Photoprotector Effect of Emulsions with Yerba-Mate (Ilex paraguariensis) Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Extracts Preparation
2.2. Formulations Preparations
2.3. Formulation Characterization
2.4. Stability Investigation
2.5. SPF Determination
2.6. Photoprotective Investigation
2.7. Statistical Analysis
3. Results and Discussion
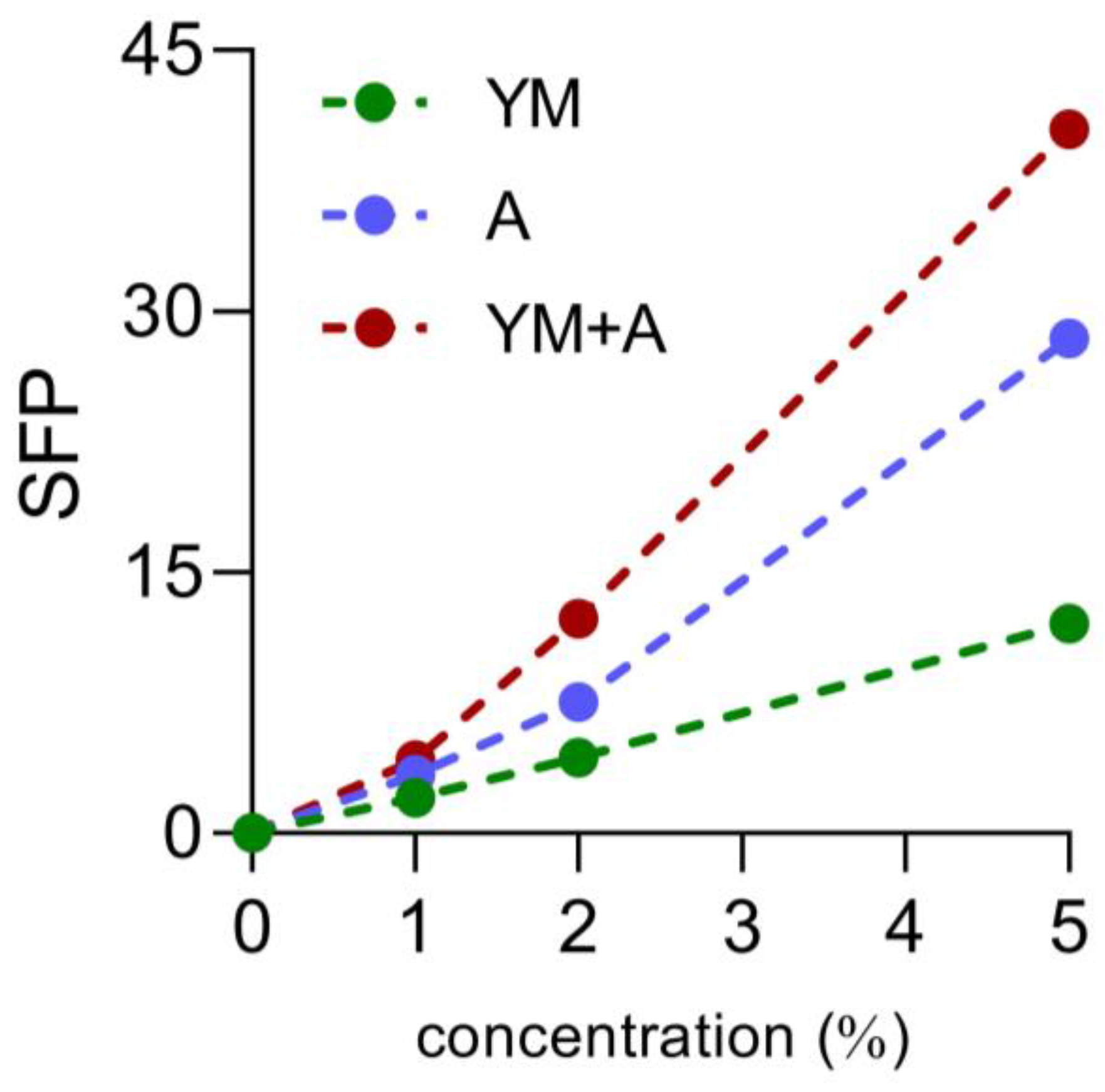
3.1. Extraction and SPF Determination
3.2. Photoprotective Effect on Resveratrol Degradation
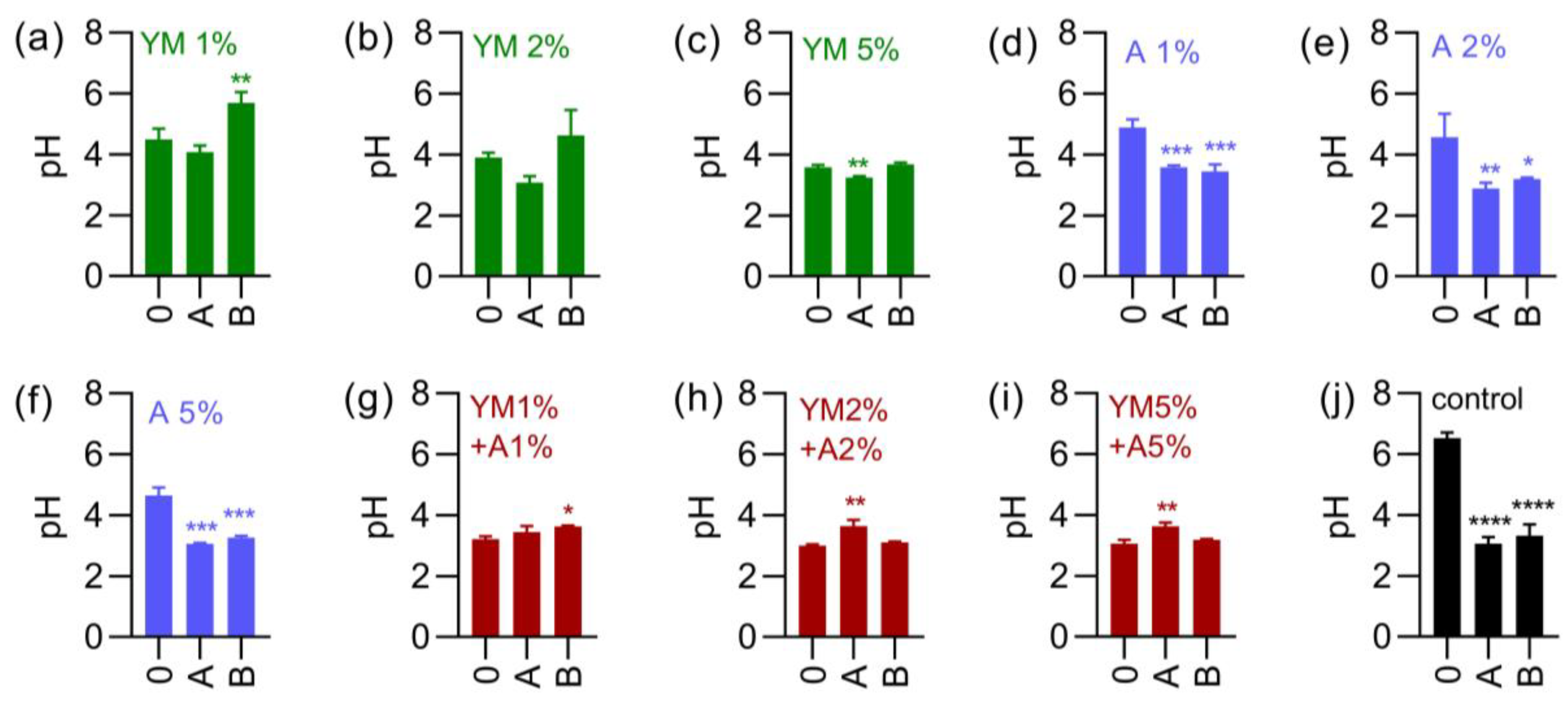
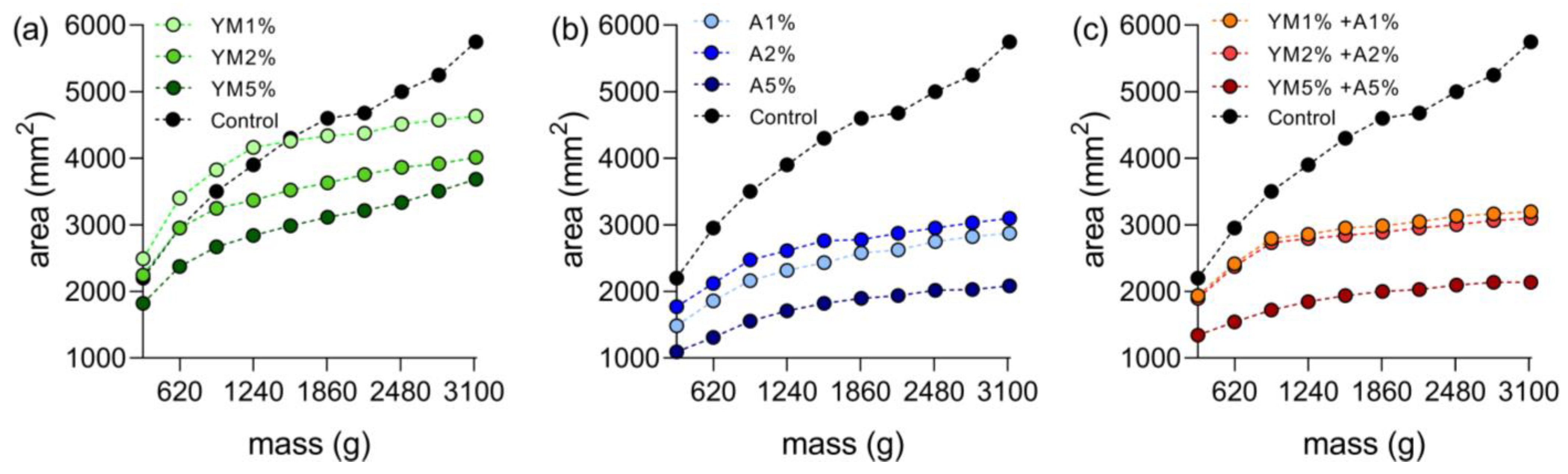
3.3. Characterization and Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Delalibera Finzer, J.R. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.I.; De Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A Comprehensive Review on Chemistry, Health Implications, and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Dartora, N.; Piovezan Borges, A.C.; Picolo, A.P.; Dallago, R.M.; Mera de Souza, L.; Valduga, A.T. Accelerated maturation of processed yerba-mate under the controlled conditions of temperature and humidity. Nutr. Food Sci. 2015, 45, 564–573. [Google Scholar] [CrossRef]
- Lewinski, C.S.; Gonçalves, I.L.; Piovezan Borges, A.C.; Dartora, N.; de Souza, L.M.; Valduga, A.T. Effects of UV light on the physic-chemical properties of yerba-mate. Nutr. Food Sci. 2015, 45, 221–228. [Google Scholar] [CrossRef]
- Magri, E.; Valduga, A.T.; Gonçalves, I.L.; Barbosa, J.Z.; Rabel, D.d.O.; Menezes, I.M.N.R.; Nascimento, P.d.A.; Oliveira, A.; Corrêa, R.S.; Motta, A.C.V. Cadmium and lead concentrations in yerba mate leaves from agroforestry and plantation systems: An international survey in South America. J. Food Compos. Anal. 2021, 96, 103702. [Google Scholar] [CrossRef]
- Faion, A.M.; Beal, P.; Ril, F.T.; Cichoski, A.J.; Cansian, R.L.; Valduga, A.T.; de Oliveira, D.; Valduga, E. Influence of the addition of natural antioxidant from mate leaves (Ilex paraguariensis St. Hill) on the chemical, microbiological and sensory characteristics of different formulations of Prato cheese. J. Food Sci. Technol. 2015, 52, 1516–1524. [Google Scholar] [CrossRef]
- Beal, P.; Faion, A.M.; Cichoski, A.J.; Cansian, R.L.; Valduga, A.T.; de Oliveira, D.; Valduga, E. Oxidative stability of fermented Italian-type sausages using mate leaves (Ilex paraguariensis St. Hil) extract as natural antioxidant. Int. J. Food Sci. Nutr. 2011, 62, 703–710. [Google Scholar] [CrossRef]
- Saguie, B.O.; Martins, R.L.; Fonseca, A.d.S.d.; Romana-Souza, B.; Monte-Alto-Costa, A. An ex vivo model of human skin photoaging induced by uva radiation compatible with summer exposure in Brazil. J. Photochem. Photobiol. B Biol. 2021, 221, 112255. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (spf). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of grape seed extract on skin fibroblasts exposed to uva light and its photostability in sunscreen formulation. J. Cosmet. Dermatol. 2021, 20, 1271–1282. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Bahuguna, A.; Kim, H.-H.; Kim, D.-I.; Kim, H.-J.; Yu, J.-M.; Jung, H.-G.; Jang, J.-Y.; Kwak, J.-H.; Park, G.-H.; et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B Biol. 2017, 174, 323–332. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; Almeida, F.B.d.; Yamasaki, P.R.; Santos, J.L.d.; Corrêa, M.A.; Chin, C.M.; Isaac, V.L.B. Can natural products improve skin photoprotection? Rodriguésia 2020, 71, 1–14. [Google Scholar] [CrossRef]
- Amador-Castro, F.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Robust natural ultraviolet filters from marine ecosystems for the formulation of environmental friendlier bio-sunscreens. Sci. Total Environ. 2020, 749, 141576. [Google Scholar] [CrossRef]
- DiNardo, J.C.; Downs, C.A. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J. Cosmet. Dermatol. 2018, 17, 15–19. [Google Scholar] [CrossRef]
- Piovezan-Borges, A.C.; Valério-Júnior, C.; Gonçalves, I.L.; Mielniczki-Pereira, A.A.; Valduga, A.T. Antioxidant potential of yerba mate (Ilex paraguariensis St. Hil.) extracts in Saccharomyces cerevisae deficient in oxidant defense genes. Braz. J. Biol. 2016, 76, 539–544. [Google Scholar] [CrossRef]
- Alice Teresa, V.; Itamar Luís, G.; Ana Cláudia Piovezan, B.; Albanin Aparecida, M.-P.; Ana Paula, P. Cytotoxic/antioxidant activity and sensorial acceptance of yerba-mate development by oxidation process. Acta Scientiarum. Technol. 2016, 38, 115–121. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Borghetti, G.S.; Knorst, M.T. Development and evaluation of physical stability from O/W lotions containing sunscreens. Rev. Bras. De Ciências Farm. 2006, 42, 531–537. [Google Scholar] [CrossRef]
- ANVISA. Guia de Estabilidade de Produtos Cosméticos; ANVISA: Brasília, Brazil, 2004; p. 54.
- Tozetto, J.T.; Tozetto, A.T.; Hoshino, B.T.; Andrighetti, C.R.; Ribeiro, E.B.; Cavalheiro, L.; Ferrarini, S.R. Extract of Punica granatum L.: An alternative to BHT as an antioxidant in semissolid emulsified systems. Química Nova 2017, 40, 97–104. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- de Souza Mansur, J.; Breder, M.N.R.; D’ Ascensão Mansur, M.C.; Azulay, R.D. Correlação entre a determinação do fator de proteção solar em seres humanos e por espectrofotometria. An. Bras. De Dermatol. 1986, 61, 167–172. [Google Scholar]
- Allan, K.E.; Lenehan, C.E.; Ellis, A.V. UV light stability of α-cyclodextrin/resveratrol host–guest complexes and isomer stability at varying pH. Aust. J. Chem. 2009, 62, 921–926. [Google Scholar] [CrossRef]
- Valduga, A.T.; Finzer, J.R.D.; Mosele, S.H. Processamento de Erva-Mate; EdiFAPES: Erechim, Brazil, 2003. [Google Scholar]
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.d.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic behavior of antioxidant phenolic compounds obtained from winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Barg, M.; Rezin, G.T.; Leffa, D.D.; Balbinot, F.; Gomes, L.M.; Carvalho-Silva, M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Streck, E.L.; et al. Evaluation of the protective effect of Ilex paraguariensis and Camellia sinensis extracts on the prevention of oxidative damage caused by ultraviolet radiation. Environ. Toxicol. Pharmacol. 2014, 37, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Cuelho, C.H.F.; Alves, G.d.A.D.; Lovatto, M.O.; Bonilha, I.F.; Barbisan, F.; da Cruz, I.B.M.; Oliveira, S.M.; Fachinetto, R.; do Canto, G.S.; Manfron, M.P. Topical formulation containing Ilex Paraguariensis extract increases metalloproteinases and myeloperoxidase activities in mice exposed to UVB radiation. J. Photochem. Photobiol. B Biol. 2018, 189, 95–103. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Lin, C.-W.; Huang, M.-C.; Shen, C.-H.; Ho, H.-O. Correlation of in vivo and in vitro measurements of sun protection factor. J. Food Drug Anal. 2003, 11, 12. [Google Scholar] [CrossRef]
- Bleasel, M.D.; Aldous, S. In vitro evaluation of sun protection factors of sunscreen agents using a novel UV spectrophotometric technique. Int. J. Cosmet. Sci. 2008, 30, 259–270. [Google Scholar] [CrossRef]
- Butiuk, A.P.; Martos, M.A.; Adachi, O.; Hours, R.A. Study of the chlorogenic acid content in yerba mate (Ilex paraguariensis St. Hil.): Effect of plant fraction, processing step and harvesting season. J. Appl. Res. Med. Aromat. Plants 2016, 3, 27–33. [Google Scholar] [CrossRef]
- Rousseau, D. Fat crystals and emulsion stability—A review. Food Res. Int. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Finnegan, M.; Duffy, E.; Morrin, A. The determination of skin surface pH via the skin volatile emission using wearable colorimetric sensors. Sens. Bio-Sens. Res. 2022, 35, 100473. [Google Scholar] [CrossRef]
- Some, I.T.; Bogaerts, P.; Hanus, R.; Hanocq, M.; Dubois, J. Improved kinetic parameter estimation in pH-profile data treatment. Int. J. Pharm. 2000, 198, 39–49. [Google Scholar] [CrossRef]



| Component | Amount (%) | |
|---|---|---|
| Phase A | Polawax | 6.0 |
| Propylparaben | 0.1 | |
| Butyl hydroxytoluene | 0.05 | |
| Octyl stearate | 5.0 | |
| Phase B | Propylene glycol | 5.0 |
| Methylparaben | 0.2 | |
| Water | up to 100 | |
| Phase C | Imidazolidinyl urea | 0.6 |
| Formulations | SPF |
|---|---|
| YM 1% | 2.05± 0.04 e |
| YM 2% | 4.35 ± 0.07 de |
| YM 5% | 12.03 ± 0.01 c |
| A1% | 3.35 ± 0.10 de |
| A2% | 7.53 ± 0.05 cd |
| A5% | 28.46 ± 5.45 b |
| YM1% + A1% | 4.22 ± 0.25 de |
| YM2% + A2% | 12.32 ± 0.07 c |
| YM5% + A5% | 40.48 ± 0.84 a |
| Control | 0.01 ± 0.04 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriolli Ribeiro, J.; Magri, E.; Gonçalves, I.L.; Paese, K.; Roman, J.; Valduga, A.T. Photoprotector Effect of Emulsions with Yerba-Mate (Ilex paraguariensis) Extract. Sci. Pharm. 2023, 91, 22. https://doi.org/10.3390/scipharm91020022
Andriolli Ribeiro J, Magri E, Gonçalves IL, Paese K, Roman J, Valduga AT. Photoprotector Effect of Emulsions with Yerba-Mate (Ilex paraguariensis) Extract. Scientia Pharmaceutica. 2023; 91(2):22. https://doi.org/10.3390/scipharm91020022
Chicago/Turabian StyleAndriolli Ribeiro, Juliana, Ederlan Magri, Itamar Luís Gonçalves, Karina Paese, Juliana Roman, and Alice Teresa Valduga. 2023. "Photoprotector Effect of Emulsions with Yerba-Mate (Ilex paraguariensis) Extract" Scientia Pharmaceutica 91, no. 2: 22. https://doi.org/10.3390/scipharm91020022
APA StyleAndriolli Ribeiro, J., Magri, E., Gonçalves, I. L., Paese, K., Roman, J., & Valduga, A. T. (2023). Photoprotector Effect of Emulsions with Yerba-Mate (Ilex paraguariensis) Extract. Scientia Pharmaceutica, 91(2), 22. https://doi.org/10.3390/scipharm91020022






