Abstract
This research sought to optimize the microwave-assisted extraction of Chatuphalathika as an herbal recipe maximizing the active compounds and the antioxidant activity by the Box–Behnken design. Three factors—microwave power, time, and cycle—were varied. Eight responses—extraction yield, total phenolic content, gallic acid content, corilagin content, chebulagic acid, chebulinic acid, IC50 from DPPH assay, and IC50 from FRAP assay—were monitored. Furthermore, cytotoxicity was evaluated to ensure the safety of the extract. After that, the optimized extract was compressed into tablets. The results showed that the optimal condition of the microwave-assisted extraction gave the simultaneous maximum extraction yield, total phenolic content, and antioxidant activity with a microwave power of 450 W for 30 s and 3 cycles. The extract obtained from the optimal condition exhibited a good safety profile although a concentration of 5 mg/mL was used. The optimized tablets were achieved when a compression force of 1500 psi and magnesium stearate of 1% were applied, and no sodium starch glycolate was added. In conclusion, the optimal green extraction method could be used for the extraction of the Chatuphalathika. Furthermore, the fabrication of Chatuphalathika tablets was successful, as the tablets had low friability with a short disintegration time.
1. Introduction
Extraction is a significant step in the chemical isolation, chemical analysis, and evaluation of the biological and pharmacological activity of plant compounds. The proper extraction procedure and techniques provide a high amount of desired plant active compounds and prevent the degradation of some thermolabile compounds [1]. The extraction techniques play an important role in the extraction yield and the content of active compounds. It also affects their activities, so the selection of extraction conditions is a key stage to be considered.
Microwaves are a form of electromagnetic radiation that varies between 0.3–300 GHz. The extraction of natural products usually uses microwaves in the range of 2.5–75 GHz. The efficiency of microwave energy principally depends on solvent content, plant matrix, extraction time, and irradiated microwave power [2]. Microwave-assisted extraction (MAE) is widely used in various fields, such as pharmacy, chemistry, medicine, material sciences, etc. MAE has been accepted as an extraction technique with many advantages over other methods, as it reduces cost and time and consumes less solvent and energy. In addition, it emits less carbon dioxide. Therefore, it is recognized as a green extraction technique [3,4,5,6,7,8]. MAE is applied in the extraction of natural active constituents. It succeeds in the extraction of several plants, e.g., Allamanda cathartica [9], Curcuma longa [10,11], Garcinia cowa [12], Foeniculum vulgare [13], Peganum harmala [14], citrus [5], etc. Furthermore, microwaves are also used for drying plant raw materials [15,16,17].
Design of experiments (DOE) is a well-recognized collection of statistical and mathematical tools that have been widely exploited to improve and optimize the extraction process. Traditional optimization of extraction procedures is carried out by the one-factor-at-time (OFAT) method, which is time-consuming and energy-demanding. Furthermore, it could lead to the loss of effects caused by the interaction of the extraction parameters. Consequently, the DOE approach can be considered a significant tool to seek a more rapid procedure for experimental process development [18].
Chatuphalathika is a Thai traditional herbal remedy. It is composed of an equal ratio of dried fruits of Terminalia chebula Retz. var. chebula, Terminalia bellirica (Gaertn.) Roxb., Phyllanthus emblica L., and Terminalia arjuna Wight and Arn. In some cases, it is called the Triphala plus T. arjuna. Antipyretic, laxative, and health promotion effects are the main indications of Chatuphalathika. The composition of these four plants is reported to have high total phenolic content and good antioxidant activity [19,20,21,22].
According to previous studies, the researchers demonstrated the interaction of phenolic compounds of plant ingredients contained in the Chatuphalathika herbal recipe [23]. The synergistic effect among its ingredients was also proven [24]. However, suitable extraction parameters were not determined. Therefore, the current study aimed to optimize the MAE of Chatuphalathika herbal recipes to maximize the total phenolic content and antioxidant activity. The cytotoxicity was also evaluated to confirm the safety of the optimal extract. Moreover, the optimized extract was used to fabricate Chatuphalathika tablets and optimize the process and formulation parameters. The researchers expected that the optimal extraction parameters could be used as guides for the extraction process for the preparation of the supplementary health products in tablets.
2. Materials and Methods
2.1. Materials
Standard markers, i.e., gallic acid, corilagin, chebulagic acid, and chebulinic acid were purchased from Chengdu Biopurify Phytochemicals Ltd., Chengdu, Sichuan, China. Acetonitrile (HPLC grade) was purchased from Fisher Chemical, Loughborough, Leicestershire, UK. Gallic acid monohydrate, Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), Methylthiazolyldiphenyltetrazolium bromide (MTT), and dimethyl sulfoxide, were purchased from Sigma-Aldrich Pte Ltd., Queenstown, Ascent, Singapore. Ferric (III) chloride hexahydrate (FeCl3·6H2O) was purchased from Merck KGaA, Frankfurter, Darmstadt, Germany. Glacial acetic acid, hydrochloric acid (HCl), and sodium acetate were purchased from Carlo Erba Reagents, Chau. du Vexin, Val-de-Reuil, France. Sodium carbonate (Na2CO3) was purchased from Ajax Finechem Pty. Ltd., Seven Hills, New South Wales, Australia. Others were analytical grade reagents and solvents. According to the pharmaceutical excipients for the tablets, microcrystalline cellulose (Comprecel® M102, Maxway Co., Ltd., Pravet, Bangkok, Thailand), magnesium stearate (Changzhou Kaide Imp. & Exp. Co., Ltd., Changzhou, Jiangsu, China), and talcum (Tabglide®, Nitika Pharmaceutical Specialities Pvt. Ltd., Civil Lines, Nagpur, India) were obtained from Sun Herb Thai Chinese Manufacturing, Muang, Pathum Thani, Thailand. Colloidal silicon dioxide was purchased from P.C. Drug Center, Khan Na Yao, Bangkok, Thailand. Sodium starch glycolate was obtained as a gift from Onimax Co., Ltd., Yannawa, Bangkok, Thailand.
2.2. Plant Sample
The plant samples were obtained from Charoensuk Osod, Nakhon Pathom Province in April 2019, except T. arjuna, which was obtained in July 2019. They were authenticated by Ajarn Nirun Vipunngeun, a plant taxonomist and lecturer, College of Pharmacy, Rangsit University. The voucher specimens were coded as CM-TC001-1-04-2019 (T. chebula), CM-TB001-1-04-2019 (T. bellirica), CM-PE001-1-04-2019 (P. emblica), and CM-TA001-1-07-2019 (T. arjuna) and deposited at the Drug and Herbal Product Research and Development Center, College of Pharmacy, Rangsit University. The dried fruits without seeds were pulverized and stored in a dry place until used.
2.3. Design of Experiments and Optimization of MAE
The Box–Behnken design was applied in the study. The three factors were microwave power (X1) of 300, 450, and 600 W; duration time (X2) of 10, 20, and 30 s; and irradiation cycle (X3) of 1, 2, and 3 cycles, for low, medium, and high levels, respectively. The stop time between each cycle was 5 s. The range of MAE condition factors was based on the preliminary study. It was ensured that the MAE parameters provided no excessive boiling and were selected to vary in the experimental design [25]. The coded and experimental values of the Box–Behnken design are shown in Table 1. Eight responses—extraction yield (Y1), total phenolic content (TPC) (Y2), gallic acid content (Y3), corilagin content (Y4), chebulagic acid content (Y5), chebulinic acid content (Y6), IC50 from DPPH assay (Y7), and IC50 from FRAP assay (Y8)—were monitored.

Table 1.
Coded values and experimental values of the Box–Behnken design: for microwave-assisted extraction, X1 is the microwave power, X2 is the duration time, and X3 is the irradiation cycle for microwave-assisted extraction; and for tablet fabrication, X1 is the compression force, X2 is the amount of sodium starch glycolate, and X3 is the amount of magnesium stearate.
A 10 g Chatuphalathika recipe powder corresponding to 2.5 g of each plant was added to a 250 mL Erlenmeyer flask. Water (100 mL) was added and agitated to ensure that the Chatuphalathika powder was completely wet. It was then placed at the center of the microwave pan before being microwaved using an open system (Samsung, MS23F300EEK, Huai Khwang, Bangkok, Thailand). The research applied the microwave power, the duration time, and the irradiation cycle according to the design. After that, the filtrate was immediately collected using Whatman® filter paper no. 1 by the vacuum filtration technique, allowed to cool to room temperature. It was frozen before being freeze-dried for 20–24 h. The remaining extract powder was used to calculate an extraction yield. The extracts were analyzed for their TPC by the colorimetric method; for the contents of gallic acid, corilagin, chebulagic acid, and chebulinic acid by validated high-performance liquid chromatography (HPLC); and for antioxidant activity by two colorimetric assays: DPPH and FRAP. Each measurement was performed in triplicate.
The data for eight responses were analyzed by Design-Expert® version 11 (Stat-Ease, Inc., Minneapolis, MN, USA). The three-dimensional response surfaces of each response were reported. The optimal condition provided the simultaneous highest extraction yield, TPC, and the lowest IC50 values from DPPH and FRAP assays were selected based on the desirability function [26]. The prediction accuracy of the optimal condition by Design-Expert® was confirmed by re-extracting the Chatuphalathika recipe. The experimental values were compared with the predicted values, and the percentage error was calculated using Equation (1).
2.4. Determination of Total Phenolic Content
The TPC was assayed by the Folin–Ciocalteu method [27]. Twenty microliters of the extracts (500 μg/mL) or gallic acid (6.25, 12.5, 25, 50, 100, and 200 μg/mL) were added to 96-well plates (n = 3). Then, 0.2 N Folin–Ciocalteu reagent (100 μL) was added and mixed. They were incubated for 6 min at room temperature. After that, 7.5% Na2CO3 aqueous solution (80 μL) was added and mixed. They were further incubated at a room temperature for 1 h. Then, they were measured for absorbance at 765 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The TPC of the plant extract was calculated from the calibration curve of gallic acid (y = 0.0059x + 0.0254, R2 = 0.9990). The content of phenolic compounds of the extract was expressed as mg gallic acid equivalents (GAE) per g extract [24,25].
2.5. HPLC Analysis of Gallic Acid, Corilagin, Chebulagic Acid, and Chebulinic Acid Contents
Validated HPLC analysis was carried out for the determination of four phenolic compounds: gallic acid, corilagin, chebulagic acid, and chebulinic acid. Each measurement was performed in triplicate. The validated HPLC analysis method was conducted similarly to previous studies [23,28]. Acetonitrile and 1% acetic acid aqueous solution were used in the mobile phase. The gradient elution system was applied, and the content of an individual compound was calculated based on the calibration curve of its standard marker.
2.6. Determination of Antioxidant Activity by DPPH Assay
The DPPH assay was slightly modified from a previous study [29]. The extracts in concentration ranges of 1.5625–100 µg/mL (100 µL) were added to a 96-well plate (n = 3). Then, 80 µM DPPH solution (100 μL) was added and mixed. The mixtures were incubated at a room temperature in the dark for 30 min. Then, they were measured for absorbance at 517 nm. The capability to scavenge the DPPH radical was calculated using Equation (2).
where ODblank is the optical density of the blank and ODextract is the optical density of the extract.
The curves between the extract concentrations vs. the percentage of DPPH scavenging were created. The IC50 was calculated from the equation of the curve [24,25].
2.7. Determination of Antioxidant Activity by FRAP Assay
The FRAP assay was modified from a previous study [30]. The extracts in concentration ranges of 0.4–25 µg/mL (20 µL) were added to 96-well plates (n = 3). A freshly prepared FRAP reagent (180 μL) composed of 30 mM acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3·6H2O in a volume ratio of 10:1:1, respectively, was then added and mixed. They were incubated at 37 °C for 15 min. They were measured for absorbance at 593 nm. The reducing powers were calculated using Equation (3).
where ODblank is the optical density of the blank and ODextract is the optical density of the extract.
The curves between the extract concentrations vs. percentage reducing power were constructed. The IC50 was calculated from the equation of the curve [24,25].
2.8. In Vitro Cytotoxicity Test
HepG2 cells at a density of 10,000 cells/well were seeded onto a 96-well culture plate and cultured overnight before being treated for 24 h with the optimal Chatuphalathika extract (0–5 mg/mL) (n = 3). Hydrogen peroxide (200 μM H2O2) was used as a positive control. Then, the culture medium was substituted with 500 µg/mL MTT solution and incubated for 3 h at 37 °C. MTT solution was then removed and 100 μL of dimethyl sulfoxide was added. It was measured using the microplate reader at 570 nm. A relative cell viability was calculated as a percentage of the vehicle control group. The average value and the standard deviation (SD) were reported [25,31].
2.9. Design of Experiments, Fabrication, and Optimization of Chatuphalathika Tablets
The Box–Behnken design was applied in the study. The three factors were compression force (X1) of 1000, 1500, and 2000 psi; the amounts of sodium starch glycolate (X2) of 0, 2, and 4%; and the amounts of magnesium stearate (X3) of 0.5, 1, and 1.5%, for low, medium, and high levels, respectively. The coded and experimental values of the Box–Behnken design are shown in Table 1.
The Chatuphalathika tablets were composed of Chatuphalathika extract powder as an active ingredient, colloidal silicon dioxide as a glidant, talcum as a glidant and antiadherent, magnesium stearate as a lubricant and antiadherent, sodium starch glycolate as a disintegrant, and microcrystalline cellulose as a diluent. A 600 mg tablet was composed of 200 mg Chatuphalathika extract powder, 1% colloidal silicon dioxide, 5% talcum, 0–4% sodium starch glycolate, 0.5–1.5% magnesium stearate, and microcrystalline cellulose, which was used to adjust the total weight of the tablets when the amounts of sodium starch glycolate and magnesium stearate were altered according to the experimental design. All ingredients were passed through a 60-mesh sieve. Initially, Chatuphalathika extract powder and microcrystalline cellulose were mixed by the geometric dilution technique. A premix of microcrystalline cellulose, colloidal silicon dioxide, talcum, sodium starch glycolate, and magnesium stearate was prepared. Then, the mixture of Chatuphalathika extract powder and microcrystalline cellulose was mixed with the premix for 3 min. The obtained mixture weighing 600 mg was compressed into a tablet using an in-house-assembled hydraulic press connected with a pressure gauge using 1000–2000 psi force and maintained for 10 s before being ejected from the die. The physicochemical properties of the obtained tablets were evaluated. Seven responses—average weight (Y1), weight variation (Y2), thickness (Y3), diameter (Y4), hardness (Y5), friability (Y6), and disintegration time (Y7)—were monitored. However, only four responses including thickness (Y3), hardness (Y5), friability (Y6), and disintegration time (Y7), were used in the optimization step.
The data of four responses were analyzed by Design-Expert® version 11. The contour plots of each of the responses, including thickness, hardness, friability, and disintegration time, were reported. The design spaces in which friability was not more than 0.5% and disintegration time was not more than 180 s were constructed. The optimal condition was selected based on the optimization desirability function [26]; thickness and hardness were set at “none” while friability and disintegration time were set at “minimize”. The optimal condition was also considered from the condition that fell in the design space. The accuracy of the prediction by Design-Expert® was confirmed by preparing the new batch of Chatuphalathika tablets according to the optimal condition. The experimental values were compared with the predicted values and a percentage error was calculated as Equation (1).
2.10. Evaluation of Chatuphalathika Tablet Properties
Seven properties, including average weight, weight variation, thickness, diameter, hardness, friability, and disintegration time were evaluated.
2.10.1. Average Weight and Weight Variation
Twenty tablets were individually weighed using an analytical balance (Entris224i-1S, Sartorius AG, Otto-Brenner-Straße, Göttingen, Germany). The average value and SD were reported. Weight variation was calculated by comparing the difference between the individual weight and the average weight using Equation (4).
2.10.2. Thickness and Diameter
Twenty tablets were tested using a thickness gauge. The average values and SD were reported.
2.10.3. Hardness
Ten tablets were tested using a hardness tester (TBH 220 TD, Erweka GmbH, Ottostraße, Heusenstamm, Germany). The average values and SD were reported.
2.10.4. Friability
Eleven tablets, from which dust was removed, were weighed (W1) using an analytical balance. Friability was tested using a friability tester (Model: CS-2, Tianjin Guoming Medicinal Equipment Co., Ltd., Hua Yuan, Tianjin, China) at 25 rpm for 4 min. Then, the tablets were removed from the drum. Dust was removed, and the tablets were weighed (W2) again. The friability was calculated using Equation (5).
2.10.5. Disintegration Time
Six tablets were evaluated using a disintegration tester (Model: BJ-2, Tianjin Guoming Medicinal Equipment Co., Ltd., Hua Yuan, Tianjin, China). Water (37 ± 0.5 °C) was used as a disintegration medium. The average values and SD were reported.
2.11. Statistical Analysis
The differences of at least three groups were analyzed using one-way analysis of variance (one-way ANOVA) by SPSS Statistics 22.0 (IBM, Madison Avenue, NY, USA). When the p-value at a 95% confidence level was less than 0.05, the data were considered to be substantially different.
3. Results
3.1. Extraction Yield, TPC, the Content of Some Phenolic Compounds, and Antioxidant Activity of Chatuphalathika Extracts
The factors and responses of the Box–Behnken design for microwave-assisted extraction are shown in Table S1 in Supplementary Materials. The mathematical models and actual equations of the responses are shown in Table 2. The extraction yield (Y1), chebulagic acid (Y5), and chebulinic acid (Y6) were fitted to the linear model while other responses were fitted to the quadratic model. The three-dimensional response surfaces of each response are shown in Figure 1. According to the response surfaces of the extraction yield, when the microwave power, the extraction time, and the irradiation cycle increased, the extraction yield also increased.

Table 2.
Mathematical models and actual equations of the responses of MAE.
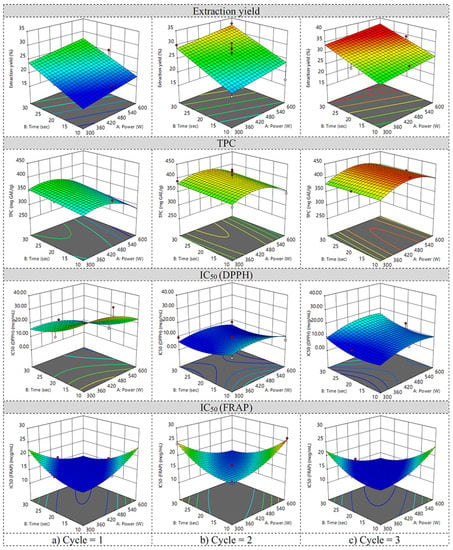
Figure 1.
Response surfaces of extraction yield, TPC, IC50 from DPPH assay, and IC50 from FRAP assay when the irradiation cycle was (a) low, (b) medium, and (c) high.
The response surfaces of TPC revealed that the microwave power and the extraction time had less effect on TPC while the irradiation cycles highly affected TPC. According to the equation of Y2 in Table 2, the irradiation cycle played the greatest effect on TPC, but the microwave power and the extraction time were comparatively lower than the irradiation cycle. X2X3, X12, X22, and X32 had a negative effect on TPC.
The HPLC chromatogram of Chatuphalathika extract is shown in Figure 2. Four phenolic compounds—gallic acid, corilagin, chebulagic acid, and chebulinic acid—were analyzed. The response surfaces of the gallic acid content revealed that the irradiation cycle had the greatest effect on gallic acid content (Figure 3). According to the equation of Y3 in Table 2, the irradiation cycle had the greatest effect on gallic acid content, followed by the extraction time and the microwave power. X1, X2, and X3 had a positive effect on the gallic acid content while other interaction and quadratic terms had a negative effect on it.
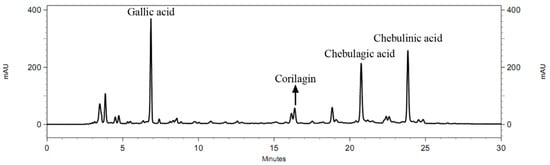
Figure 2.
HPLC chromatogram of the Chatuphalathika extract obtained from the optimal condition (2 mg/mL).
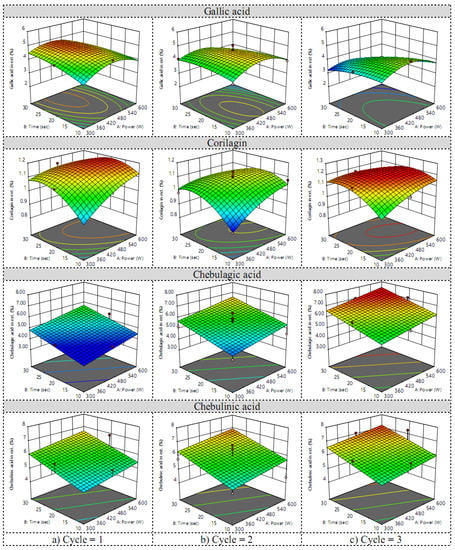
Figure 3.
Response surfaces of gallic acid, corilagin, chebulagic acid, and chebulinic acid contents when the irradiation cycle was (a) low, (b) medium, and (c) high.
The response surfaces of the corilagin content revealed that, when the microwave power and the extraction time increased, the corilagin content also increased. The high content of corilagin was found at a low and high irradiation cycle: 1 cycle and 3 cycles (Figure 3). According to the equation of Y4 in Table 2, the extraction time had a better positive effect on Y4 when compared with the microwave power and the irradiation cycle. The terms X1, X2, ad X32 had a positive effect on the corilagin content, while other linear, interaction, and quadratic terms harmed the corilagin content.
When the microwave power, the extraction time, and the irradiation cycle increased, both chebulagic acid and chebulinic acid contents increased (Figure 3). Among the three factors, the irradiation cycle had a greater effect on the extraction yield than the extraction time and the microwave power, corresponding to the equation of Y5 and Y6 in Table 2. Furthermore, perturbation plots of the Box–Behnken design for microwave-assisted extraction are shown in Figure S1 in Supplementary Materials.
In the case of IC50, a low value was required to ensure a good antioxidant activity. The response surfaces of IC50 from the DPPH and FRAP assays are shown in Figure 1. The result showed that a low IC50 value obtained from the DPPH assay was achieved when a medium to high irradiation cycle was applied. Meanwhile, a low IC50 value obtained from the FRAP assay was achieved when a low or high irradiation cycle was applied. According to the equation of Y7 in Table 2, a good antioxidant activity (or low IC50 value) based on the DPPH assay was achieved by X2, X3, and X12. Meanwhile, a good antioxidant activity based on the FRAP assay was achieved by X1, X1X2, X1X3, X2X3, and X32.
3.2. Optimal Condition of MAE
The optimal condition provided a simultaneous high extraction yield, a high TPC, and the greatest antioxidant activity. The factors—microwave power, time, and irradiation cycle—were set at “is in range” and responses—extraction yield (Y1) and TPC (Y2)—were set at “maximize”, while IC50 from the DPPH assay (Y7) and IC50 from the FRAP assay (Y8) were set at “minimize”, with a microwave power of 450 W for 30 s and repeated for 3 cycles. The gallic acid, corilagin, chebulagic acid, and chebulinic acid contents (Y3 to Y6) were not used in the optimization process because the TPC (Y2) covered the Y3 to Y6. Verification of the predicted optimal condition was performed to ensure the accuracy of the prediction of computer software. The percentage prediction error of all responses was less than 15%. The percentage error seemed to be relatively high due to the small value that caused a huge percentage error. However, the research suggested that all experimental values were close to the predicted values (Table 3).

Table 3.
Data verification to confirm the prediction accuracy of the microwave-assisted extraction.
The extract obtained from the optimal condition was also analyzed for the phenolic compound contents. The HPLC chromatogram of the Chatuphalathika extract obtained from the optimal condition is shown in Figure 2. The optimal extract contained gallic acid, corilagin, chebulagic acid, and chebulinic acid of 3.30 ± 0.16%, 1.19 ± 0.06%, 7.21 ± 0.49%, and 7.11 ± 1.09%, respectively.
3.3. In Vitro Cytotoxicity
Chatuphalathika extracts had no toxic effects on HepG2 cells at concentrations up to 5 mg/mL, with a cell viability of 86.22 ± 0.78%; thus, the IC50 values of both extracts could not be determined from the study (Figure 4). A significant decrease in the cell viability in the presence of Chatuphalathika extract was observed at a concentration of 0.0001 mg/mL, and the cell viability further decreased up to a concentration of 5 mg/mL, compared with the non-treated group. Meanwhile, the positive control, 200 μM hydrogen peroxide, yielded a cell viability of only 62.82 ± 0.64% (Figure 4).
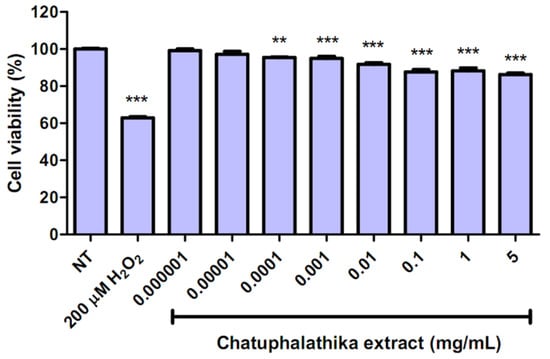
Figure 4.
In vitro cytotoxicity of Chatuphalathika extracts in HepG2 cells compared with the non-treated (NT) group and the positive control group (200 μM hydrogen peroxide). The significance is presented as ** and *** when p < 0.01 and p < 0.001, respectively.
3.4. Optimal Fabricated Chatuphalathika Tablets
Regarding the average weight (Y1), the weight variation (Y2), and the diameter (Y4), the average weight per tablet was about 600 mg with a diameter of 12.7 mm. No tablets had a weight variation of more than 5% of the average weight. The factors and responses of the Box–Behnken design for tablet fabrication are shown in Table S2 in Supplementary Materials. Among the optimized responses, the tablet thickness (Y3), friability (Y6), and disintegration time (Y7) were fitted to the quadratic model while hardness (Y5) was fitted to the linear model. The contour plots of the four responses of the fabricated tablets are shown in Figure 5. Furthermore, perturbation plots of the Box–Behnken design for tablet fabrication are shown in Figure S2 in Supplementary Materials. When compression force increased, tablet thickness and friability decreased, whereas hardness and disintegration time increased in all levels of magnesium stearate. An increasing amount of sodium starch glycolate seemed to have no effect on tablet thickness, hardness, and disintegration time, but increased tablet friability. The amount of magnesium stearate had more effect on tablet friability than the disintegration time and thickness. However, it seemed to have no effect on tablet hardness. According to the equation in Table 4, the terms that decreased tablet thickness (Y3) were X1, X2, X1X3, and X32. The terms X1 and X3 increased tablet hardness (Y5) while X2 decreased Y5. The terms decreasing tablet friability (Y6) were X1, X1X2, and X1X3, while the others increased tablet friability. The terms that shortened the disintegration time (Y7) were X1, X2, X3, X2X3, and X22. Among these terms, X3 affected Y7 the most.
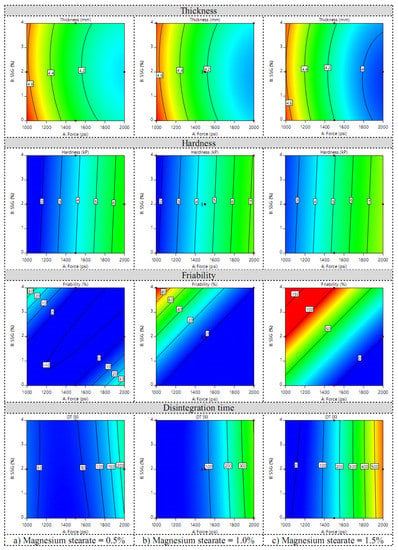
Figure 5.
Contour plots of tablet thickness, hardness, friability, and disintegration time when the amount of magnesium stearate was (a) low, (b) medium, and (c) high.

Table 4.
Mathematical models and actual equations of the responses of tablet fabrication.
Design spaces with friability of more than 0.5% and disintegration times of more than 180 s are shown in Figure 6. It was found that when the amount of magnesium stearate increased, the area of the design space decreased. In this case, the compression force of 1500 psi and magnesium stearate of 1%, without sodium glycolate added, were the optimal condition used to verify the result due to the fact that it was within the design space, yielding low friability and shortening the disintegration time.
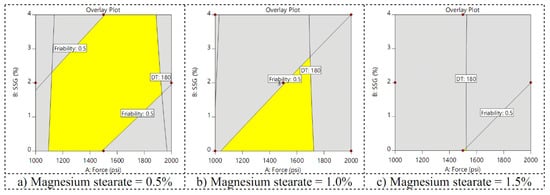
Figure 6.
Design spaces in which friability was not more than 0.5% and the disintegration time was not more than 180 s when the amount of magnesium stearate was (a) low, (b) medium, and (c) high.
The verification of data as shown in Table 5 was conducted to confirm the accuracy of the prediction of tablet fabrication. The percentage prediction error of each response was less than 10%, except the friability, in which a huge percentage error was found. This phenomenon could be because, when a prediction value was 0, the percentage error was always 100% for any experimental values. However, the tablet friability was lower than 1%, so it was acceptable.

Table 5.
Data verification to confirm the prediction accuracy of the tablet fabrication.
4. Discussion
The factors investigated in the present study were important parameters that affected the yield of active compounds, which consequently affected the biological or pharmacological activity of the extract. Previous studies reported an increase in the microwave power when the TPC of plants increased. However, the optimal extraction time was found in some cases, especially when some conditions might induce the decomposition of some bioactive compounds [32]. A similar effect was also observed in the MAE of TPC from Chromolaena odorata leaves [33]. A previous study demonstrated that, based on one-factor consideration, when the microwave power or the extraction time increased, TPC decreased while the interaction between microwave power and extraction time increased TPC [34].
MAE operates based on the direct effect on active compounds by dipole rotation and ionic conduction, which lead to power dissipation inside the plant matrix and extraction solvent, resulting in heating and molecular movement [35]. The plant matrix could absorb microwave energy and cause internal superheating, resulting in cell disruption and causing active compounds to leach out from plant materials [36]. Therefore, some plant active compounds could be destroyed by the extreme heat generated by microwave energy [32]. The mechanism of the phenomenon that microwave power had a negative effect on plant active compound content was also described previously [37,38]. The very high microwave power induced the plant samples to be thermally compromised, leading to a decrease in the yield of active compounds.
An increasing microwave time could promote the decomposition of some plant active compounds; TPC decreased when microwave time increased [32]. Some other studies reported that, with an increasing microwave power, total curcuminoids were highly extracted at a low and high rather than at medium microwave power. An increase in the irradiation cycle increased the total curcuminoids content. However, curcuminoids could be destroyed from the excessive irradiation time [10]. All the above data indicated the importance of the optimization of MAE parameters for plant extraction.
A previous work reported comparable gallic acid and corilagin contents, while higher chebulagic acid and chebulinic acid of Chatuphalathika extract: 3.54%, 1.33%, 5.93%, and 4.48%, respectively, from the decoction method [23]. The decoction technique takes more time (approximately 15 min for three times) and energy, while MAE takes less than a minute and less energy. The yield of phenolic compounds can be increased by increasing consecutive extraction times [10,12]. However, the present study extracted the plants only one time, making yields of chebulagic acid and chebulinic acid lower than those by the decoction method. Therefore, MAE is an alternative method for the extraction of Chatuphalathika.
According to the cytotoxicity test, the ISO 10993-5 standard defined that cytotoxicity is considered when cell viability is reduced by more than 30% [39]. Therefore, it could be concluded that optimized Chatuphalathika extract was safe based on in vitro data. To the best of our knowledge, there is no evidence of cytotoxicity data on Chatuphalathika extract on HepG2 cells. There is only cytotoxicity data on a Triphala remedy (Chatuphalathika without T. arjuna) on HepG2 cells. Triphala extract exhibited an IC50 value of 77.63 µg/mL, which was close to the IC50 values of P. emblica and T. chebula, while T. bellirica had an IC50 value of 129.3 µg/mL [40]. The clinical trial showed that Triphala aqueous extract is safe for healthy volunteers, raises HDL-C levels, and decreases blood sugar [41]. However, the present work revealed that Chatuphalathika extracts had no toxic effects on HepG2 cells even though a high concentration of up to 5 mg/mL was used.
The factors of tablet fabrication investigated in this research were compression force, the amount of sodium starch glycolate, and the amount of magnesium stearate. Normally, an increasing compression force decreased tablet thickness and friability, but increased tablet hardness and disintegration time [42,43,44,45]. According to previous studies, an increasing compression force did not affect the disintegration time when sodium starch glycolate was used [46]. However, this research found that the interaction of compression force and the amount of sodium starch glycolate (X1X2) seemed to increase the disintegration time but decrease friability.
Sodium starch glycolate was used as a disintegrant because it could shorten the drug disintegration time by a swelling mechanism. When the tablet contacted the aqueous medium, disintegrant with rapid water uptake swelled and generated pressure, pushed apart particles, and finally broke up the tablet [47,48]. The effectiveness of many disintegrants was affected by the hydrophobic ingredients such as magnesium stearate and talcum. However, sodium starch glycolate was not affected by some hydrophobic ingredients [46,49]. The research found that the quadratic term of sodium starch glycolate (X22) decreased the disintegration time. Furthermore, the interaction of sodium starch glycolate and magnesium stearate (X2X3) decreased the disintegration time while increasing friability. Nevertheless, the fabricated Chatuphalathika tablets rapidly disintegrated naturally, so sodium starch glycolate was unnecessary for this formulation. Magnesium stearate is a lubricant used to decrease friction forces between particles [50]. Because of its hydrophobic property, it could prolong the disintegration time [50], especially when a high compression force was applied, as found in this research.
The tablets prepared from the direct compression method had higher friability compared with the wet granulation method. However, low friability was the desired tablet property. Generally, friability should not be more than 1%; however, the friability of the design space construction in the optimization step was set at not higher than 0.5%, to ensure that the tablet had sufficient physical strength and did not lose components due to abrasion, friction, or mechanical shock [51]. Moreover, the tablet disintegration time should not be more than 30 min for a plain tablet. This research found that the longest disintegration time was approximately 11 min, indicating that the disintegration time of fabricated direct compressed tablets was acceptable. However, a shorter disintegration time was required for a plain tablet to ensure the drug absorption and action compared with a tablet with a longer disintegration time. Therefore, according to the criteria for the construction of the design space, friability and disintegration time must not exceed 0.5% and 3 min, respectively.
The developed tablet included a microcrystalline cellulose diluent which also exhibited disintegrant property, so the developed tablet disintegrated rapidly. Hence, disintegrants and sodium starch glycolate were not required. The disintegrant is an expensive ingredient. Adding no disintegrants could reduce production costs. The optimization process and design space, using low or high compression forces of 1000 and 2000 psi, could not reach the design space for all magnesium stearate levels. Therefore, a compression force of 1500 psi was selected. A comparison among different magnesium stearate levels revealed that using low magnesium stearate (0.5%) yielded broader design space than medium or high magnesium stearate. However, when 0% sodium starch glycolate was selected, the interest point was outside the design space when using 0.5% magnesium stearate. Therefore, the compression force of 1500 psi, 0% sodium glycolate, and 1% magnesium stearate were selected as the optimal conditions of the tablet fabrication. The verification of data indicated the fabrication of optimized direct compressed Chatuphalathika tablets was accurate and precise. In summary, the optimized Chatuphalathika extract could be used to fabricate tablets with suitable properties, as it showed low friability and a shortened disintegration time.
5. Conclusions
The optimal condition of MAE that gave the simultaneous maximum extraction yield, total phenolic content, and antioxidant activity, was a microwave power of 450 W for 30 s and 3 cycles. Chatuphalathika extract obtained from the optimal condition gave a cell viability of more than 85%, although a high concentration (5 mg/mL) was used, indicating a good safety profile. The optimal extract was further used to fabricate tablets by the direct compression technique. The optimized tablet was achieved when a compression force of 1500 psi was used, magnesium stearate of 1% was added, and sodium starch glycolate was not added. The fabricated tablet had low friability with a shortened disintegration time. In conclusion, the optimal extraction condition was suitable and effective for Chatuphalathika extraction due to its low energy consumption and short duration. This research was successfully conducted, since the fabricated Chatuphalathika tablets had low friability and a shortened disintegration time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm91020017/s1, Table S1: Factors and responses of the Box–Behnken design for microwave-assisted extraction; Table S2: Factors and responses of the Box–Behnken design for tablet fabrication; Figure S1: Perturbation plots of the Box–Behnken design for microwave-assisted extraction; (a) extraction yield, (b) TPC, (c) gallic acid content, (d) corilagin content, (e) chebulagic acid content, (f) chebulinic acid content, (g) IC50 from DPPH assay, and (h) IC50 from FRAP assay; Figure S2: Perturbation plots of each response of the Box–Behnken design for tablet fabrication; (a) tablet thickness, (b) hardness, (c) friability, and (d) disintegration time.
Author Contributions
Conceptualization, C.M. and T.S.; methodology, C.M., T.W. and A.N.; software, C.M. and J.S.; validation, C.M., T.W. and A.N.; formal analysis, C.M., P.K. (Piyapa Keawchay), C.P., P.K. (Pariyakorn Kamnoedthapaya) and A.N.; investigation, C.M., P.K. (Piyapa Keawchay), C.P., P.K. (Pariyakorn Kamnoedthapaya) and A.N.; resources, N.C. and T.S.; data curation, C.M., P.K. (Piyapa Keawchay), C.P., P.K. (Pariyakorn Kamnoedthapaya) and J.S.; writing—original draft preparation, all authors; writing—review and editing, C.M.; visualization, C.M., J.S., N.C. and T.S.; supervision, C.M. and T.S.; project administration, C.M.; funding acquisition, C.M. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the College of Pharmacy, Rangsit University, research funding for undergraduate students (the academic year 2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Ajarn Nirun Vipunngeun for the authentication of plant samples used in this research. The authors would also like to thank Hanafarah Salaemae, Pakawat Pinwiwat, and Ekkawat Ingudomnukul for their research assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Uzel, R.A. Microwave-assisted green extraction technology for sustainable food processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; You, K.Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Extraction|Microwave-assisted extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 67–77. [Google Scholar]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Song, Z.; Wei, X.; Xie, M.; Zhao, X.; Sun, J.; Mao, Y.; Wang, X.; Wang, W. Study on the microwave extraction process and product distribution of essential oils from citrus peel. Chem. Eng. Process. Process Intensif. 2022, 171, 108726. [Google Scholar] [CrossRef]
- Bener, M.; Burak Şen, F.; Nur Önem, A.; Bekdeşer, B.; Esin Çelik, S.; Lalikoglu, M.; Selim Aşçı, Y.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Souza, O.A.; Ramalhão, V.G.d.S.; Trentin, L.d.M.; Funari, C.S.; Carneiro, R.L.; Bolzani, V.d.S.; Rinaldo, D. Combining natural deep eutectic solvent and microwave irradiation towards the eco-friendly and optimized extraction of bioactive phenolics from Eugenia uniflora L. Sustain. Chem. Pharm. 2022, 26, 100618. [Google Scholar] [CrossRef]
- Pengdee, C.; Sritularak, B.; Putalun, W. Optimization of microwave-assisted extraction of phenolic compounds in Dendrobium formosum Roxb. ex Lindl. and glucose uptake activity. S. Afr. J. Bot. 2020, 132, 423–431. [Google Scholar] [CrossRef]
- Bonomini, T.J.; Góes, J.A.; Machado, M.d.S.; Silva, R.M.L.d.; Malheiros, A. Development and optimization of a microwave-assisted extraction of plumieride from Allamanda cathartica L. flowers. Quím. Nova 2018, 41, 36–42. [Google Scholar] [CrossRef]
- Lateh, L.; Yuenyongsawad, S.; Chen, H.; Panichayupakaranant, P. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019, 15, 730–735. [Google Scholar] [CrossRef]
- Kanchanathawornviboon, X.; Monton, C.; Urairong, H. Microwave-assisted extraction of curcuminoids from organic Curcuma longa L. in different oil types for cosmetic purpose: An optimization approach. J. Curr. Sci. Technol. 2021, 11, 71–89. [Google Scholar] [CrossRef]
- Sae-Lim, P.; Yuenyongsawad, S.; Panichayupakaranant, P. Chamuangone-enriched Garcinia cowa leaf extract with rice bran oil: Extraction and cytotoxic activity against cancer cells. Pharmacogn. Mag. 2019, 15, 183–188. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Ansari, M.; Ghaffar, N.; Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J. King Saud Univ. Sci. 2020, 32, 1451–1458. [Google Scholar] [CrossRef]
- Shang, X.; Guo, X.; Li, B.; Pan, H.; Zhang, J.; Zhang, Y.; Miao, X. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. J. Ethnopharmacol. 2016, 192, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Monton, C.; Luprasong, C.; Charoenchai, L. Acceleration of turmeric drying using convection and microwave-assisted drying technique: An optimization approach. J. Food Process. Preserv. 2019, 43, e14096. [Google Scholar] [CrossRef]
- Monton, C.; Luprasong, C.; Charoenchai, L. Convection combined microwave drying affect quality of volatile oil compositions and quantity of curcuminoids of turmeric raw material. Rev. Bras. Farmacogn. 2019, 29, 434–440. [Google Scholar] [CrossRef]
- Charoenchai, L.; Monton, C.; Luprasong, C.; Kraisintu, K. Pretreatment study of turmeric rhizomes and optimization of drying methods using microwave oven and hot air oven to obtain high quality of turmeric powder. J. Curr. Sci. Technol. 2020, 10, 49–57. [Google Scholar] [CrossRef]
- Rosa, R.; Ferrari, E.; Veronesi, P. From field to shelf: How microwave-assisted extraction techniques foster an integrated green approach. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; You, K.Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Amalraj, A.; Gopi, S. Medicinal properties of Terminalia arjuna (Roxb.) Wight & Arn.: A review. J. Tradit. Complement. Med. 2017, 7, 65–78. [Google Scholar] [CrossRef]
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.M.R. Terminalia bellirica fruit extracts: In-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complement. Altern. Med. 2018, 18, 325. [Google Scholar] [CrossRef]
- Naik, G.H.; Priyadarsini, K.I.; Naik, D.B.; Gangabhagirathi, R.; Mohan, H. Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine 2004, 11, 530–538. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Monton, C.; Suksaeree, J. Interaction of plant ingredients contained in Chatuphalathika herbal remedy based on chemical analysis aspect: Four-component simplex lattice design. Adv. Tradit. Med. 2021, 21, 535–544. [Google Scholar] [CrossRef]
- Suksaeree, J.; Wunnakup, T.; Monton, C. Synergistic antioxidant activity of plant compositions contained in Chatuphalathika herbal recipe: Terminalia chebula Retz. var. chebula, Terminalia arjuna Wight and Arn., Terminalia bellirica (Gaertn.) Roxb., and Phyllanthus emblica L. Adv. Tradit. Med. 2022, 22, 547–556. [Google Scholar] [CrossRef]
- Monton, C.; Kittiratpattana, P.; Nakyai, S.; Sutapakul, T.; Navabhatra, A.; Wunnakup, T.; Chankana, N.; Suksaeree, J. Microwave-assisted extraction of Clausena anisata leaves and Vernonia cinerea whole plants to maximize nitrate content: Optimization approach, antioxidant activity, and cytotoxicity. Adv. Tradit. Med. 2022, 22, 697–711. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Monton, C.; Wunnakup, T.; Suksaeree, J.; Charoenchai, L.; Chankana, N. Investigation of the interaction of herbal ingredients contained in Triphala recipe using simplex lattice design: Chemical analysis point of view. Int. J. Food Sci. 2020, 2020, 5104624. [Google Scholar] [CrossRef]
- Lacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Chiangsom, A.; Maniratanachote, R.; Meksuriyen, D.; Luechapudiporn, R.; Kulthong, K.; Aueviriyavit, S.; Oda, S.; Yokoi, T.; Lawanprasert, S. Protective effect of Phikud Navakot extract against hydrogen peroxide-induced oxidative stress in HepG2 cells. Thai J. Pharm. Sci. 2019, 4, 186–194. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Abdul Mudalip, S.K. Optimizing microwave-assisted extraction conditions to obtain phenolic-rich extract from Chromolaena odorata leaves. Chem. Eng. Technol. 2019, 42, 1733–1740. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Alara, J.A. Two-level factorial screening of microwave-assisted extraction parameters for the recovery of phenolic compounds from Vernonia cinerea leaf. J. Chem. Eng. Ind. Biotechnol. 2019, 25, 16–28. [Google Scholar] [CrossRef]
- Deo, S.; Janghel, A.; Raut, P.; Bhosle, D.; Verma, C.; Kumar, S.S.; Agrawal, M.; Amit, N.; Sharma, M.; Giri, T.; et al. Emerging microwave assisted extraction (MAE) techniques as an innovative green technologies for the effective extraction of the active phytopharmaceuticals. Res. J. Pharm. Technol. 2015, 8, 655–666. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crop. Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Yang, L.; Sun, X.; Yang, F.; Zhao, C.; Zhang, L.; Zu, Y. Application of ionic liquids in the microwave-assisted extraction of proanthocyanidins from Larix gmelini bark. Int. J. Mol. Sci. 2012, 13, 5163–5178. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crop. Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Sahragard, A.; Alavi, Z.; Abolhassanzadeh, Z.; Moein, M.; Mohammadi-Bardbori, A.; Omidi, M.; Zarshenas, M.M. Assessment of the cytotoxic activity of Triphala: A semisolid traditional formulation on HepG2 cancer cell line. BioMed Res. Int. 2021, 2021, 6689568. [Google Scholar] [CrossRef]
- Phetkate, P.; Kummalue, T.; Rinthong, P.; Kietinun, S.; Sriyakul, K. Study of the safety of oral Triphala aqueous extract on healthy volunteers. J. Integr. Med. 2020, 18, 35–40. [Google Scholar] [CrossRef]
- Manley, L.; Hilden, J.; Valero, P.; Kramer, T. Tablet compression force as a process analytical technology (PAT): 100% inspection and control of tablet weight uniformity. J. Pharm. Sci. 2019, 108, 485–493. [Google Scholar] [CrossRef]
- Marais, A.F.; Song, M.; Villiers, M.M.d. Effect of compression force, humidity and disintegrant concentration on the disintegration and dissolution of directly compressed furosemide tablets using croscarmellose sodium as disintegrant. Trop. J. Pharm. Res. 2003, 2, 125–135. [Google Scholar]
- Suksaeree, J.; Monton, C.; Chankana, N.; Charoenchai, L. Microcrystalline cellulose promotes superior direct compressed Boesenbergia rotunda (L.) Mansf. extract tablet properties to spray-dried rice starch and spray-dried lactose. Arab J. Basic Appl. Sci. 2023, 30, 13–25. [Google Scholar] [CrossRef]
- Suksaeree, J.; Monton, C.; Charoenchai, L.; Chankana, N.; Wunnakup, T. Optimization of process and formulation variables for Semha-Pinas extract effervescent tablets using the Box-Behnken design. AAPS PharmSciTech 2023, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Markl, D.; Zeitler, J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [PubMed]
- Apeji, Y.E.; Zechariah, F.D.; Anyebe, S.N.; Tytler, B.; Olowosulu, A.K.; Oyi, A.R. Effect of mode of superdisintegrant incorporation on tableting properties of metronidazole granules. Pharm. Sci. Asia 2019, 46, 25–32. [Google Scholar] [CrossRef]
- Rojas, J.; Guisao, S.; Ruge, V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y. Lubricants in pharmaceutical solid dosage forms. Lubricants 2014, 2, 21–43. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Sun, C.C. Validation and applications of an expedited tablet friability method. Int. J. Pharm. 2015, 484, 146–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
