the formulation did not change. The measurements were carried out using Zatasizer Pro (Malvern Panalytical Ltd., Malvern, UK) equipped with a He-Ne laser with a maximum power of 10 mW operating at a wavelength of 633 nm with a fixed scattering light angle of 173°. All measurements were performed at 24.0 ± 0.1° After five measurements, the average value was taken as the surface charge and size of the capsules.
2.5. Release of the Drug in Conditions Close to the Physiological Ones
The released amount of drug from the capsules was estimated by using two different experimental procedures. First, after the adsorption of aptamer on the capsule surface, a small volume of the dispersion (2 mL) was centrifuged (15,000 rpm, 21,382 g, at 15 °C for 90 min). The cream was extracted and diluted in a physiological solution (Unolab Manufacturing, S.L., Madrid, Spain) to the same volume as before centrifugation. The first sample was taken 15 h after the preparation of the dispersion. The solution of capsules was centrifuged and the cream was re-dispersed again in a physiological solution (2 mL). The procedure was repeated after 24 h. The free drug concentration in samples was estimated by UV-vis spectroscopy using appropriate calibration curves.
According to the second procedure (dialysis method), after adsorption of aptamer, an aliquot from the dispersion (1 mL) was added into a dialysis tube (D-Tube™ Dialyser Midi, MWCO 3.5 kDa, Sigma Aldrich) and incubated with 25 mL buffer pH 7.00 (Chem-Lab NV, Belgium) at room temperature (24 °C) and 37 °C with a stirring speed 50 rpm. Aliquots (2 mL) were drawn at predetermined time points and the medium was immediately replenished with fresh buffer. The concentration of free drug in samples was estimated by UV-vis spectroscopy.
2.6. Transmission Electron Microscopy (TEM)
The produced capsules were visualized by TEM. The samples for the TEM studies were prepared by dropping and drying a drop of suspension of capsules on formvar-covered TEM grids. The images were captured using High-Resolution Scanning Transmission electron microscope HR STEM JEOL JEM 2100 for investigations of surface morphology.
2.7. Microbiology Studies
2.7.1. Host Cell Culture
Human colon carcinoma cells (HCT-8) were obtained from the American Type Culture Collection (ATCC). Permanent HCT-8 [HRT-18] (ATCC-CCL-244, LGC Standards) were maintained at 37 °C and 5% CO2 using sterile RPMI 1640 (Roswell Park Memorial Institute Medium, ATCC-30-2001) supplemented with 0.3 mg/mL L-glutamine (Sigma-Aldrich, Darmstadt, Germany), 10% horse serum (ATCC-30-2021), 100 UI penicillin, and 0.1 mg streptomycin/mL (both purchased from Sigma-Aldrich).
2.7.2. Viruses
Human Coronavirus OC43 (HCoV-OC43) (ATCC: VR-1558) strain was propagated in HCT-8 cells in RPMI 1640 supplemented with 2% horse serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were lysed 5 days after infection by 2 freeze and thaw cycles and the virus was titrated according to the Reed and Muench formula. Virus and mock aliquots were stored at −80 °C.
2.7.3. Cytotoxicity Assay
Confluent monolayer cell culture in a 96-well plate (Costar®, Corning Inc., Kennebunk, ME, USA) was treated with 0.1 mL/well containing a maintenance medium that did not contain/or contained decreasing concentrations of test substances. The cells were incubated at 37 °C and 5% CO2 for 5 days. After microscopic evaluation, the medium containing the test compound was removed, and the cells were washed and incubated with neutral red, at 37 °C for 3 h. After incubation, the neutral red dye was removed and the cells were washed with PBS and 0.15 mL/well desorbing solution (1% glacial acetic acid and 49% ethanol in distilled water) was added. The optical density (OD) of each well was registered at 540 nm in a microplate reader (Biotek Organon, West Chester, PA, USA). Then, 50% cytotoxic concentration (CC50) was defined as the concentration of the material that reduces cell viability by 50% compared to untreated controls. Each sample was tested in triplicate with four wells for cell culture on a test sample.
The maximum tolerable concentration (MTC) of the extracts was also determined, which was the concentration at which they did not affect the cell monolayer and in the sample, it looks like the cells in the control sample (untreated with compounds).
2.7.4. Antiviral Activity Assay
The cytopathic effect (CPE) inhibition test was used to assess the tested compounds’ antiviral activity. Confluent cell monolayer in 96-well plates was infected with 100 cell culture infectious dose 50% (CCID
50) in 0.1 mL (coronavirus OC43 strain). After 120 min of virus adsorption, the tested compound was added in various concentrations and cells were incubated for 5 days at 33 °C and 5% CO
2. The cytopathic effect was estimated using a neutral red dye uptake assay and the percentage of CPE inhibition was calculated:
where OD
test sample is the mean value of the ODs of the wells inoculated with a virus and treated with the test sample in the respective concentration, OD
virus control is the mean value of the ODs of the virus control wells (with no compound in the medium), and OD
toxicity control is the mean value of the ODs of the wells not inoculated with a virus but treated with the corresponding concentration of the test compound. The concentration of the test substance that inhibited 50% of viral replication when compared to the virus control is labelled as IC50. The selectivity index (SI) was calculated from the ratio CC
50/IC
50.
2.7.5. Virucidal Assay
Samples of 1 mL containing HCoV (CCID 50), and samples in their maximal tolerable concentration (MTC) were mixed in a 1:1 ratio and, subsequently, stored at room temperature for different time intervals. The residual infectious virus content in each sample was determined by the end-point dilution method of Reed and Muench [
24] and Δlgs as compared to the untreated controls were evaluated.
2.7.6. Effect on the Viral Adsorption
Twenty-four-well plates containing HCT-8 cell monolayer were pre-cooled to 4 °C and inoculated with CCID50 of HCoV. In parallel, they were treated with tested samples at their MTC and incubated at 4 °C for the time of virus adsorption. At various time intervals (15, 30, 45, and 60 min), the cells were washed with PBS to remove both the compound and the unattached virus, and the cells were then covered with a support medium and incubated at 37 °C and 5% CO2 for 24 h. After freezing and thawing three times, the infectious viral titer of each sample was determined by the final dilution method. Δlgs was determined compared to the viral control (untreated with the compounds). Each sample was prepared in four replicates.
2.7.7. Statistical Analysis
Data on cytotoxicity and antiviral effects were analyzed statistically. The values of CC50 and IC50 were presented as means ± SD.
4. Conclusions
The present study addressed the design and characterization of model chitosan-based formulations suitable for encapsulation and target delivery of remdesivir in a potential therapy of coronavirus infection.
The reproducible procedure was developed for the formation of stable drug-loaded structures by subsequent (predominantly) electrostatic adsorption of oppositely charged components (chitosan, SBECD-REM complexes, and aptamer) on oil-core capsules. The influence of the molecular weight of chitosan on the properties of the produced structures was studied. Variation in the hydrodynamic size and surface charge of the structures was registered after each deposition step.
The complexes of SBECD-REM were loaded into the chitosan layer of the capsules because of the electrostatic attraction with the oppositely charged chitosan monomers. The encapsulation efficiencies of the drug were estimated at ca. 23% and 45% for capsules produced from chitosan with medium and low molecular weight (CS-M and CS-L), respectively.
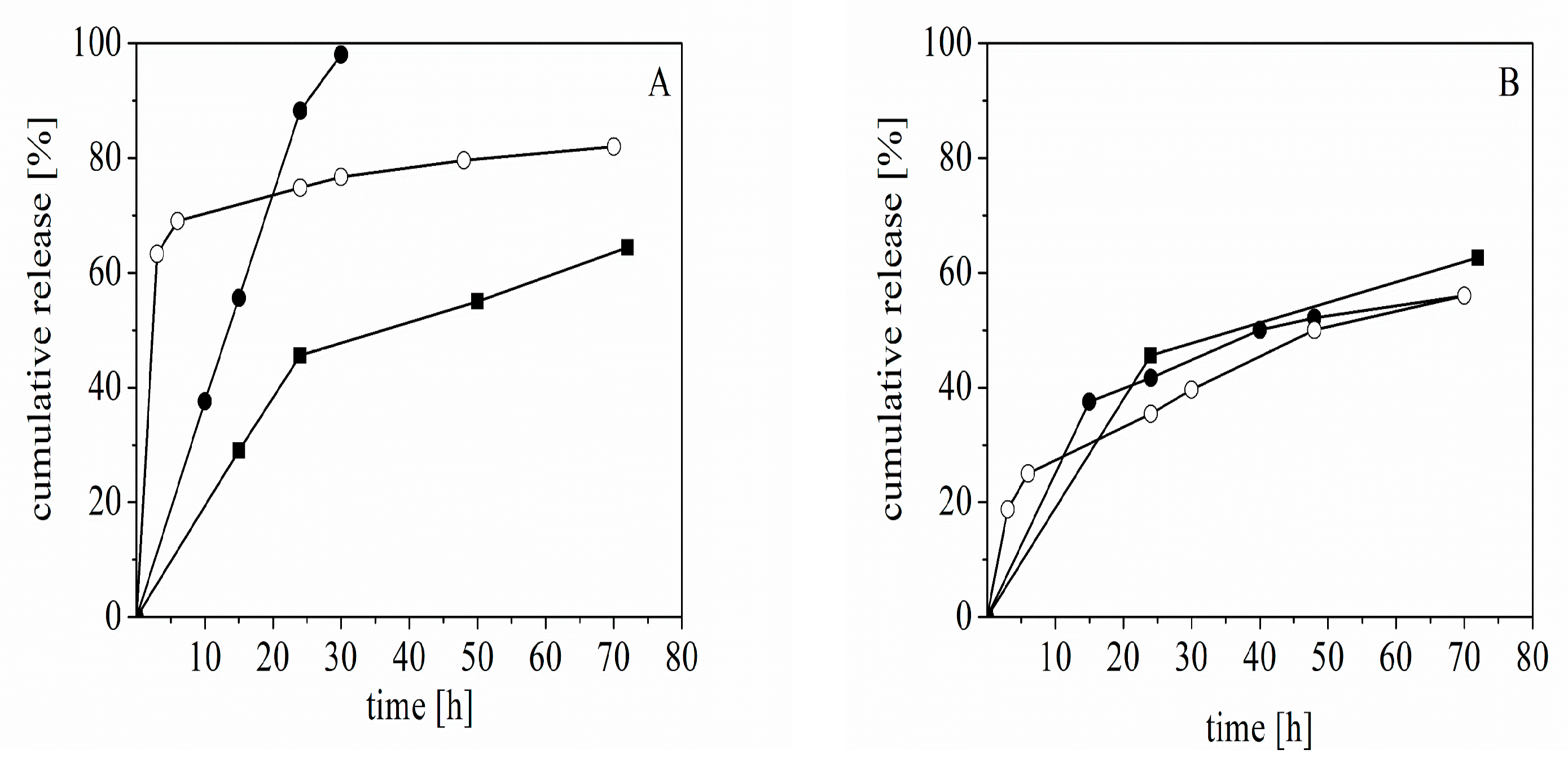
The released amount of REM from the capsules was investigated in a physiological solution and buffer pH 7.0. The experimental results indicated that the released amount of REM from capsules formed from CS-L strongly depended on the pH and temperature, whereas the released amount of drug from capsules of CSM almost did not depend on the variation in the experimental conditions. Complete release of the drug from the capsules was achieved in a dispersion of capsules formed from CS-L incubated in phosphate buffer at 37 °C (after 30 h).
The cytotoxic assay indicated that all tested substances were not toxic towards HCT-8 cells. According to the experimental results, the solution of chitosan CS-L showed lower cytotoxicity compared to chitosan CS-M. Moreover, the toxicities of drug-loaded and unloaded capsules on the cells were close.
The investigations on the influence of the produced capsules on the replication cycle of HCoV-OC43 showed that the drug-loaded capsules formed from chitosan CS-L showed low activity, whereas capsules with chitosan CS-M had stronger inhibitory activity against virus replication compared to pure drug.
The analysis of the virucidal activity of the samples against the viability of the extracellular HCoV-OC43 virions indicated that the pure chitosan solutions had a weak inhibition effect. However, in spite of the presence of chitosan in the structure of capsules, the results showed that their influence on the virions was negligible. Moreover, there was no difference between the behavior of the loaded and unloaded capsules.
The investigation of the effect of the substances and capsules on viral adsorption on the cell membrane indicated that the chitosan solutions inhibit the adsorption of the virus. Moreover, unloaded capsules, loaded capsules, and the pure drug did not affect the adsorption of HCoV-OC43 on HCT-8 cells.
The main conclusion from the study was that the physicochemical and antiviral properties of the produced composite capsules strongly depend on the properties of the chitosan in their structure, and we supposed that the capsules formed from CS-L were more suitable for the potential pharmacological application.