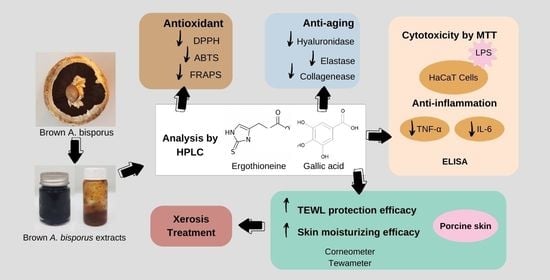
Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Brown A. bisporus Preparation
2.3. Brown A. bisporus Extraction
2.4. Measurement of Total Polysaccharides Content, Total Phenolics Content, and Total Flavonoids Content
2.4.1. Determination of Total Polysaccharides Content
2.4.2. Determination of Total Phenolics Content
2.4.3. Determination of Total Flavonoids Content
2.5. Determination of Chemical Components by High Performance Liquid Chromatography (HPLC)
2.6. Determination of Antioxidant Activity
2.6.1. DPPH Radical Scavenging Assay
2.6.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6.3. ABTS Radical Scavenging Assay
2.7. Determination of Anti-Aging Activities
2.7.1. Hyaluronidase Inhibitory Assay
2.7.2. Collagenase Inhibitory Assay
2.7.3. Elastase Inhibitory Assay
2.8. Cell Culture
2.8.1. Determination of Cytotoxicity
2.8.2. Quantification of IL-6 and TNF-α Secretion by ELISA
2.9. Statistical Analysis
3. Results and Discussion
3.1. Brown A. bisporus Extraction
3.2. Measurement of Total Polysaccharides, Total Phenolics, and Total Flavonoids Contents
3.2.1. Total Polysaccharides Content of Brown A. bisporus Extracts
3.2.2. Total Phenolics and Flavonoids Contents of Brown A. bisporus Extracts
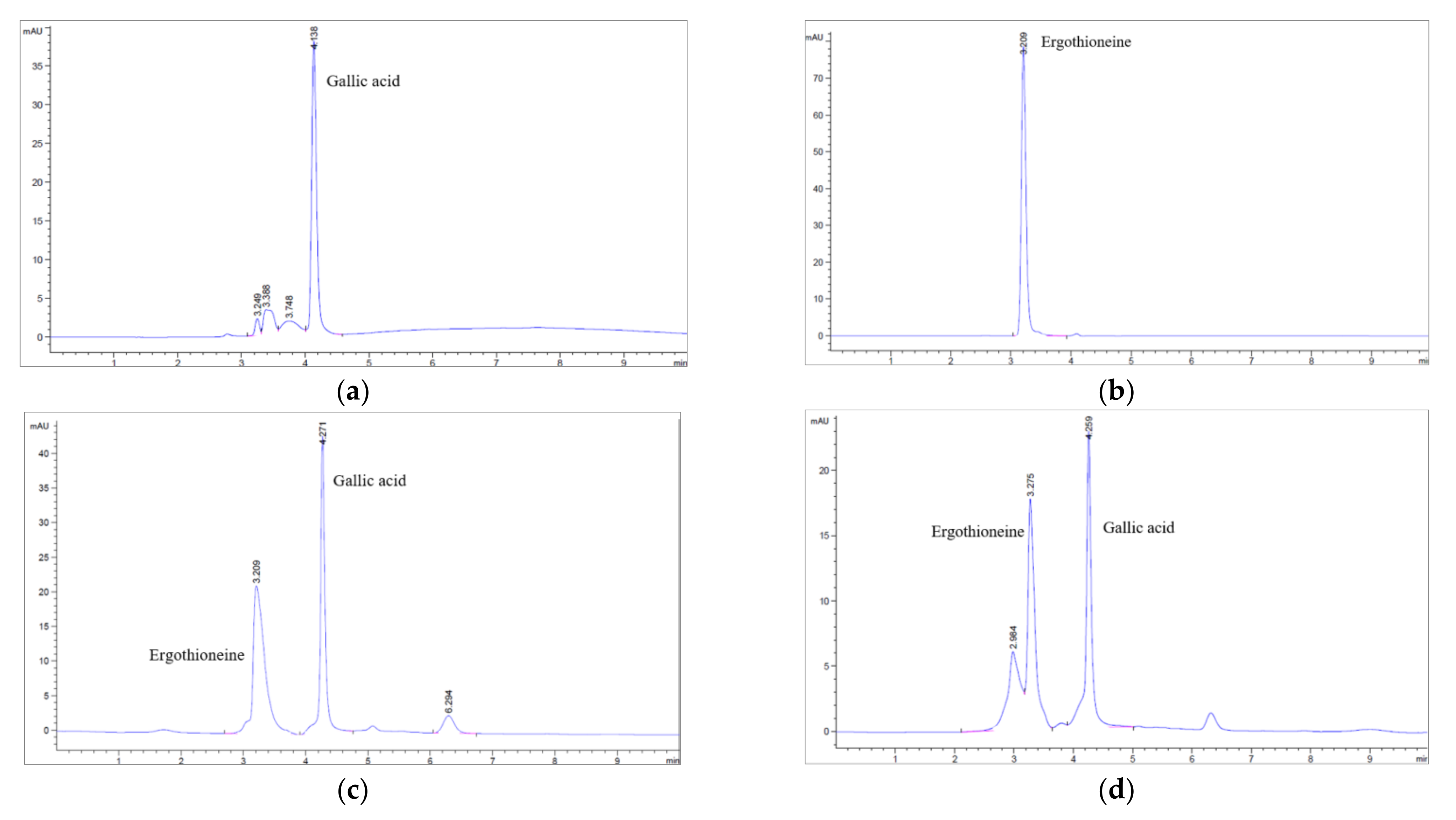
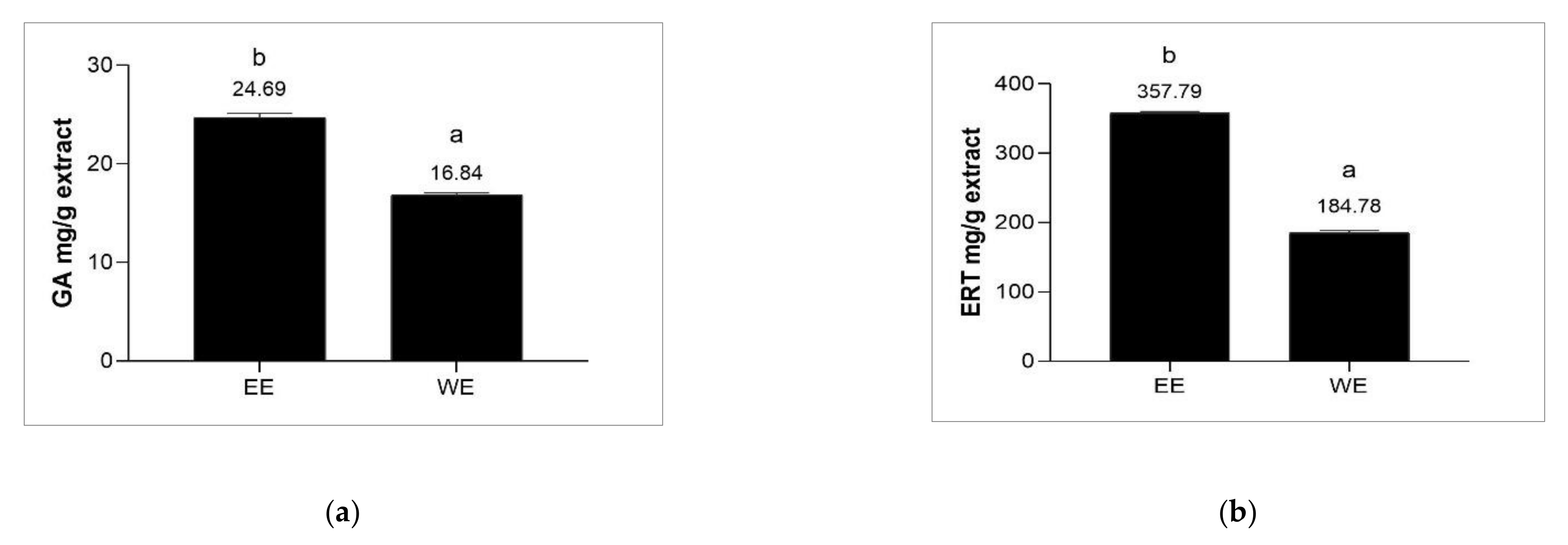
3.3. Determination of Ergothioneine and Gallic Acid Contents of Brown A. bisporus Extracts
3.4. Antioxidant Activity of Brown A. bisporus Extracts
3.5. Determination of Anti-Aging Activities
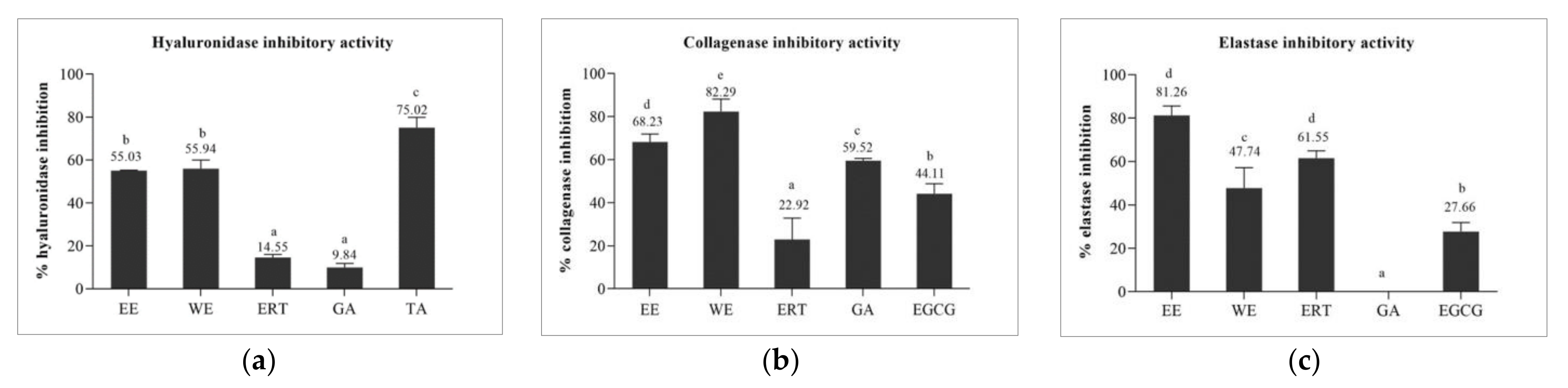
3.5.1. Hyaluronidase Inhibitory Activity of Brown A. bisporus Extracts
3.5.2. Collagenase Inhibitory and Elastase Inhibitory Activities of Brown A. bisporus Extracts
3.6. Cell Culture
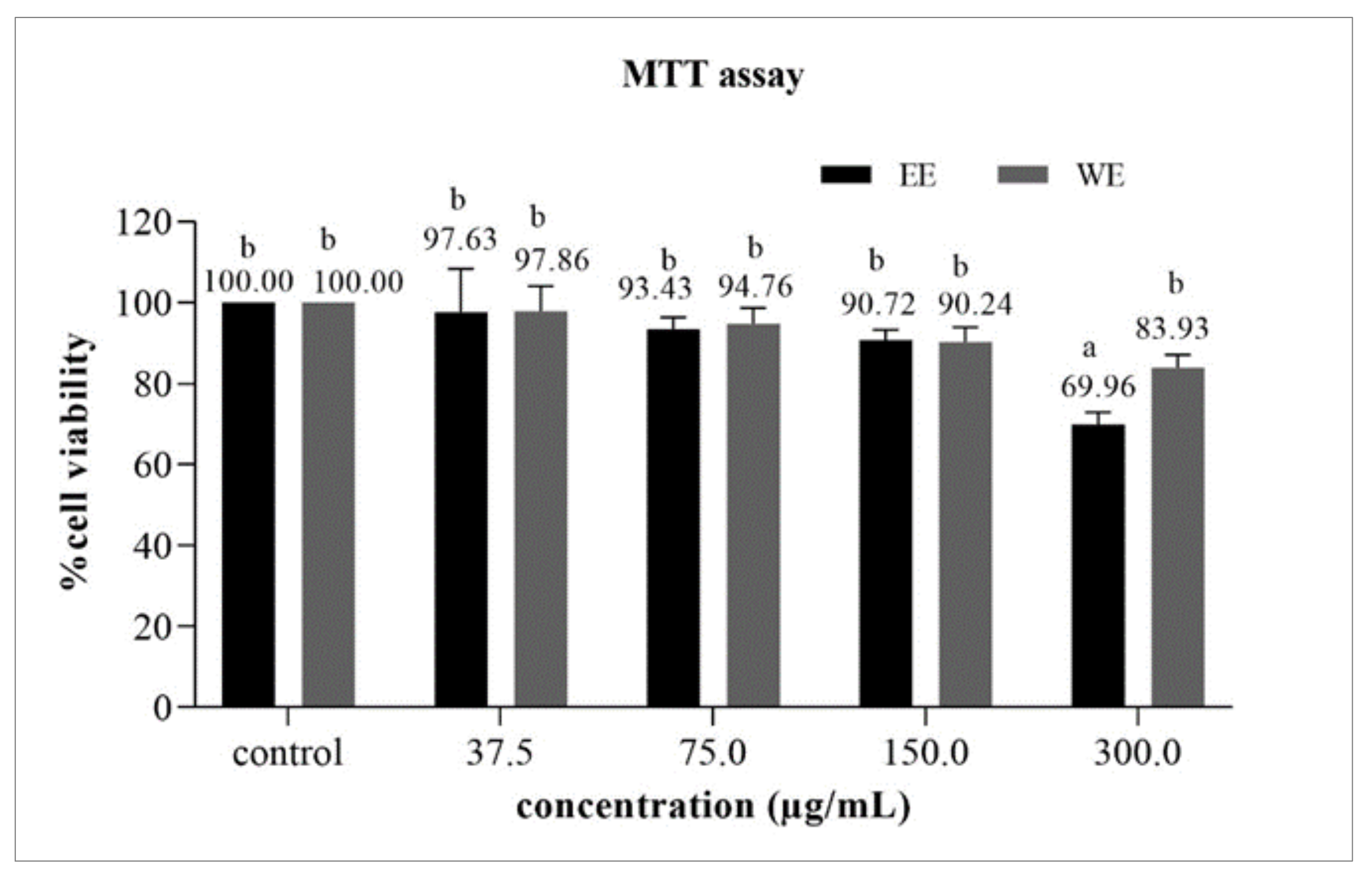
3.6.1. Cytotoxicity Test by MTT Assay
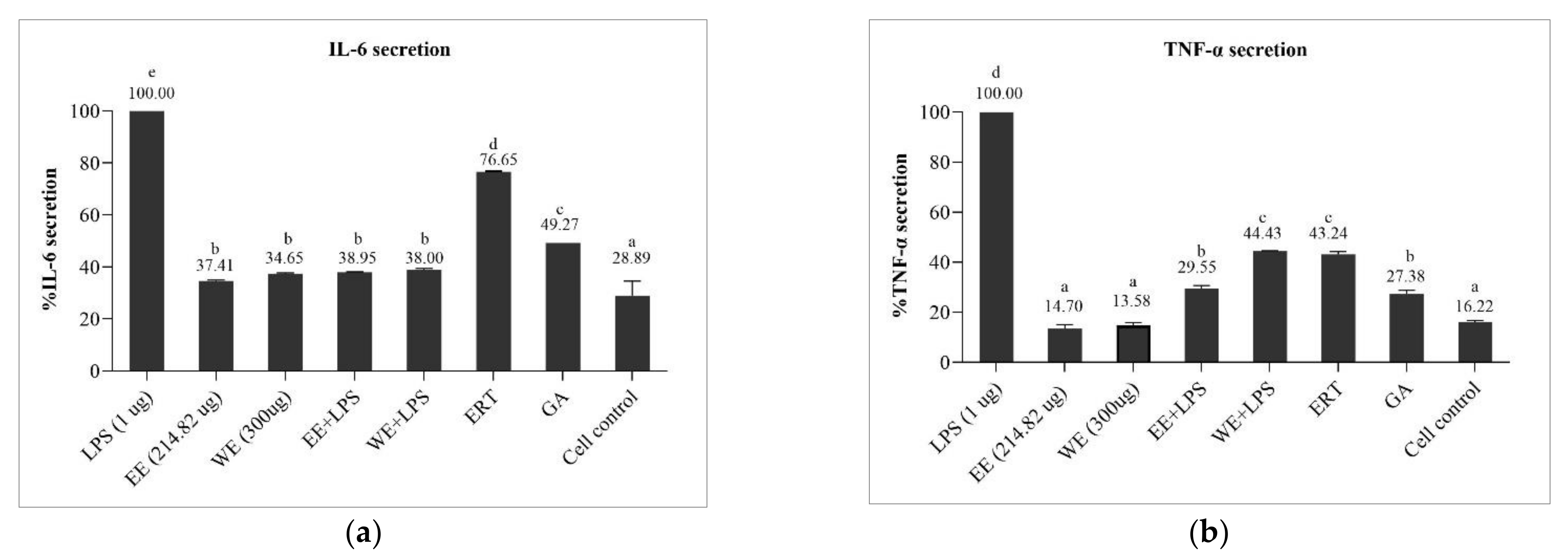
3.6.2. Preventive Effect of Brown A. bisporus Extracts against Inflammation in LPS-Induced HaCaT Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barco, D.; Giménez-Arnau, A. Xerosis: A dysfunction of the epidermal barrier. Actas Dermosifiliogr. (Engl. Ed.) 2008, 99, 671–682. [Google Scholar] [CrossRef]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.; Peng, G.; Yue, H.; Nguyen, L.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, J.; Wang, L.; Li, B.; Guo, J.; Guan, X.; Han, Q.; Zhang, H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-α), IL-6 and IL-1β levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014, 37, 347–354. [Google Scholar] [CrossRef]
- Keshari, S.; Wang, Y.; Herr, D.R.; Wang, S.-M.; Yang, W.-C.; Chuang, T.-H.; Chen, C.-L.; Huang, C.-M. Skin Cutibacterium acnes mediates fermentation to suppress the calcium phosphate-induced itching: A butyric acid derivative with potential for uremic pruritus. J. Clin. Med. 2020, 9, 312. [Google Scholar] [CrossRef]
- Jung, K.; Kim, S.-H.; Joo, K.-M.; Lim, S.-H.; Shin, J.-H.; Roh, J.; Kim, E.; Park, C.W.; Kim, W. Oral intake of enzymatically decomposed AP collagen peptides improves skin moisture and ceramide and natural moisturizing factor contents in the stratum corneum. Nutrients 2021, 13, 4372. [Google Scholar] [CrossRef] [PubMed]
- Oikarinen, A.; Haapasaari, K.; Sutinen, M.; Tasanen, K. The molecular basis of glucocorticoid-induced skin atrophy: Topical glucocorticoid apparently decreases both collagen synthesis and the corresponding collagen mRNA level in human skin in vivo. Br. J. Dermatol. 1998, 139, 1106–1110. [Google Scholar] [CrossRef]
- Waktola, G.; Temesgen, T. Application of mushroom as food and medicine. Biotechnol. Adv. Microbiol 2018, 11, 555817. [Google Scholar] [CrossRef]
- Muszynska, B.; Kala, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and biological properties of Agaricus bisporus fruiting bodies-a review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Gan, C.; Nurul Amira, B.; Asmah, R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). Int. Food Res. J. 2013, 20, 1095–1102. [Google Scholar]
- Hassan, M.J.; Karim, M.M.; Islam, M.A.; Pramanik, M.H.R.; Hossain, M.A. Changes in root porosity and water soluble carbohydrates in rice (Oryza sativa L.) under submergence stress: Responses of rice as under submergence stress. J. Bangladesh Agric. Univ. 2019, 17, 539–544. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Tom, N.; Alnoumani, H.A.; Were, L. Interactions between mushroom powder, sodium chloride, and bovine proteins and their effects on lipid oxidation products and consumer acceptability. Food Sci. Technol. 2018, 98, 219–224. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of stability and transdermal delivery of bioactive compounds in green robusta coffee beans extract loaded nanostructured lipid carriers. J. Nanotechnol. 2018, 2018, 7865024. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of cosmeceutical potentials of selected mushroom fruitbody extracts through evaluation of antioxidant, anti-hyaluronidase and anti-tyrosinase activity. Multidiscip. J. 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Vajrabhaya, L.-O.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009, 107, 42–48. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Merdivan, S.; Bettin, P.; Preisitsch, M.; Lindequist, U. Quality control of medicinal mushrooms: Comparison of different methods for the quantification of polysaccharides/β-glucans. Planta Med. 2014, 80, 17–22. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzym. Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Abugri, D.A.; McElhenney, W.H. Extraction of total phenolic and flavonoids from edible wild and cultivated medicinal mushrooms as affected by different solvents. J. Nat. Prod. Plant Resour. 2013, 3, 37–42. [Google Scholar]
- Mircea, C.; Cioanca, O.; Iancu, C.; Tataringa, G.; Hancianu, M. In vitro antioxidant activity of some extracts obtained from Agaricus bisporus brown, Pleurotus ostreatus and Fomes fomentarius. Farmacia 2015, 63, 927–933. [Google Scholar]
- Cheung, B.; Kwan, M.; Chan, R.; Sea, M.; Woo, J. Potential of Asian natural products for health in aging. Mol. Basis Nutr. Aging 2016, 2016, 659–676. [Google Scholar]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef]
- Ferrari, G.P.; Soares, A.A.; Bazanella, G.D.S.; Bracht, A.; Souza, C.D.; Boer, C.G.; Peralta, R. Antioxidant properties of the most common edible mushrooms consumed in Brazil. In Mushrooms: Types, Properties and Nutrition, 1st ed.; Andres, S., Baumann, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 285–297. [Google Scholar]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.-P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 1–7. [Google Scholar] [CrossRef]
- Tyner, H.; Patel, R. Hyaluronidase in clinical isolates of Propionibacterium acnes. Int. J. Bacteriol. 2015, 2015, 18918. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Goldstein, B.S.; Leotta, D.F. Skin response to mechanical stress: Adaptation rather than breakdown—A review of the literature. J. Rehabil. Res. Dev. 1995, 32, 214. [Google Scholar] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Szymański, Ł.; Cios, A.; Ciepielak, M.; Stankiewicz, W. Cytokines and apoptosis in atopic dermatitis. Adv. Dermatol. Allergol. Postępy Dermatol. Alergol. 2021, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Um, J.-Y.; Cho, J.-S.; Lee, S.H.; Lee, S.H.; Lee, H.-M. Histamine promotes the release of interleukin-6 via the H1R/p38 and NF-κB pathways in nasal fibroblasts. Allergy Asthma Immunol. Res. 2014, 6, 567–572. [Google Scholar] [CrossRef]
- Hu, L.; Mauro, T.M.; Dang, E.; Man, G.; Zhang, J.; Lee, D.; Wang, G.; Feingold, K.R.; Elias, P.M.; Man, M.-Q. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J. Investig. Dermatol. 2017, 137, 1277–1285. [Google Scholar] [CrossRef]

| Brown A. bisporus Extracts | Yield (%) |
|---|---|
| EE | 2.64 ± 1.39 |
| WE | 73.89 ± 3.22 |
| Brown A. bisporus Extracts | Total Polysaccharides Content (mg Glucose/g Extract) | Total Phenolics Content (mg GAE/g Extract) | Total Flavonoids Content (mg QE/g Extract) |
|---|---|---|---|
| EE | 370.00 ± 0.30 a | 184.87 ± 0.10 a | 16.11 ± 0.21 a |
| WE | 734.00 ± 0.03 b | 190.90 ± 0.07 a | 12.92 ± 0.02 a |
| Brown A. bisporus Extracts | DPPH IC50 (mg/mL) | ABTS TEAC Value (µM Trolox/g Extract) | FRAP FRAP Value (mg FeSO4/g Extract) |
|---|---|---|---|
| EE | 0.30 ± 0.04 b | 8.06 ± 0.08 a | 390.50 ± 0.32 b |
| WE | 1.22 ± 0.82 c | 4.59 ± 0.34 a | 124.36 ± 0.77 a |
| Standards | |||
| Ergothioneine | 0.01 ± 0.05 a | 125.64 ± 0.69 b | 646.36 ± 0.06 d |
| Gallic acid | 0.02 ± 0.02 a | 493.46 ± 0.01 c | 544.53 ± 0.12 c |
| Trolox | 0.02 ± 0.08 a | - | 481.81 ± 0.20 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitthikan, N.; Leelapornpisid, P.; Naksuriya, O.; Intasai, N.; Kiattisin, K. Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment. Sci. Pharm. 2022, 90, 59. https://doi.org/10.3390/scipharm90040059
Nitthikan N, Leelapornpisid P, Naksuriya O, Intasai N, Kiattisin K. Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment. Scientia Pharmaceutica. 2022; 90(4):59. https://doi.org/10.3390/scipharm90040059
Chicago/Turabian StyleNitthikan, Nichcha, Pimporn Leelapornpisid, Ornchuma Naksuriya, Nutjeera Intasai, and Kanokwan Kiattisin. 2022. "Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment" Scientia Pharmaceutica 90, no. 4: 59. https://doi.org/10.3390/scipharm90040059
APA StyleNitthikan, N., Leelapornpisid, P., Naksuriya, O., Intasai, N., & Kiattisin, K. (2022). Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment. Scientia Pharmaceutica, 90(4), 59. https://doi.org/10.3390/scipharm90040059