In Vitro Cytotoxicity and Antioxidant Studies of Dovyallis caffra-Mediated Cassiterite (SnO2) Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
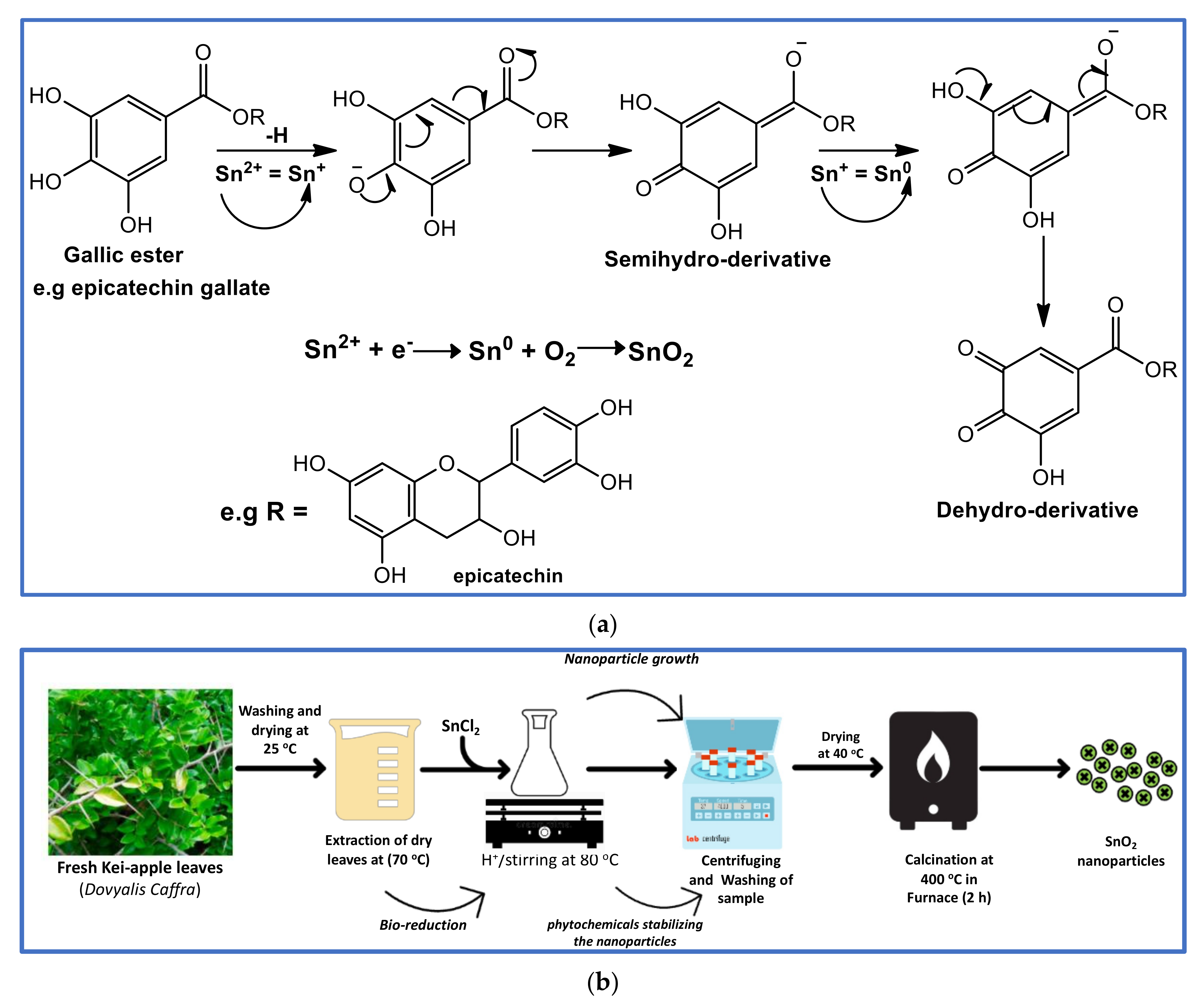
2.1. Plant Preparation and Synthesis of SnO2 Nanoparticles, Using Leaf Extracts of Dovyallis c.
2.2. Characterization of SnO2 Nanoparticles
2.3. Cytotoxicity Study Using MTT Assay
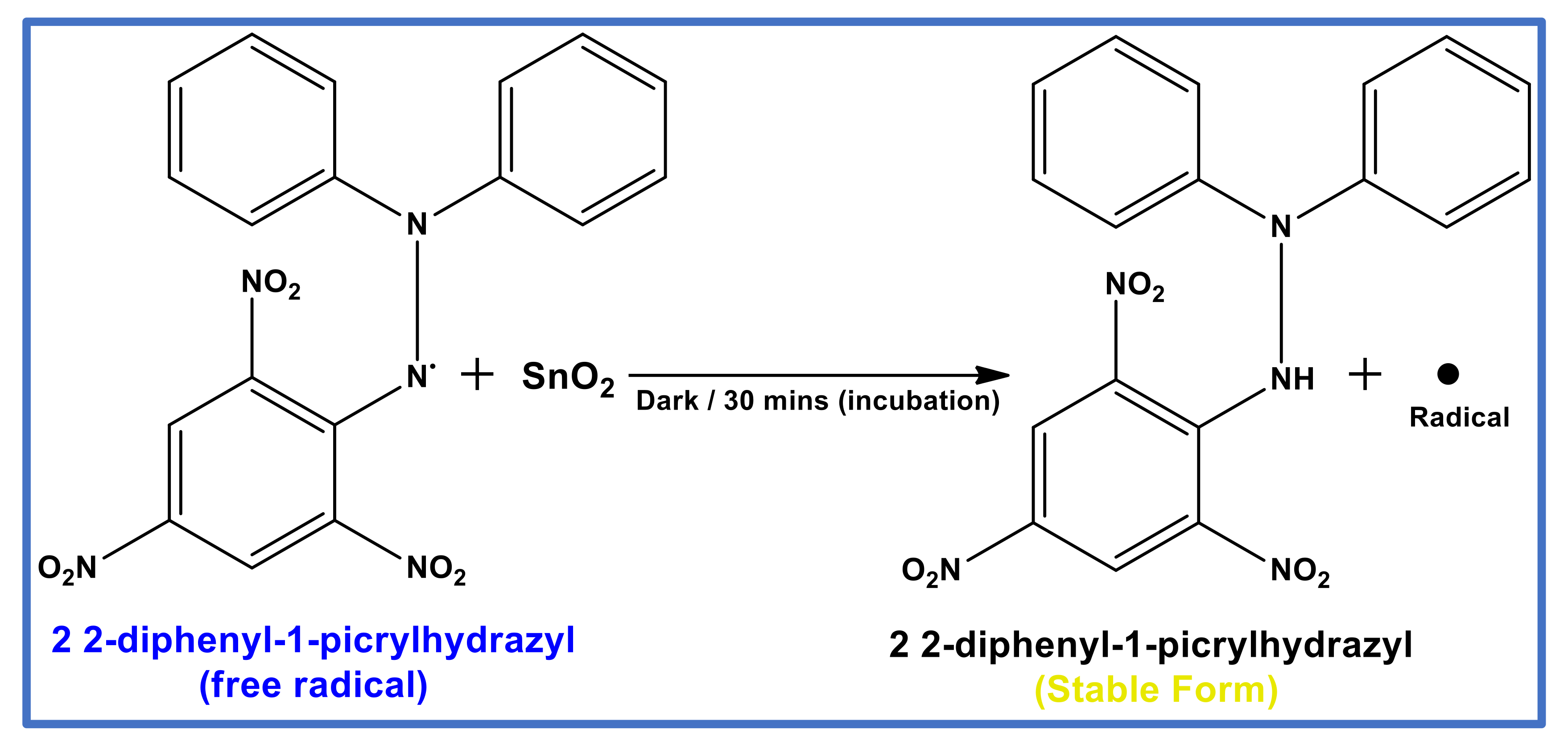
2.4. Antioxidant Potential Study of SnO2 NPs, Using DPPH Assay
3. Results and Discussions
3.1. Synthesis
3.2. XRD Analysis Study of SnO2 NPs
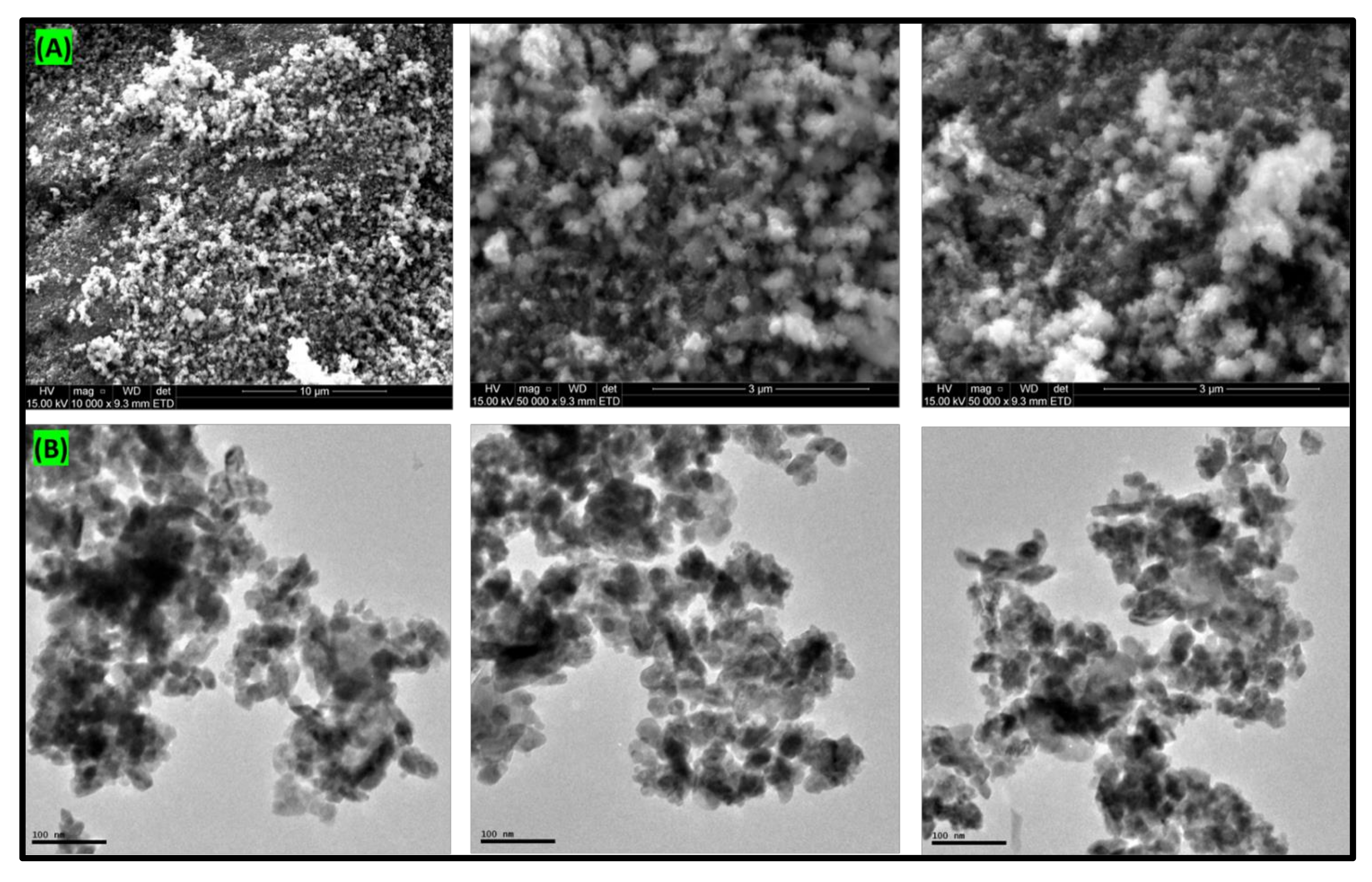
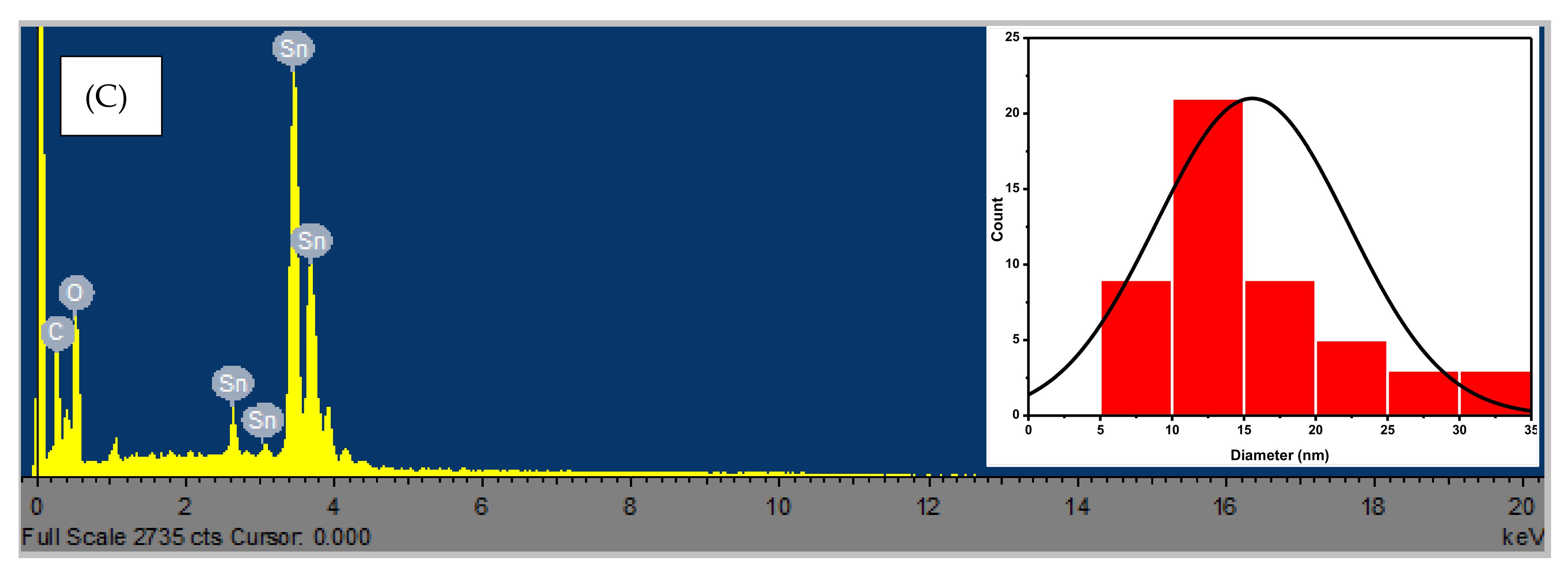
3.3. Microscopic Analysis
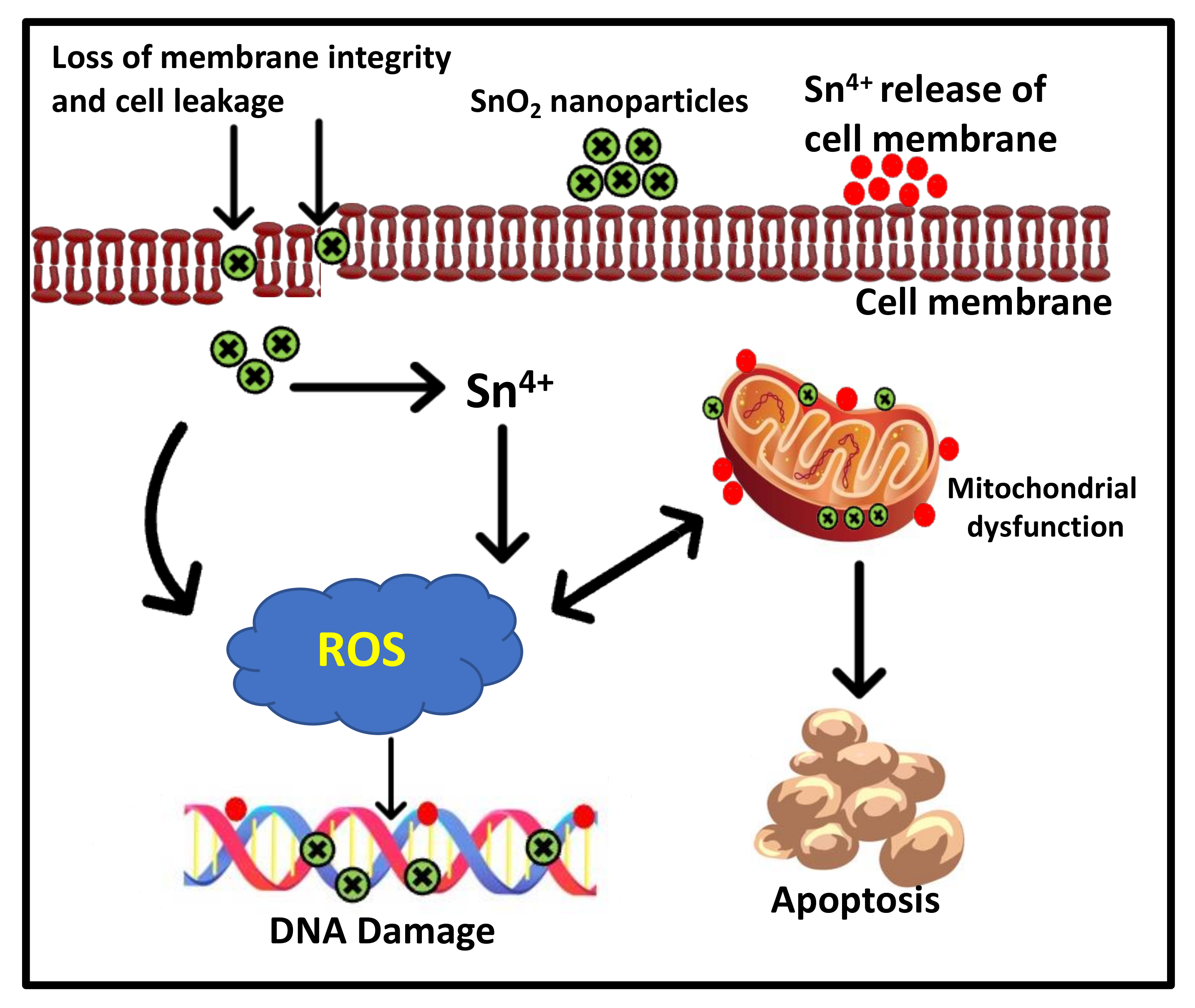
3.4. Cytotoxicity of SnO2 Nanoparticles
3.5. Free Radical Scavenging Potential of SnO2 Nanoparticles, Using DPPH Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical Uses, Biological Activities and Chemical Properties of Kei-Apple [Dovyalis caffra (Hook.f. & Harv.) Sim]: An Indigenous Fruit Tree of Southern Africa. J. Ethnopharmacol. 2019, 241, 111963. [Google Scholar] [CrossRef]
- Loots, D.T.; Van Der Westhuizen, F.H.; Jerling, J. Polyphenol Composition and Antioxidant Activity of Kei-Apple (Dovyalis caffra) Juice. J. Agric. Food Chem. 2006, 54, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; Tadros, L.K.; Dawood, D.H. Phytochemical Constituents, Antioxidant Activity and Safety Evaluation of Kei-Apple Fruit (Dovyalis caffra). Food Chem. 2018, 265, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. In Vitro α-Glucosidase Enzyme Inhibition and Anti-Inflammatory Studies of Mn3O4 Nanoparticles Mediated Using Extract of Dalbergiella welwitschia. Results Chem. 2022, 4, 100497. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C. ZnO Nanoparticles Mediated by Aqueous Extracts of Dovyalis caffra Fruits and the Photocatalytic Evaluations. Mater. Res. Express 2019, 6, 125091. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Majeed Khan, M.A.; Alhadlaq, H.A. Oxidative Stress Mediated Cytotoxicity of Tin (IV) Oxide (SnO2) Nanoparticles in Human Breast Cancer (MCF-7) Cells. Colloids Surf. B Biointerfaces 2018, 172, 152–160. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Sundaramoorthy, P.; Poudel, B.K.; Youn, Y.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Multimodal Selenium Nanoshell-Capped Au@mSiO2 Nanoplatform for NIR-Responsive Chemo-Photothermal Therapy against Metastatic Breast Cancer. NPG Asia Mater. 2018, 10, 197–216. [Google Scholar] [CrossRef]
- Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective Cytotoxic Effect of ZnO Nanoparticles on Glioma Cells. Nano Res. 2009, 2, 882–890. [Google Scholar] [CrossRef]
- Matussin, S.N.; Harunsani, M.H.; Tan, A.L.; Mohammad, A.; Cho, M.H.; Khan, M.M. Photoantioxidant Studies of SnO2 Nanoparticles Fabricated Using Aqueous Leaf Extract of Tradescantia spathacea. Solid State Sci. 2020, 105, 106279. [Google Scholar] [CrossRef]
- Goel, A.; Tomar, S. Green Synthesis and Characterization of Murraya koenigii Leaf Extract Mediated IrO2, SnO2, and Ir-SnO2 Nanoparticles. Inorg. Nano-Met. Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanwal, S.; Rizwan, K.; Shahid, S. Enhanced Antimicrobial, Antioxidant, in Vivo Antitumor and in Vitro Anticancer Effects against Breast Cancer Cell Line by Green Synthesized Un-Doped SnO2 and Co-Doped SnO2 Nanoparticles from Clerodendrum inerme. Microb Pathog. 2018, 125, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties. Molecules 2022, 27, 3206. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Bio-Inspired Synthesis and Cytotoxic Evaluation of Silver-Gold Bimetallic Nanoparticles Using Kei-Apple (Dovyalis caffra) Fruits. Inorg. Chem. Commun. 2019, 109, 107569. [Google Scholar] [CrossRef]
- Waweru, D.M.; Arimi, J.M.; Marete, E.; Jacquier, J.C.; Harbourne, N. Current Status of Utilization and Potential of Dovyalis caffra Fruit: Major Focus on Kenya—A Review. Sci. Afr. 2022, 16, e01097. [Google Scholar] [CrossRef]
- Selvakumari, J.C.; Ahila, M.; Malligavathy, M.; Padiyan, D.P. Structural, Morphological, and Optical Properties of Tin(IV) Oxide Nanoparticles Synthesized Using Camellia sinensis Extract: A Green Approach. Int. J. Miner. Metall. 2017, 24, 1043–1051. [Google Scholar] [CrossRef]
- Beer, T. Polyphenols, Ascorbate and Antioxidant Capacity of the Kei-Apple (Dovyalis caffra). Master’s Thesis, Potchefstroom Campus of the North-West University, Potchefstroom, South Africa, 2006. [Google Scholar]
- Suresh, K.C.; Surendhiran, S.; Manoj Kumar, P.; Ranjth Kumar, E.; Khadar, Y.A.S.; Balamurugan, A. Green Synthesis of SnO2 Nanoparticles Using Delonix elata Leaf Extract: Evaluation of Its Structural, Optical, Morphological and Photocatalytic Properties. SN Appl. Sci. 2020, 2, 1735. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Hidayat, H.; Sagadevan, S.; Ghazali, S.A.I.S.M.; Oh, W.C.; Doong, R.A. Flower-like SnO2 Nanoparticle Biofabrication Using Pometia pinnata Leaf Extract and Study on Its Photocatalytic and Antibacterial Activities. Nanomaterials 2021, 11, 3012. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Sahroni, I.; Saba, A.K.; Rahman, R.; Fatimah, I. Biofabricated SnO2 nanoparticles Using Red Spinach (Amaranthus tricolor L.) Extract and the Study on Photocatalytic and Electrochemical Sensing Activity. Mater. Res. Express 2020, 7, 7. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Ameen, F.; Alsamhary, K.; Dawoud, T.; Al-Khattaf, F.; AlNadhari, S. Tin Oxide Nanoparticles (SnO2-NPs) Synthesis Using Galaxaura elongata and Its Anti-Microbial and Cytotoxicity Study: A Greenery Approach. Appl. Nanosci. 2023, 13, 519–527. [Google Scholar] [CrossRef]
- Bhosale, T.T.; Shinde, H.M.; Gavade, N.L.; Babar, S.B.; Gawade, V.V.; Sabale, S.R.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of SnO2 Nanoparticles by Aqueous Leaf Extract of Calotropis gigantea for Photocatalytic Applications. J. Mater. Sci. Mater. Electron. 2018, 29, 6826–6834. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Ranjan, S.; Dasgupta, N. Cytotoxicity Study of Piper nigrum Seed Mediated Synthesized SnO2 Nanoparticles towards Colorectal (HCT116) and Lung Cancer (A549) Cell Lines. J. Photochem. Photobiol. B 2017, 166, 158–168. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T.; Gebretnsae, H.G. Green and Cost—Effective Synthesis of Tin Oxide Nanoparticles: A Review on the Synthesis Methodologies, Mechanism of Formation, and Their Potential Applications. Nanoscale Res. Lett. 2021, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Antioxidant and Catalytic Activity of Biosynthesized CuO Nanoparticles Using Extract of Galeopsidis herba. J. Inorg. Organomet Polym. Mater. 2018, 28, 812–819. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile Green Synthesis of Zinc Oxide Nanoparticles by Eucalyptus globulus and Their Photocatalytic and Antioxidant Activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Phytosynthesis and Applications of Bioactive SnO2 Nanoparticles. Mater. Charact. 2015, 101, 97–105. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Wang, X.; Yuan, M.; Zhang, Y.; Xu, Z.; Li, G.; Liu, T. Reactive Oxygen Species Mediated Oxidative Stress Links Diabetes and Atrial Fibrillation. Mol. Med. Rep. 2018, 17, 4933–4940. [Google Scholar] [CrossRef]
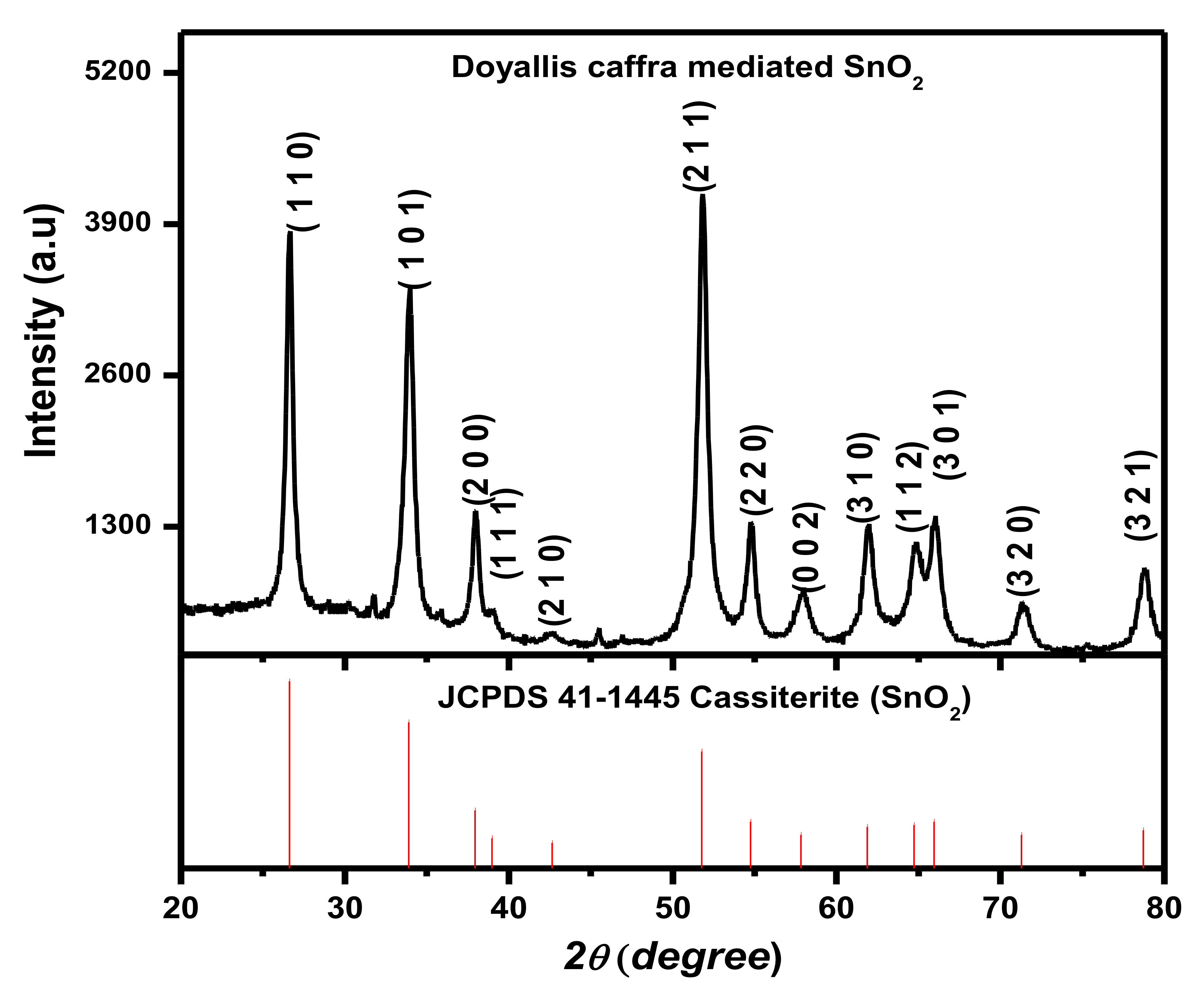
| S/No | Peaks (2θ) | hkl | FWHM | Crystallite Size (D) nm | D (Average) nm |
|---|---|---|---|---|---|
| 1 | 26.11 | 110 | 0.63 | 12.35 | 10.11 |
| 2 | 33.89 | 101 | 0.77 | 9.29 | |
| 3 | 37.95 | 200 | 0.54 | 13.88 | |
| 4 | 38.96 | 111 | 0.58 | 12.99 | |
| 5 | 42.64 | 210 | 7.73 | 0.96 | |
| 6 | 51.78 | 211 | 0.77 | 9.28 | |
| 7 | 54.76 | 220 | 0.56 | 12.51 | |
| 8 | 57.82 | 002 | 0.58 | 11.98 | |
| 9 | 61.87 | 310 | 0.66 | 10.30 | |
| 10 | 64.72 | 112 | 0.89 | 7.49 | |
| 11 | 65.94 | 301 | 0.69 | 9.58 | |
| 12 | 71.28 | 202 | 0.41 | 15.67 | |
| 13 | 78.71 | 321 | 0.56 | 10.97 |
| Samples (µg mL−1) | Control (Cells Only) | Control (Cells + DMSO) | 100 | 80 | 40 | 20 | IC50 |
|---|---|---|---|---|---|---|---|
| SnO2 (%) | 100.00 | 100.59 | 57.26 | 60.43 | 70.91 | 73.72 | 62.33 |
| 5-FUC (%) | 100.00 | 100.59 | 37.23 | 53.81 | 73.06 | 98.44 | 71.21 |
| Samples (mg mL−1) | 3.13 | 6.3 | 12.5 | 25 | 50 | IC50 |
|---|---|---|---|---|---|---|
| SnO2 (%) | 23.32 | 28.59 | 33.52 | 42.71 | 46.98 | 4.68 |
| Ascorbic acid (%) | 4.08 | 12.63 | 34.88 | 39.25 | 52.75 | 4.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, J.O. In Vitro Cytotoxicity and Antioxidant Studies of Dovyallis caffra-Mediated Cassiterite (SnO2) Nanoparticles. Sci. Pharm. 2023, 91, 34. https://doi.org/10.3390/scipharm91030034
Adeyemi JO. In Vitro Cytotoxicity and Antioxidant Studies of Dovyallis caffra-Mediated Cassiterite (SnO2) Nanoparticles. Scientia Pharmaceutica. 2023; 91(3):34. https://doi.org/10.3390/scipharm91030034
Chicago/Turabian StyleAdeyemi, Jerry O. 2023. "In Vitro Cytotoxicity and Antioxidant Studies of Dovyallis caffra-Mediated Cassiterite (SnO2) Nanoparticles" Scientia Pharmaceutica 91, no. 3: 34. https://doi.org/10.3390/scipharm91030034
APA StyleAdeyemi, J. O. (2023). In Vitro Cytotoxicity and Antioxidant Studies of Dovyallis caffra-Mediated Cassiterite (SnO2) Nanoparticles. Scientia Pharmaceutica, 91(3), 34. https://doi.org/10.3390/scipharm91030034






