In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs
Abstract
1. Introduction
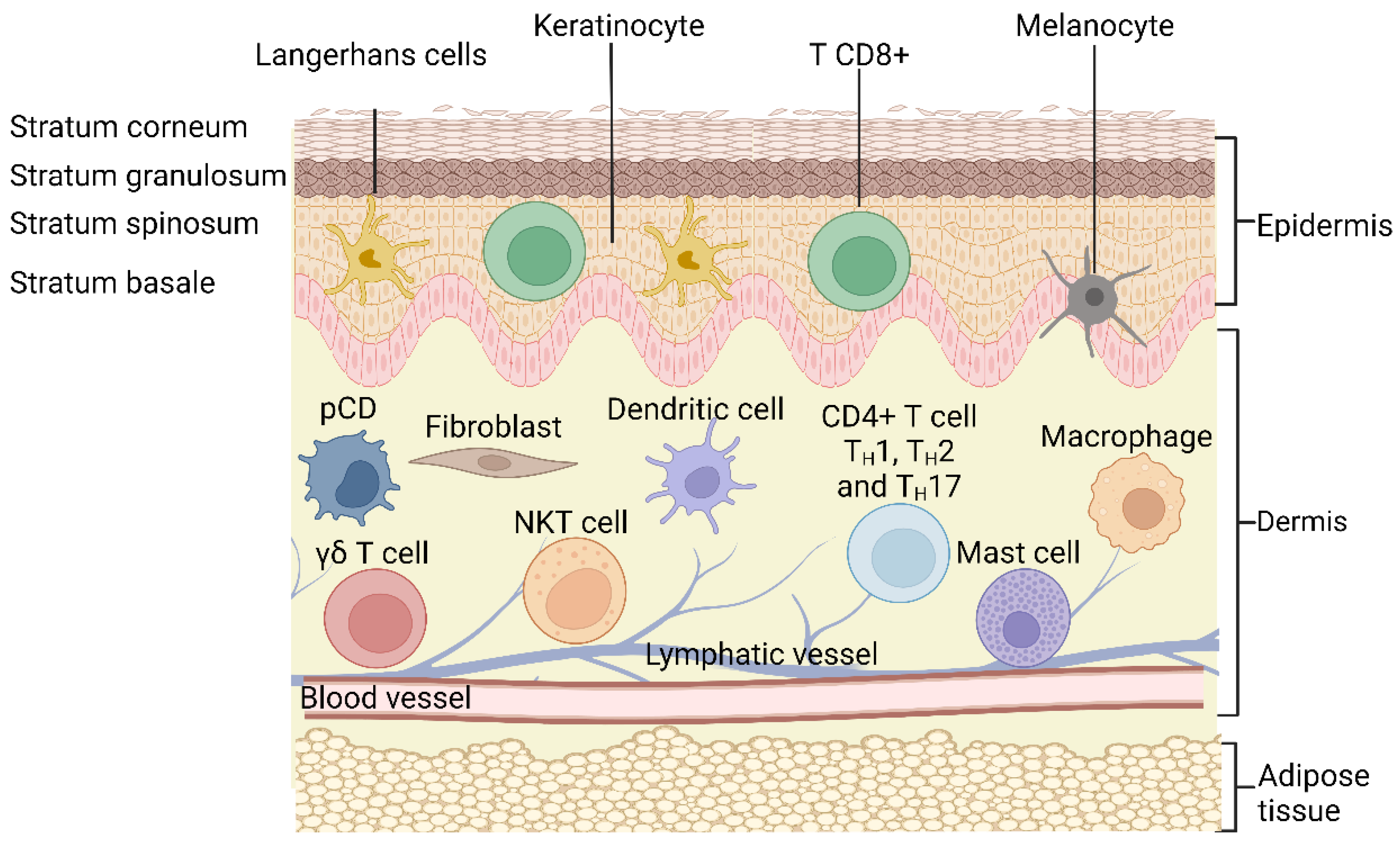
2. Structure of the Skin
3. Cutaneous Inflammation
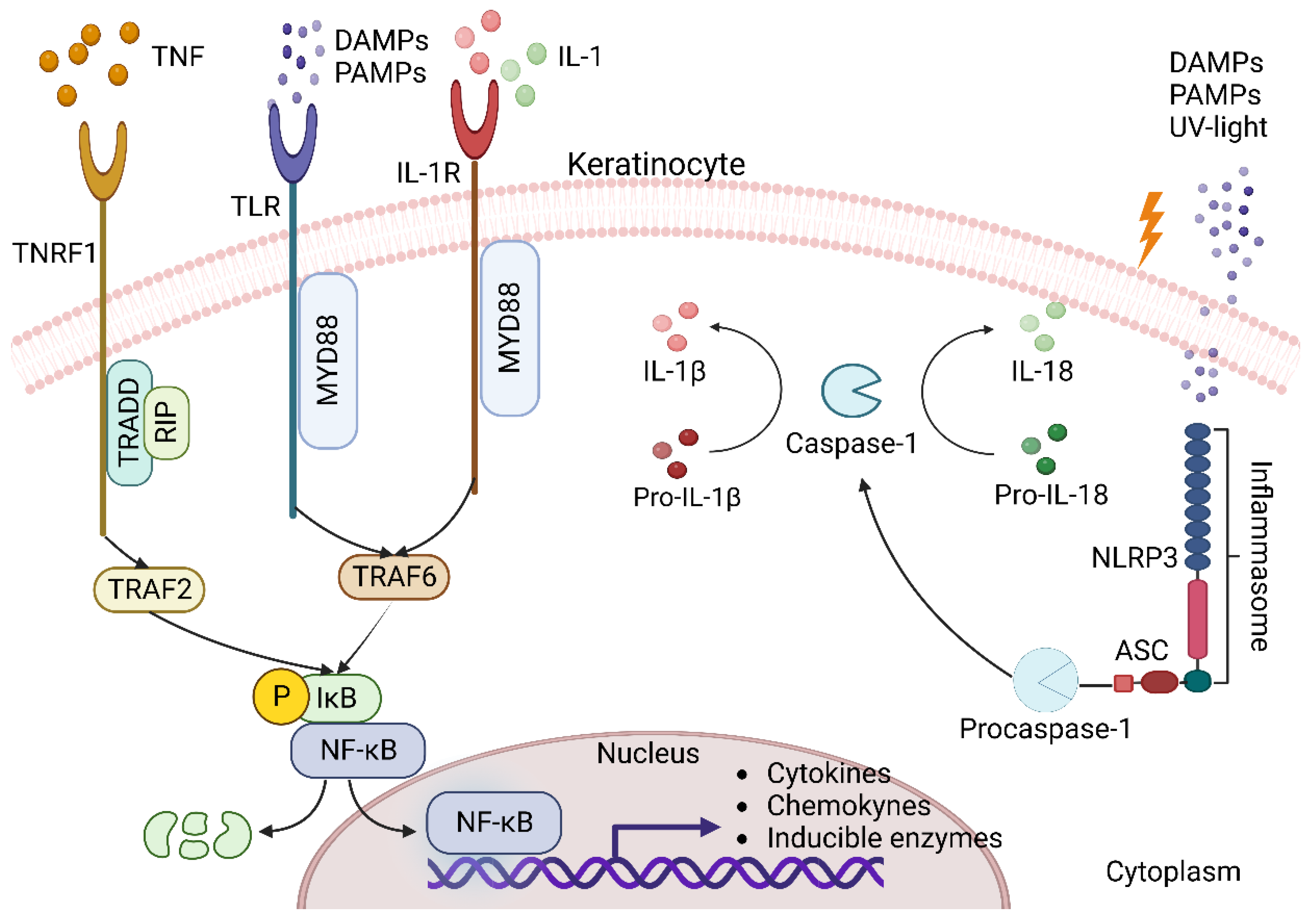
3.1. Keratinocytes as Triggers of Inflammation in the Skin
3.2. Dendritic Cells
3.3. Macrophages
3.4. Mast Cells
3.5. T Cells
3.6. The Skin Microbiome in Inflammatory Skin Diseases
4. Ethical and Legal Considerations
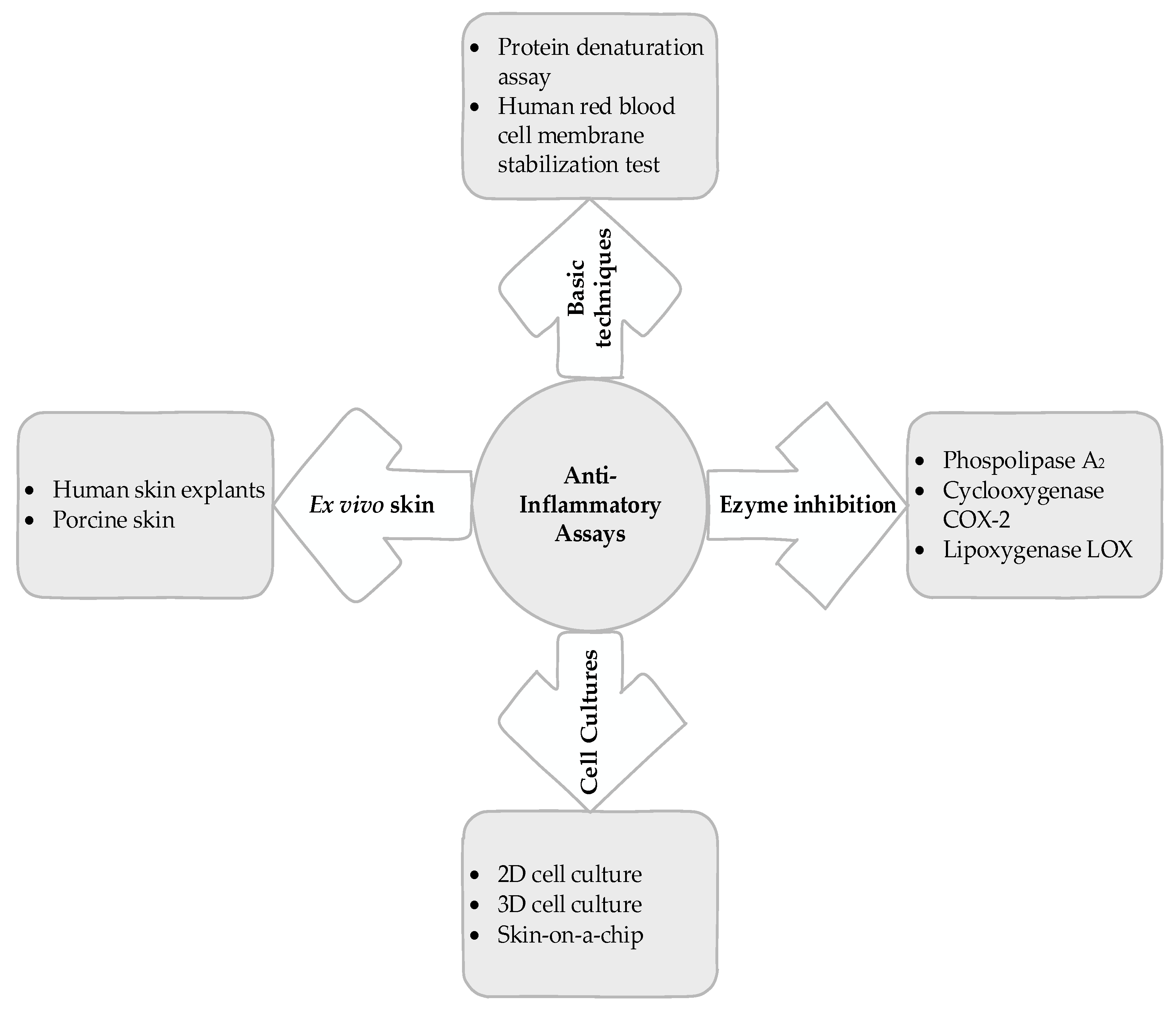
5. Basic Techniques
5.1. Protein Denaturation Assay
5.2. Human Red Blood Cell Membrane Stabilization Test
6. Enzyme Inhibition
6.1. Phospholipase A2 Inhibitory Activity
6.2. COX-2 Inhibitory Activity
6.3. Lipoxygenase (LOX) Inhibitory Activity
7. Cell Cultures
7.1. Two-Dimensional Cell Culture
7.2. Three-Dimensional Cell Culture
7.3. Skin on a Chip
8. Ex Vivo Skin Tests
8.1. Human Skin
8.2. Pig Skin
9. Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García Barreno, P. Inflamación. Cienc. Exact. Fís. Nat. 2008, 102, 91–159. Available online: http://www.rac.es/ficheros/doc/00681.pdf (accessed on 21 January 2023).
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A.; Kuby, J. Kuby Immunology, 6th ed.; W.H. Freeman: New York, NY, USA, 2007; pp. 350–356. [Google Scholar]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved Corrosion Performance of Biodegradable Magnesium in Simulated Inflammatory Condition via Drug-Loaded Plasma Electrolytic Oxidation Coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Bahrampour, S.; Mozafari, M.; Gasik, M. Surface Functionalization of Anodized Tantalum with Mn3O4 Nanoparticles for Effective Corrosion Protection in Simulated Inflammatory Condition. Ceram. Int. 2022, 48, 3148–3156. [Google Scholar] [CrossRef]
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic Inflammation after Trauma. Injury 2007, 38, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.I.; Uzor, P.F.; Ikechukwu, P.; Obi, B.C.; Osadebe, P.O. In Vitro and In Vivo Models for Anti-Inflammation: An Evaluative Review. ITPS 2019, 2, 3–15. [Google Scholar] [CrossRef]
- Heard, C.M. An Ex Vivo Skin Model to Probe Modulation of Local Cutaneous Arachidonic Acid Inflammation Pathway. J. Biol. Methods 2020, 7, e138. [Google Scholar] [CrossRef]
- Richardson, M. Understanding the Structure and Function of the Skin. Nurs. Times 2003, 99, 46–48. [Google Scholar] [PubMed]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-Based Topical Drug Delivery for the Treatment of Skin Diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.S.; Diamond, A.; Russell, A.; Jameson, J.M. Human Aβ and Γδ T Cells in Skin Immunity and Disease. Front. Immunol. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin Immune Sentinels in Health and Disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Yuki, T.; Tobiishi, M.; Kusaka-Kikushima, A.; Ota, Y.; Tokura, Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS ONE 2016, 11, e0161759. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Juráňová, J.; Franková, J.; Ulrichová, J. The Role of Keratinocytes in Inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms Regulating Skin Immunity and Inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The Multitasking Organ: Recent Insights into Skin Immune Function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in Immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Riva, F.; Bonavita, E.; Gentile, S.; Mantovani, A. Decoys and Regulatory “Receptors” of the IL-1/Toll-Like Receptor Superfamily. Front. Immunol. 2013, 4, 180. [Google Scholar] [CrossRef]
- Pobezinskaya, Y.L.; Liu, Z. The Role of TRADD in Death Receptor Signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Montaño Estrada, L.D.; Fortoul Van der Goes, T.I.; Rendón Huerta, E.P. ¿Qué son los inflamosomas? El NLRP3 como ejemplo. Rev. Fac. Med. UNAM 2016, 60, 42–49. [Google Scholar]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Derm. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Tomura, M.; Hata, A.; Matsuoka, S.; Shand, F.H.W.; Nakanishi, Y.; Ikebuchi, R.; Ueha, S.; Tsutsui, H.; Inaba, K.; Matsushima, K.; et al. Tracking and Quantification of Dendritic Cell Migration and Antigen Trafficking between the Skin and Lymph Nodes. Sci. Rep. 2014, 4, 6030. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Aerts, J.L.; Breckpot, K.; Goyvaerts, C.; Knol, E.; Van Wijk, F.; Gutermuth, J. T-cell Subsets in the Skin and Their Role in Inflammatory Skin Disorders. Allergy 2022, 77, 827–842. [Google Scholar] [CrossRef]
- Seidel, J.A.; Vukmanovic-Stejic, M.; Muller-Durovic, B.; Patel, N.; Fuentes-Duculan, J.; Henson, S.M.; Krueger, J.G.; Rustin, M.H.A.; Nestle, F.O.; Lacy, K.E.; et al. Skin Resident Memory CD8+ T Cells Are Phenotypically and Functionally Distinct from Circulating Populations and Lack Immediate Cytotoxic Function. Clin. Exp. Immunol. 2018, 194, 79–92. [Google Scholar] [CrossRef]
- Matos, T.R.; O’Malley, J.T.; Lowry, E.L.; Hamm, D.; Kirsch, I.R.; Robins, H.S.; Kupper, T.S.; Krueger, J.G.; Clark, R.A. Clinically Resolved Psoriatic Lesions Contain Psoriasis-Specific IL-17–Producing Aβ T Cell Clones. J. Clin. Investig. 2017, 127, 4031–4041. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.; Wang, T.; Zheng, J.; et al. Pivotal Role of Dermal IL-17-Producing Γδ T Cells in Skin Inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Ferček, I.; Lugović-Mihić, L.; Tambić-Andrašević, A.; Ćesić, D.; Grginić, A.G.; Bešlić, I.; Mravak-Stipetić, M.; Mihatov-Štefanović, I.; Buntić, A.-M.; Čivljak, R. Features of the Skin Microbiota in Common Inflammatory Skin Diseases. Life 2021, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Bourrain, M.; Ribet, V.; Calvez, A.; Lebaron, P.; Schmitt, A.-M. Balance between Beneficial Microflora and Staphylococcus Aureus Colonisation: In Vivo Evaluation in Patients with Atopic Dermatitis during Hydrotherapy. Eur. J. Dermatol. 2013, 23, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Fahlén, A.; Engstrand, L.; Baker, B.S.; Powles, A.; Fry, L. Comparison of Bacterial Microbiota in Skin Biopsies from Normal and Psoriatic Skin. Arch. Dermatol. Res. 2012, 304, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Heredia Antúnez, A.P.; Cantón, B.V.; Santillán Doherty, P. Retos de Los Comités de Ética En Investigación En Animales. Experiencia de México. Rev. Bioética Derecho 2021, 51, 99–121. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Balls, M. It’s Time to Reconsider The Principles of Humane Experimental Technique. Altern. Lab. Anim. 2020, 48, 40–46. [Google Scholar] [CrossRef]
- Osman, N.; Sidik, N.; Awal, A.; Adam, N.; Rezali, N. In Vitro Xanthine Oxidase (XO) and Albumin Denaturation Inhibition Assay of Barringtonia racemosa L. and Total Phenolic Content Analysis for Potential Anti-Inflammatory Use in Gouty Arthritis. J. Intercult. Ethnopharmacol. 2016, 5, 343. [Google Scholar] [CrossRef]
- Samuel, A.J.; Mulla, N. Formulation and Evaluation of Herbal Topical Gel Containing Leaves Extract of Andrographis Paniculata. J. Drug Deliv. Ther. 2020, 10, 48–51. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Arya, J.C.; Pal, S.; Dogra, P.; Atteri, S.; Patial, B.; Babu, G.; Meena, A.K. Preparation, standardization and evaluation of preliminary anti-inflammatory activity of herbal formulation of Citrullus colocynthis. J. Adv. Sci. Res. 2021, 12, 127–131. [Google Scholar]
- Babu, S.; Noor, A. Aloe Barbadensis Miller Peptide/Polypeptide Fraction Alleviates Inflammation through Inhibition of Proinflammatory Cytokines and Mediators in Vitro and in Rats with Freund’s Adjuvant-Induced Hind Paw Edema. Asian. Pac. J. Trop. Biomed. 2019, 9, 524. [Google Scholar] [CrossRef]
- Moni, J.N.R.; Adnan, M.; Tareq, A.M.; Kabir, M.I.; Reza, A.S.M.A.; Nasrin, M.S.; Chowdhury, K.H.; Sayem, S.A.J.; Rahman, M.A.; Alam, A.K.; et al. Therapeutic Potentials of Syzygium Fruticosum Fruit (Seed) Reflected into an Array of Pharmacological Assays and Prospective Receptors-Mediated Pathways. Life 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, B.; Silvestrini, M. Medical Implications of the Relationships among Protein Denaturation, Necrosis and Inflammation: An Intriguing Story. In Tendons-Trauma, Inflammation, Degeneration, and Treatment; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- García Candela, J.L.E.; Pariona Velarde, C.D.; Londoñe Bailon, R.P. Actividad antiinflamatoria in vitro de los polisacáridos sulfatados de Patallus mollis extraídos mediante digestión enzimática. Rev. Peru. Med. Integr. 2017, 2, 759–764. [Google Scholar] [CrossRef]
- Phanse, M.A. In-Vivo and in-Vitro Screening of Medicinal Plants for Their Anti-Inflammatory Activity: An Overview. J. Appl. Pharm. Sci. 2012, 2, 19–33. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. The Anti-Inflammatory Potential of Cinnamomum Camphora (L.) J.Presl Essential Oil in Vitro and in Vivo. J. Ethnopharmacol. 2021, 267, 113516. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Modak, D.; Chattaraj, S.; Nandi, D.; Sarkar, A.; Roy, J.; Chaudhuri, T.K.; Bhattacharjee, S. Aloe Vera Gel Homogenate Shows Anti-Inflammatory Activity through Lysosomal Membrane Stabilization and Downregulation of TNF-α and Cox-2 Gene Expressions in Inflammatory Arthritic Animals. Future J. Pharm. Sci. 2021, 7, 12. [Google Scholar] [CrossRef]
- Dan, P.; Rosenblat, G.; Yedgar, S. Phospholipase A2 Activities in Skin Physiology and Pathology. Eur. J. Pharmacol. 2012, 691, 1–8. [Google Scholar] [CrossRef]
- Shao, S.; Chen, J.; Swindell, W.R.; Tsoi, L.C.; Xing, X.; Ma, F.; Uppala, R.; Sarkar, M.K.; Plazyo, O.; Billi, A.C.; et al. Phospholipase A2 Enzymes Represent a Shared Pathogenic Pathway in Psoriasis and Pityriasis Rubra Pilaris. JCI Insight 2021, 6, e151911. [Google Scholar] [CrossRef] [PubMed]
- Thibane, V.S.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Modulation of the Enzyme Activity of Secretory Phospholipase A2, Lipoxygenase and Cyclooxygenase Involved in Inflammation and Disease by Extracts from Some Medicinal Plants Used for Skincare and Beauty. S. Afr. J. Bot. 2019, 120, 198–203. [Google Scholar] [CrossRef]
- Acheva, A.; Schettino, G.; Prise, K.M. Pro-Inflammatory Signaling in a 3D Organotypic Skin Model after Low LET Irradiation—NF-κB, COX-2 Activation, and Impact on Cell Differentiation. Front. Immunol. 2017, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Termer, M.; Carola, C.; Salazar, A.; Keck, C.M.; Hemberger, J.; von Hagen, J. Activity-Guided Characterization of COX-2 Inhibitory Compounds in Waltheria indica L. Extracts. Molecules 2021, 26, 7240. [Google Scholar] [CrossRef]
- Suleyman, H.; Demircan, B.; Karagoz, Y. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247. [Google Scholar] [PubMed]
- Kola-Mustapha, A.T.; Khalid-Salako, F.A. Herbal Emulgels Incorporated with Cola millenii K. Schum Stem Bark Ethanol Extract Potentially for the Management of Rheumatoid Arthritis in-Vitro. Phytomed. Plus 2021, 1, 100033. [Google Scholar] [CrossRef]
- Chandrakanthan, M.; Handunnetti, S.M.; Premakumara, G.S.A.; Kathirgamanathar, S. Topical Anti-Inflammatory Activity of Essential Oils of Alpinia calcarata Rosc., Its Main Constituents, and Possible Mechanism of Action. Evid. Based Complement. Altern. Med. 2020, 2020, 2035671. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The Role of Lipoxygenases in Pathophysiology; New Insights and Future Perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Wisastra, R.; Dekker, F. Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef]
- Wu, M.Y.; Lin, T.H.; Chiu, Y.C.; Liou, H.C.; Yang, R.S.; Fu, W.M. Involvement of 15-Lipoxygenase in the Inflammatory Arthritis. J. Cell. Biochem. 2012, 113, 2279–2289. [Google Scholar] [CrossRef]
- Krieg, P.; Fürstenberger, G. The Role of Lipoxygenases in Epidermis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 390–400. [Google Scholar] [CrossRef]
- Toda, K.; Tsukayama, I.; Nagasaki, Y.; Konoike, Y.; Tamenobu, A.; Ganeko, N.; Ito, H.; Kawakami, Y.; Takahashi, Y.; Miki, Y.; et al. Red-Kerneled Rice Proanthocyanidin Inhibits Arachidonate 5-Lipoxygenase and Decreases Psoriasis-like Skin Inflammation. Arch. Biochem. Biophys. 2020, 689, 108307. [Google Scholar] [CrossRef]
- De Vuyst, E.; Salmon, M.; Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Atopic Dermatitis Studies through In Vitro Models. Front. Med. 2017, 4, 119. [Google Scholar] [CrossRef]
- Teimouri, A.; Yeung, P.; Agu, R. 2D vs. 3D Cell Culture Models for In Vitro Topical (Dermatological) Medication Testing. In Cell Culture; Ali Mehanna, R., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Carola, C.; Salazar, A.; Rakers, C.; Himbert, F.; Do, Q.T.; Bernard, P.; von Hagen, J. A Cornflower Extract Containing N-Feruloylserotonin Reduces Inflammation in Human Skin by Neutralizing CCL17 and CCL22 and Inhibiting COX-2 and 5-LOX. Mediat. Inflamm. 2021, 2021, 6652791. [Google Scholar] [CrossRef]
- Chanput, W.; Peters, V.; Wichers, H. THP-1 and U937 Cells. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 147–159. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In Vitro Anti-Inflammatory and Skin Protective Properties of Virgin Coconut Oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Mokdad, R.; Seguin, C.; Fournel, S.; Frisch, B.; Heurtault, B.; Hadjsadok, A. Anti-Inflammatory Effects of Free and Liposome-Encapsulated Algerian Thermal Waters in RAW 264.7 Macrophages. Int. J. Pharm. 2022, 614, 121452. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Hsu, D.K.; Chong, J.; Huynh, M.; Mendoza, L.; Yamada, D.; Hwang, S.T. A Monocyte-Keratinocyte-Derived Co-Culture Assay Accurately Identifies Efficacies of BET Inhibitors as Therapeutic Candidates for Psoriasiform Dermatitis. J. Dermatol. Sci. 2020, 100, 31–38. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Griffoni, C.; Neidhart, B.; Yang, K.; Groeber-Becker, F.; Maniura-Weber, K.; Dandekar, T.; Walles, H.; Rottmar, M. In Vitro Skin Culture Media Influence the Viability and Inflammatory Response of Primary Macrophages. Sci. Rep. 2021, 11, 7070. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, D.H.; Shin, J.U. In Vitro Models Mimicking Immune Response in the Skin. Yonsei Med. J. 2021, 62, 969. [Google Scholar] [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; van den Bogaard, E.H. 3D Skin Models for 3R Research: The Potential of 3D Reconstructed Skin Models to Study Skin Barrier Function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hennies, H.C.; Poumay, Y. Skin Disease Models In Vitro and Inflammatory Mechanisms: Predictability for Drug Development. In Organotypic Models in Drug Development; Schäfer-Korting, M., Stuchi Maria-Engler, S., Landsiedel, R., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2021; Volume 265, pp. 187–218. [Google Scholar] [CrossRef]
- Clarysse, K.; Pfaff, C.M.; Marquardt, Y.; Huth, L.; Kortekaas Krohn, I.; Kluwig, D.; Lüscher, B.; Gutermuth, J.; Baron, J. JAK1/3 Inhibition Preserves Epidermal Morphology in Full-Thickness 3D Skin Models of Atopic Dermatitis and Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H.J.P.M. Crosstalk between Keratinocytes and T Cells in a 3D Microenvironment: A Model to Study Inflammatory Skin Diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef]
- Schmidt, C. Out of Your Skin. Nat. Biotechnol. 2020, 38, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Haisma, E.M.; Rietveld, M.H.; de Breij, A.; van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and Antimicrobial Responses to Methicillin-Resistant Staphylococcus Aureus in an In Vitro Wound Infection Model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-Chip Models: General Overview and Future Perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Zhang, Q.; Sito, L.; Mao, M.; He, J.; Zhang, Y.S.; Zhao, X. Current Advances in Skin-on-a-Chip Models for Drug Testing. Microphysiol. Syst. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wiraja, C.; Zheng, M.; Singh, G.; Yong, K.; Xu, C. Recent Progress in Skin-on-a-Chip Platforms. Adv. Ther. 2022, 5, 2100138. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-Chip Model Simulating Inflammation, Edema and Drug-Based Treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef]
- Biglari, S.; Le, T.Y.L.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; De Bernard, M.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthc. Mater. 2019, 8, 1801307. [Google Scholar] [CrossRef]
- Eberlin, S.; da Silva, M.S.; Facchini, G.; da Silva, G.H.; Pinheiro, A.L.T.A.; Eberlin, S.; da Pinheiro, A.S. The Ex Vivo Skin Model as an Alternative Tool for the Efficacy and Safety Evaluation of Topical Products. Altern. Lab. Anim. 2020, 48, 10–22. [Google Scholar] [CrossRef]
- Neil, J.E.; Brown, M.B.; Williams, A.C. Human Skin Explant Model for the Investigation of Topical Therapeutics. Sci. Rep. 2020, 10, 21192. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.E.; Brown, M.B.; Lenn, J.D.; Williams, A.C. Accelerating Topical Formulation Development for Inflammatory Dermatoses; an Ex Vivo Human Skin Culture Model Consistent with Clinical Therapeutics. Int. J. Pharm. 2022, 618, 121648. [Google Scholar] [CrossRef]
- Anitua, E.; Pino, A.; Aspe, L.; Martínez, M.; García, A.; Goñi, F.; Troya, M. Anti-Inflammatory Effect of Different PRGF Formulations on Cutaneous Surface. J. Tissue Viability 2021, 30, 183–189. [Google Scholar] [CrossRef]
- Trompezinski, S.; Weber, S.; Cadars, B.; Larue, F.; Ardiet, N.; Chavagnac-Bonneville, M.; Sayag, M.; Jourdan, E. Assessment of a New Biological Complex Efficacy on Dysseborrhea, Inflammation, and Propionibacterium Acnes Proliferation. Clin. Cosmet. Investig. Dermatol. 2016, 9, 233–239. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and Guinea Pig Skin as Surrogates for Human in Vitro Penetration Studies: A Quantitative Review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The Pig: A Model for Human Infectious Diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.M. Ex Vivo Live Full-Thickness Porcine Skin Model as a Versatile In Vitro Testing Method for Skin Barrier Research. Int. J. Mol. Sci. 2021, 2, 657. [Google Scholar] [CrossRef] [PubMed]
- Ouitas, N.A.; Heard, C.M. A Novel Ex Vivo Skin Model for the Assessment of the Potential Transcutaneous Anti-Inflammatory Effect of Topically Applied Harpagophytum procumbens Extract. Int. J. Pharm. 2009, 376, 63–68. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-Inflammatory Activity of Punica granatum L. (Pomegranate) Rind Extracts Applied Topically to Ex Vivo Skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Miyake, T.; Shimada, M. 3D Organoid Culture Using Skin Keratinocytes Derived from Human Induced Pluripotent Stem Cells. In Induced Pluripotent Stem (iPS) Cells; Nagy, A., Turksen, K., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2021; Volume 2454, pp. 285–295. [Google Scholar] [CrossRef]
- Gledhill, K.; Guo, Z.; Umegaki-Arao, N.; Higgins, C.A.; Itoh, M.; Christiano, A.M. Melanin Transfer in Human 3D Skin Equivalents Generated Exclusively from Induced Pluripotent Stem Cells. PLoS ONE 2015, 10, e0136713. [Google Scholar] [CrossRef]
- Abaci, H.E.; Gledhill, K.; Guo, Z.; Christiano, A.M.; Shuler, M.L. Pumpless Microfluidic Platform for Drug Testing on Human Skin Equivalents. Lab Chip 2015, 15, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Muller, Q.; Beaudet, M.J.; De Serres-Bérard, T.; Bellenfant, S.; Flacher, V.; Berthod, F. Development of an Innervated Tissue-Engineered Skin with Human Sensory Neurons and Schwann Cells Differentiated from IPS Cells. Acta Biomater. 2018, 82, 93–101. [Google Scholar] [CrossRef]
- Mohsen-Kanson, T.; Hafner, A.-L.; Wdziekonski, B.; Takashima, Y.; Villageois, P.; Carrière, A.; Svensson, M.; Bagnis, C.; Chignon-Sicard, B.; Svensson, P.A.; et al. Differentiation of Human Induced Pluripotent Stem Cells into Brown and White Adipocytes: Role of Pax3. Stem Cells 2014, 32, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.H.; Yoshida, T.; De Benedetto, A.; Beck, L.A. The Cutaneous Innate Immune Response in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2013, 131, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
| Technique | Advantage | Limitations |
|---|---|---|
| Basic techniques and enzyme inhibition in vitro |
|
|
| 2D Cell culture |
|
|
| 3D Cell culture |
|
|
| Skin-on-a-chip |
|
|
| Ex vivo skin tests |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Salas, J.L.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; González-Laredo, R.F.; Medina-Torres, L.; Gallegos-Infante, J.A. In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs. Sci. Pharm. 2023, 91, 20. https://doi.org/10.3390/scipharm91020020
Pérez-Salas JL, Moreno-Jiménez MR, Rocha-Guzmán NE, González-Laredo RF, Medina-Torres L, Gallegos-Infante JA. In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs. Scientia Pharmaceutica. 2023; 91(2):20. https://doi.org/10.3390/scipharm91020020
Chicago/Turabian StylePérez-Salas, Juan Luis, Martha Rocío Moreno-Jiménez, Nuria Elizabeth Rocha-Guzmán, Rubén Francisco González-Laredo, Luis Medina-Torres, and José Alberto Gallegos-Infante. 2023. "In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs" Scientia Pharmaceutica 91, no. 2: 20. https://doi.org/10.3390/scipharm91020020
APA StylePérez-Salas, J. L., Moreno-Jiménez, M. R., Rocha-Guzmán, N. E., González-Laredo, R. F., Medina-Torres, L., & Gallegos-Infante, J. A. (2023). In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs. Scientia Pharmaceutica, 91(2), 20. https://doi.org/10.3390/scipharm91020020







