Retinoic Acid Potentiates the Therapeutic Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) against Cisplatin-Induced Hepatotoxicity in Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Drugs and Chemicals
2.2. Experimental Animals and Study Design
2.3. Isolation, Expansion and Characterization of Rat BM-MSCs
2.4. Evaluation of Oxidative Stress Marker
2.5. Evaluation of Antioxidant Activities
2.6. Gene Expression by Real-Time Polymerase Chain Reaction
2.7. Histopathology and Caspase-3, p53,Bcl2 and IL1beta Immunohistochemistry
2.8. Statistical Analysis
3. Results
3.1. Effect of BM-MSCs and RA and Combination of Both on Liver Functions in Cis-Induced Hepatotoxicity
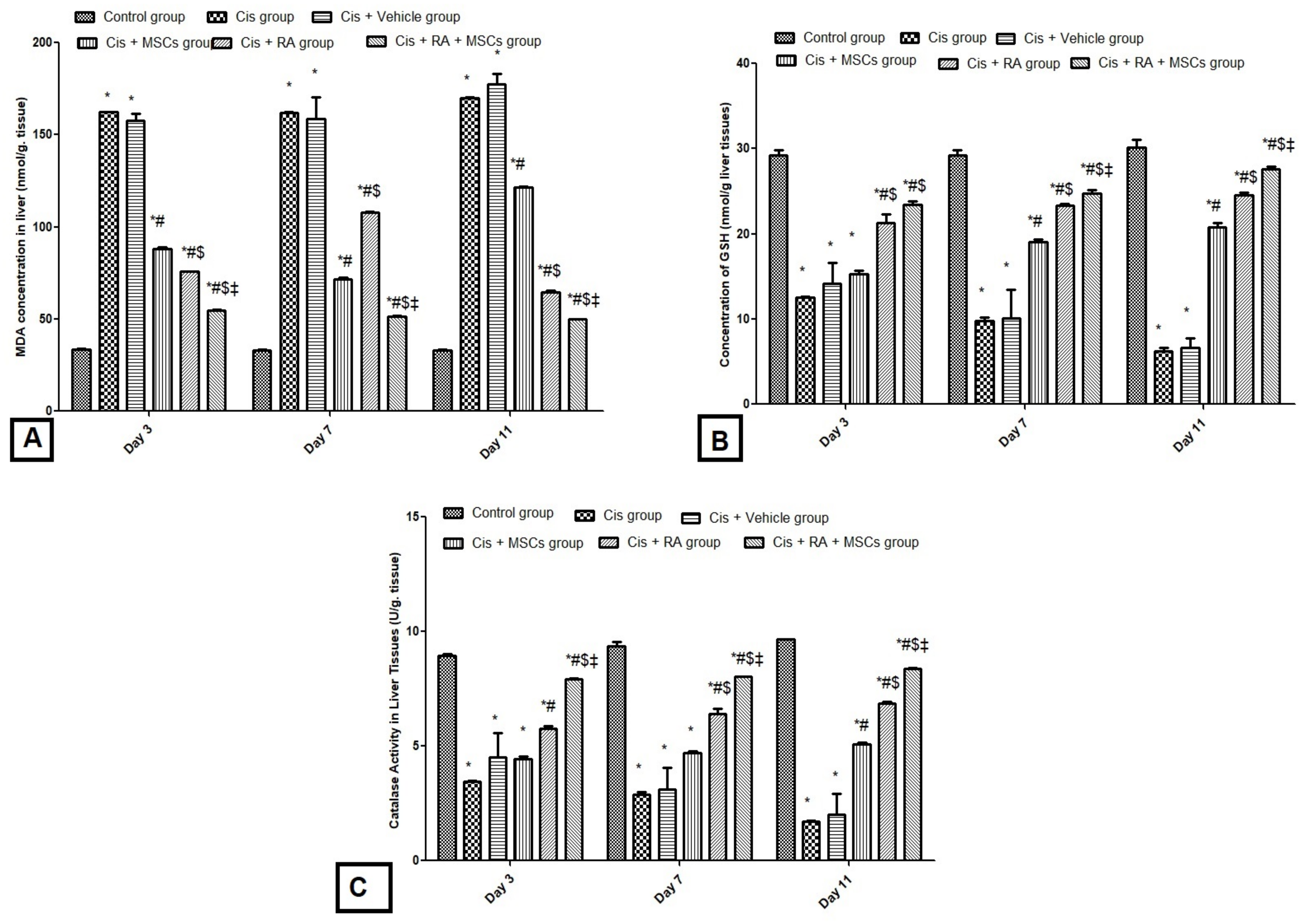
3.2. Effect of BM-MSCs and RA and Combination of Both on Oxidative Stress Markers (GSH, CAT and MDA) in Cis-Induced Hepatotoxicity
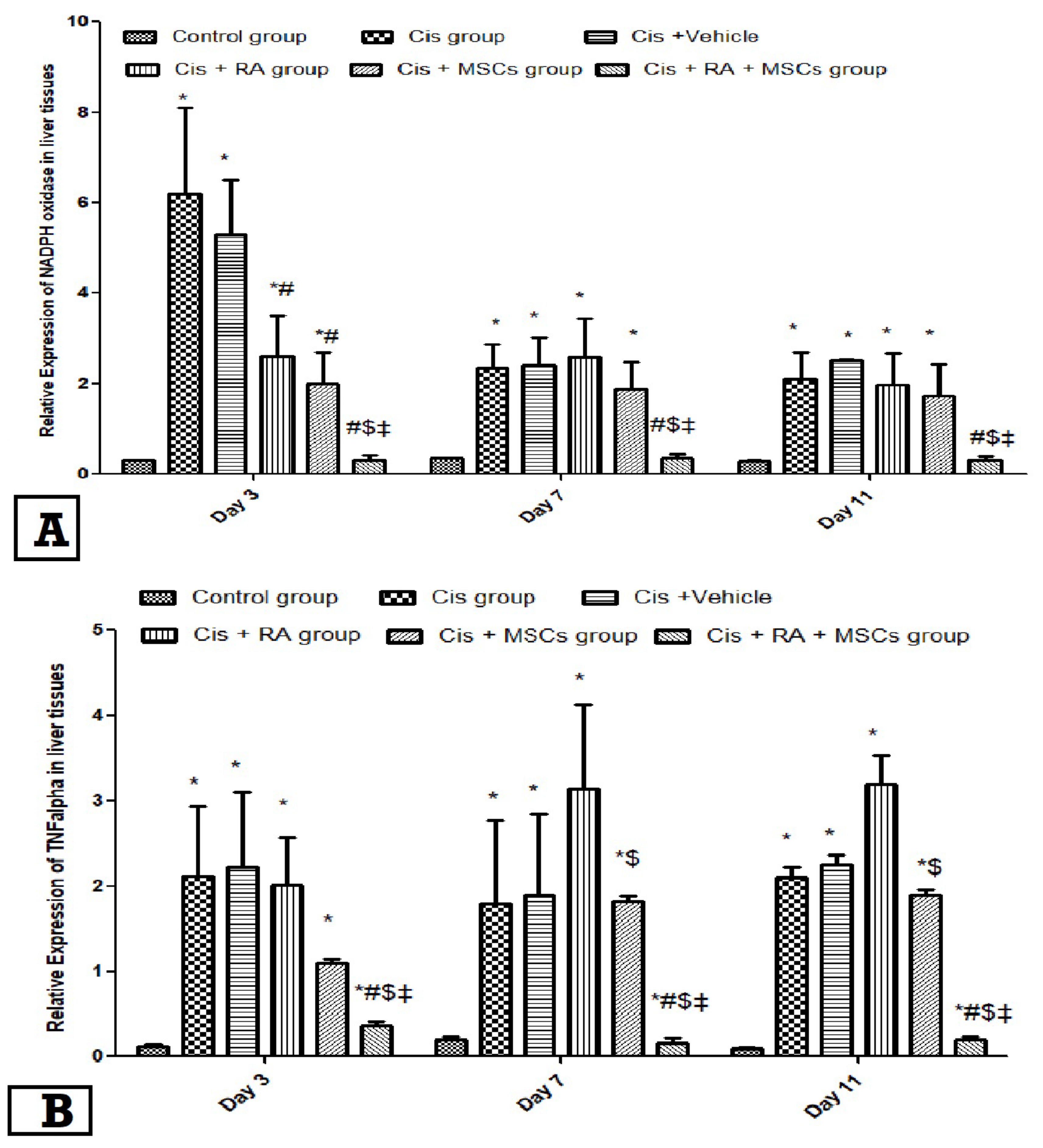
3.3. Effect of BM-MSCs and RA and Combination of Both on NADPH Oxidase and TNF-α Expression in Cis-Induced Hepatotoxicity
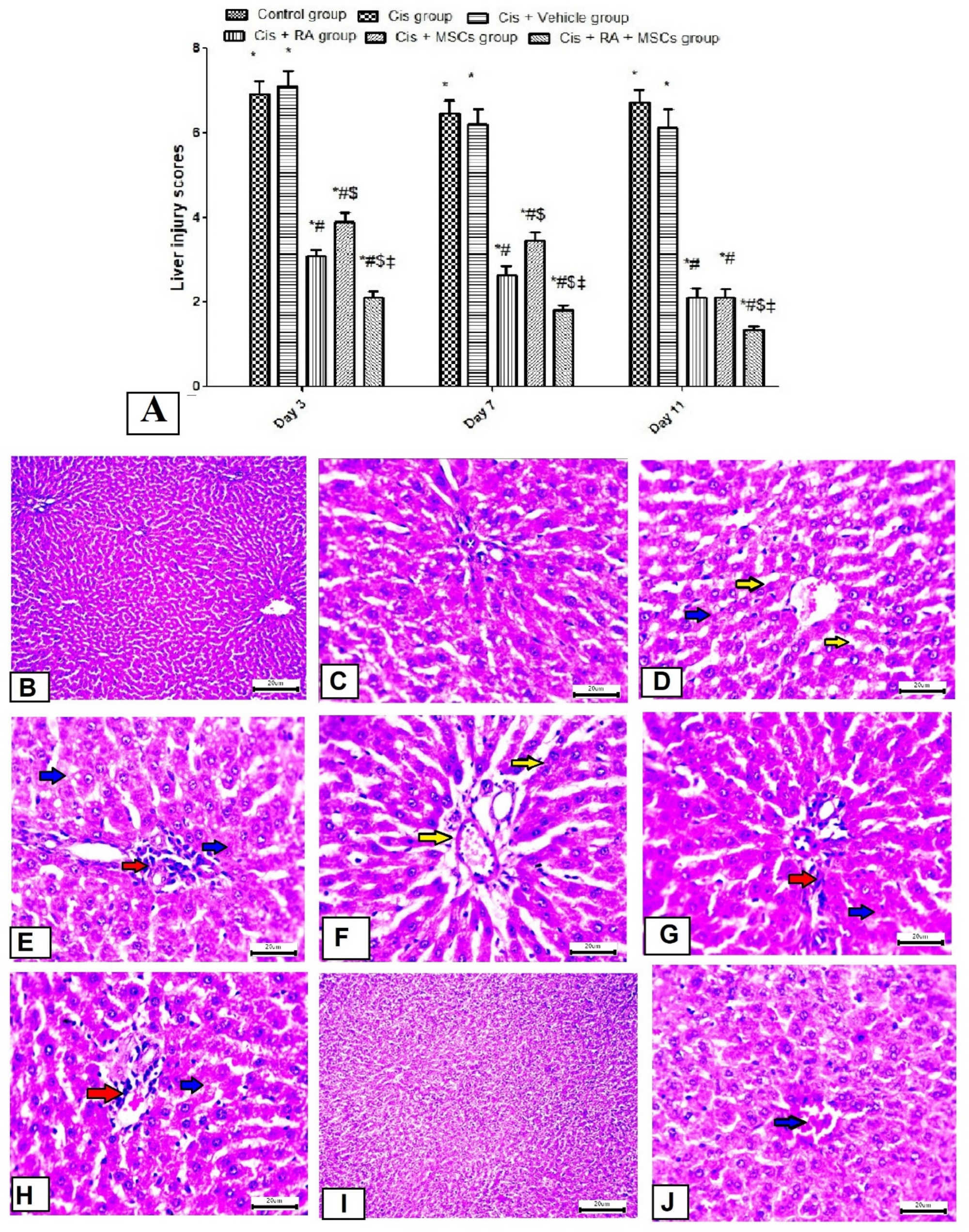
3.4. Effect of BM-MSCs and RA and Combination of Both on Liver Morphology in Cis-Induced Hepatotoxicity
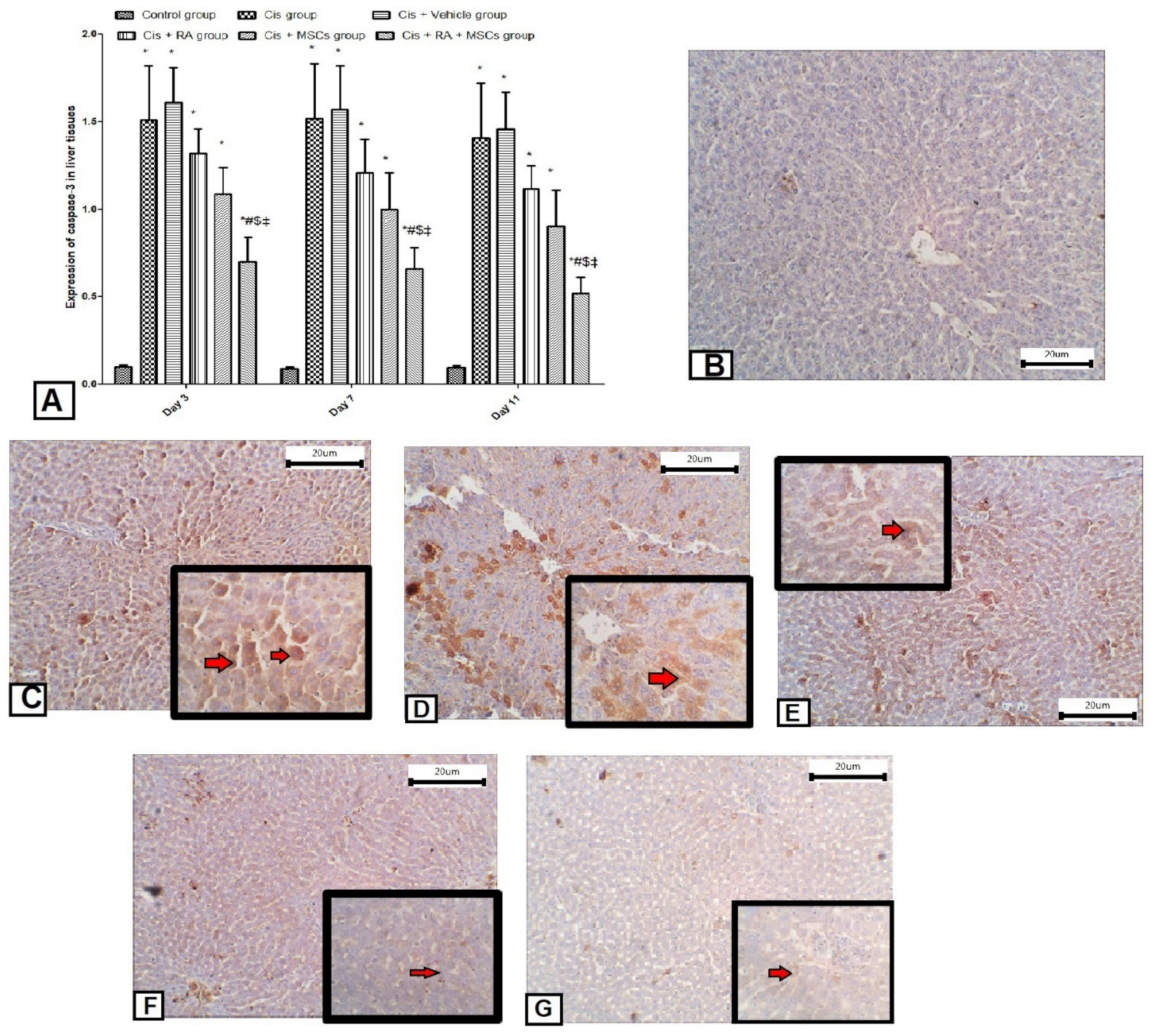
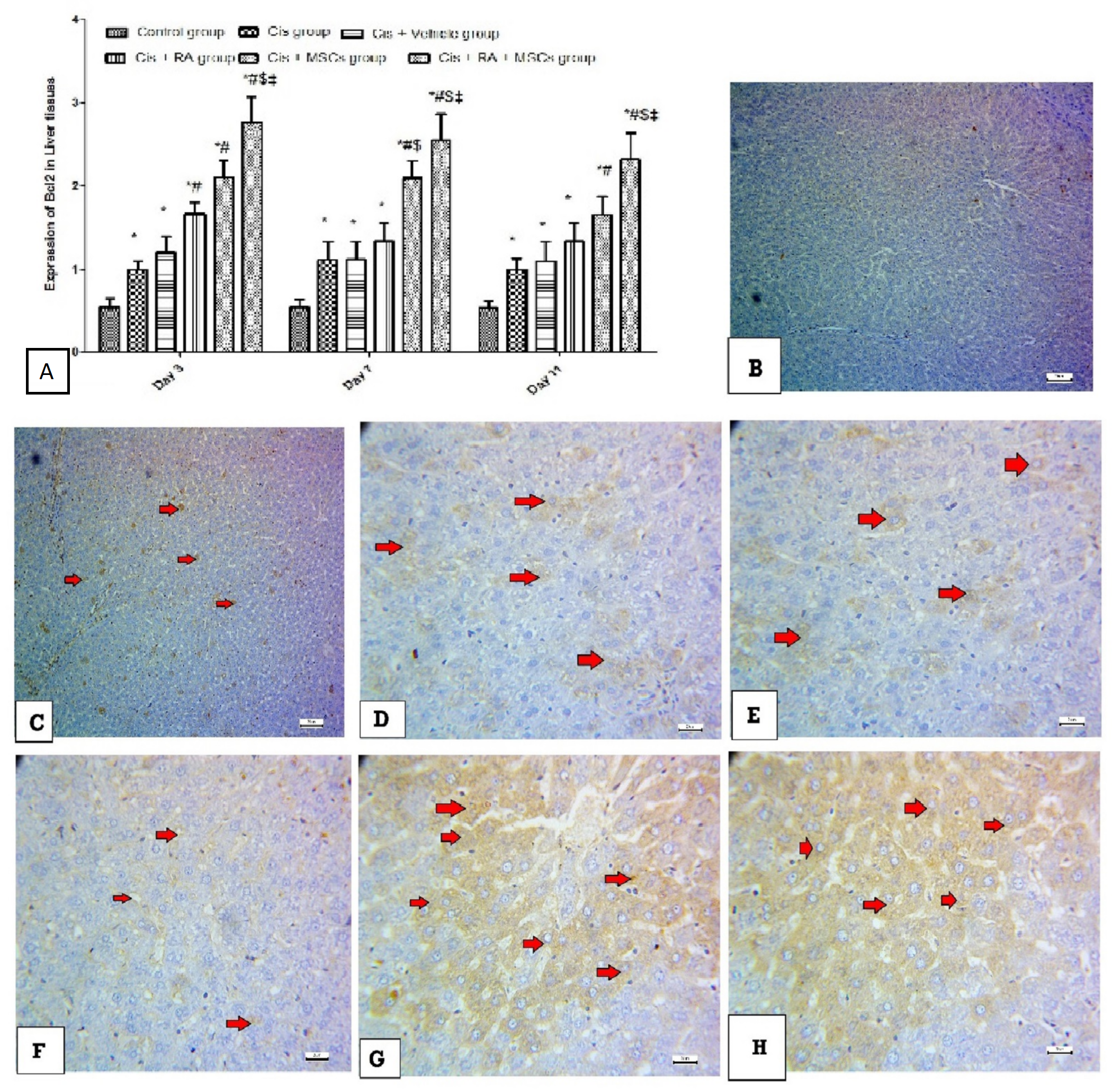
3.5. Effect of BM-MSCs and RA and Combination of Both on Apoptotic Markers (Caspase-3, p53 and Bcl2) in Cis-Induced Hepatotoxicity
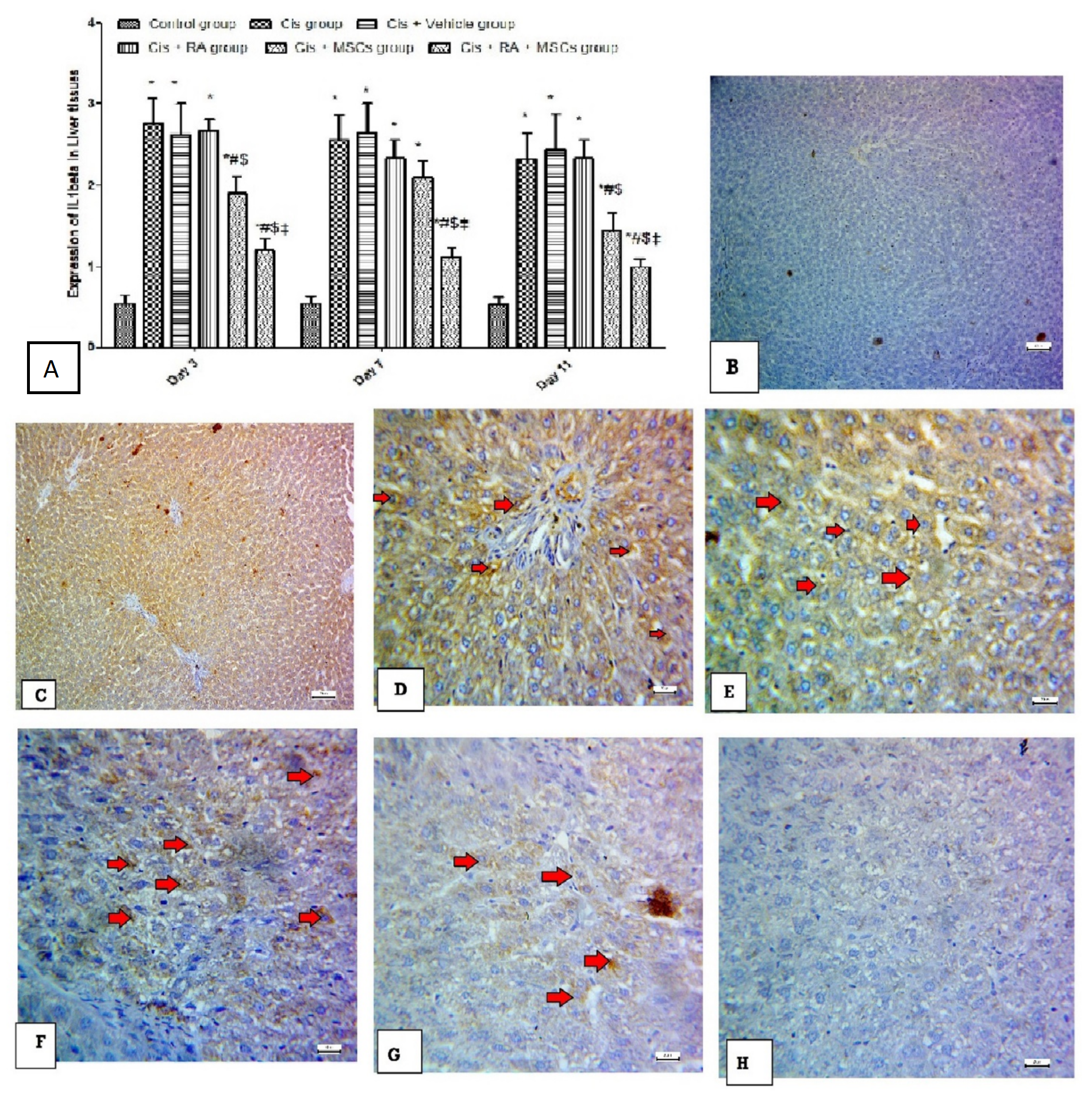
3.6. Effect of BM-MSCs and RA and Combination of Both on Inflammatory Marker (IL-1Beta) in Cis-Induced Hepatotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, S.L. Liver fibrosis–from bench to bedside. J. Hepatol. 2003, 38, 38–53. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.Y.; Najjar, T.A.; Alashari, M. Role of non-selective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Cure, M.C.; Cüre, E.; Kalkan, Y.; Kirbas, A.; Tumkaya, L.; Yilmaz, A.; Turkyilmaz, A.K.; Sehitoglu, I.; Yuce, S. Infliximab modulates cisplatin-induced hepatotoxicity in rats. Balk. Med. J. 2016, 33, 504. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Punsawad, C.; Koomhin, P.; Suwannalert, P. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complementary Altern. Med. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Parvez, S. Mitochondrial dysfunction mediated cisplatin induced toxicity: Modulatory role of curcumin. Food Chem. Toxicol. 2013, 53, 334–342. [Google Scholar] [CrossRef]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef]
- Pan, X.; Dai, Y.; Li, X.; Niu, N.; Li, W.; Liu, F.; Zhao, Y.; Yu, Z. Inhibition of arsenic induced-rat liver injury by grape seed exact through suppression of NADPH oxidase and TGF-β/Smad activation. Toxicol. Appl. Pharmacol. 2011, 254, 323–331. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.W.; Jung, Y.S.; Kwon, D.Y.; Park, H.K.; Ryu, C.S.; Kim, S.K.; Oh, G.T.; Kim, Y.C. Ethanol-induced liver injury and changes in sulfur amino acid metabolomics in glutathione peroxidase and catalase double knockout mice. J. Hepatol. 2009, 50, 1184–1191. [Google Scholar] [CrossRef]
- Tsai, P.C.; Fu, T.W.; Chen, Y.M.A.; Ko, T.L.; Chen, T.H.; Shih, Y.H.; Hung, S.-C.; Fu, Y.-C. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transplant. 2009, 15, 484–495. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.-S.; Verfaillie, C.M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Investig. 2002, 109, 1291–1302. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009. [Google Scholar] [CrossRef]
- Venkataramana, N.K.; Kumar, S.K.; Balaraju, S.; Radhakrishnan, R.C.; Bansal, A.; Dixit, A.; Rao, D.K.; Das, M.; Jan, M.; Gupta, P.K.; et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010, 155, 62–70. [Google Scholar] [CrossRef]
- Elwakeel, E.E.; Mohamed, A.Z. The Hepatotoxic Effect Induced by Methotrexate Therapy and Protective Role of Bone Marrow-Derived Mesenchymal Stem Cells in Adult Male Albino Rats. Histological and Ultrastructural Study. J. Am. Sci. 2018, 14, 91–98. [Google Scholar]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 213–221. [Google Scholar] [CrossRef]
- Noy, N. Between death and survival: Retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 2010, 30, 201–217. [Google Scholar] [CrossRef]
- Sarhan, M.; El Serougy, H.; Hussein, A.M.; El-Dosoky, M.; Sobh, M.A.; Fouad, S.A.; ElHusseini, F.; Sobh, M. Impact of bone-marrow-derived mesenchymal stem cells on adriamycin-induced chronic nephropathy. Can. J. Physiol. Pharmacol. 2014, 92, 733–743. [Google Scholar] [CrossRef]
- Ying, M.; Wang, S.; Sang, Y.; Sun, P.; Lal, B.; Goodwin, C.R.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Laterra, J.; Xia, S. Regulation of glioblastoma stem cells by retinoic acid: Role for Notch pathway inhibition. Oncogene 2011, 30, 3454–3467. [Google Scholar] [CrossRef]
- Ewees, M.G.; Abdelghany, T.M.; Abdel-Aziz, A.-A.H.; Abdel-Bakky, M.S. All-trans retinoic acid mitigates methotrexate-induced liver injury in rats; relevance of retinoic acid signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 931–938. [Google Scholar] [CrossRef]
- Hong, S.H.; Gang, E.J.; Jeong, J.A.; Ahn, C.; Hwang, S.H.; Yang, I.H.; Park, H.K.; Han, H.; Kim, H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem. Biophys. Res. Commun. 2005, 330, 1153–1161. [Google Scholar] [CrossRef]
- Seo, M.J.; Suh, S.Y.; Bae, Y.C.; Jung, J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 328, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, J.; Zhang, B.-Q.; Zhang, H.; Bi, Y.; Kang, Q.; Wang, N.; Bie, P.; Yang, Z.; Wang, H.; et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J. Biomed. Mater. Res. Part A 2014, 102, 1017–1025. [Google Scholar] [CrossRef]
- Çayır, K.; Karadeniz, A.; Şimşek, N.; Yıldırım, S.; Karakuş, E.; Kara, A.; Akkoyun, H.T.; Sengul, E. Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J. Med. Food 2011, 14, 1254–1262. [Google Scholar] [CrossRef]
- Huang, S.; Xu, L.; Sun, Y.; Wu, T.; Wang, K.; Li, G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J. Orthop. Transl. 2015, 3, 26–33. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Hassan, M.H.; Al-Gayyar, M.; El-Gayar, M.A.; Ibrahim, T. Effect of simvastatin on inflammatory cytokines balance in air pouch granuloma model. Inflamm. Allergy Drug Targets 2014, 13, 74–79. [Google Scholar] [CrossRef]
- Alsheblak, M.M.; Elsherbiny, N.; El-Karef, A.; El-Shishtawy, M.M. Protective effects of L-carnosine on CCl4-induced hepatic injury in rats. Eur. Cytokine Netw. 2016, 27, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Abbasi, M.M.; Hassanalilou, T.; Khordadmehr, M.; Vardin, A.M.; Kohlan, A.B.; Khalili, L. Effects of Cornus mas Fruit Hydro-Methanolic Extract on Liver Antioxidants and Histopathologic Changes Induced by Cisplatin in Rats. Indian J. Clin. Biochem. 2019, 35, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, Y.; Akbulut, S.; Aksungur, Z.; Erdemli, M.E.; Ozhan, O.; Parlakpinar, H.; Vardi, N.; Turkoz, Y. Protective effect of dexpanthenol against cisplatin-induced hepatotoxicity. Exp. Ther. Med. 2018, 16, 4049–4057. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.; Arab, H.; Arafa, E.-S. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: Targeting MAPKs and apoptosis. PLoS ONE 2016, 11, e0151649. [Google Scholar] [CrossRef]
- Hassan, H.M.; Al-Wahaibi, L.H.; A Elmorsy, M.; Mahran, Y.F. Suppression of cisplatin-induced hepatic injury in rats through alarmin high-mobility group box-1 pathway by Ganoderma lucidum: Theoretical and experimental study. Drug Des. Dev. Ther. 2020, 14, 2335. [Google Scholar] [CrossRef]
- Niu, C.; Ma, M.; Han, X.; Li, H.; Wang, Z. Hyperin protects against cisplatin-induced liver injury in mice1. Acta Cir. Bras. 2017, 32, 633–640. [Google Scholar] [CrossRef]
- Ko, J.-W.; Lee, I.-C.; Park, S.-H.; Moon, C.; Kang, S.-S.; Kim, S.-H.; Kim, J.-C. Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats. Lab. Anim. Res. 2014, 30, 174–180. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; Sayed, A. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complementary Altern. Med. 2014, 14, 282. [Google Scholar] [CrossRef]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.; E Dikalova, A.; Sohal, R.S.; E Hatch, G.; Jones, D.P.; Mason, R.P.; Barrett, J. Biomarkers of oxidative stress study: Are plasma antioxidants markers of CCl4 poisoning? Free Radic. Biol. Med. 2000, 28, 838–845. [Google Scholar] [CrossRef]
- Moustafa, F.; Sobh, M.-A.; Abouelkheir, M.; Khater, Y.; Mahmoud, K.; Saad, M.-A. Study of the effect of route of administration of mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague Dawley rats. Int. J. Stem Cells 2016, 9, 79. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Qin, X.; Cheng, J.; He, Y.; Jiang, Y. NADPH oxidase signaling pathway mediates mesenchymal stem cell-induced inhibition of hepatic stellate cell activation. Stem Cells Int. 2018, 2018, 1239143. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor B pathway in inflammation. Inflamm. Biol. Group 2009, 1, a001651. [Google Scholar] [CrossRef]
- Freyman, T.; Polin, G.; Osman, H.; Crary, J.; Lu, M.; Cheng, L.; Palasis, M.; Wilensky, R. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006, 27, 1114–1122. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Coffey, J.W.; Sullivan, A.C. Inflammation and Collagenase Production in Rats with Adjuvant Arthritis Reduced with 13- cis -Retinoic Acid. Science 1983, 221, 756–758. [Google Scholar] [CrossRef]
- Orfanos, C.; Bauer, R. Evidence for anti-inflammatory activities of oral synthetic retinoids: Experimental findings and clinical experience. Br. J. Dermatol. 1983, 109, 55–60. [Google Scholar]
- Mehta, K.; McQueen, T.; Tucker, S.; Pandita, R.; Aggarwal, B. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J. Leukoc. Biol. 1994, 55, 336–342. [Google Scholar] [CrossRef]
- Motomura, K.; Sakai, H.; Isobe, H.; Nawata, H. Effects of retinoids on the production of tumour necrosis factor-alpha and nitric oxide by lipopolysaccharide-stimulated rat Kupffer cells in vitro: Evidence for participation of retinoid X receptor signalling pathway. Cell Biochem. Funct. 1997, 15, 95–101. [Google Scholar] [CrossRef]
- Eckle, V.-S.; Buchmann, A.; Bursch, W.; Schulte-Hermann, R.; Schwarz, M. Immunohistochemical detection of activated caspases in apoptotic hepatocytes in rat liver. Toxicol. Pathol. 2004, 32, 9–15. [Google Scholar] [CrossRef]
- Berardis, S.; Sattwika, P.D.; Najimi, M.; Sokal, E. Use of mesenchymal stem cells to treat liver fibrosis: Current situation and future prospects. World J. Gastroenterol. 2015, 21, 742. [Google Scholar] [CrossRef] [PubMed]
- Kallis, Y.N.; Alison, M.R.; Forbes, S.J. Bone marrow stem cells and liver disease. Gut 2007, 56, 716–724. [Google Scholar] [CrossRef]
- Alisi, A.; Leoni, S.; Piacentani, A.; Devirgiliis, L.C. Retinoic acid modulates the cell-cycle in fetal rat hepatocytes and HepG2 cells by regulating cyclin-cdk activities. Liver Int. 2003, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tonge, P.D.; Andrews, P.W. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation 2010, 80, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Bauerbach, E.; Plath, M.; Steuber, M.; Heers, C.; Tegtmeier, F.; Krieglstein, J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free. Radic. Biol. Med. 2001, 30, 1067–1077. [Google Scholar] [CrossRef]



| Accession No. | Primer Sequence | Product Length | Annealing Temperature | |
|---|---|---|---|---|
| GAPDH | NM_053524.1 | F: TGCCACTCAGAAGACTGTGG | 85 | 59 |
| R: GGATGCAGGGATGATGTTCT | ||||
| TNF-α | NM_012675.3 | F: TCTTCAAGGGACAAGGCTGC | 104 | 60 |
| R: CTTGATGGCAGAGAGGAGGC | ||||
| NADPH | NM_017008.4 | F: TGTTGGGCCTAGGATTGTGT | 119 | 60 |
| R: CTTCTGTGATCCGCGAAGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzam, M.M.; Hussein, A.M.; Marghani, B.H.; Barakat, N.M.; Khedr, M.M.M.; Heakel, N.A. Retinoic Acid Potentiates the Therapeutic Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) against Cisplatin-Induced Hepatotoxicity in Rats. Sci. Pharm. 2022, 90, 58. https://doi.org/10.3390/scipharm90040058
Azzam MM, Hussein AM, Marghani BH, Barakat NM, Khedr MMM, Heakel NA. Retinoic Acid Potentiates the Therapeutic Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) against Cisplatin-Induced Hepatotoxicity in Rats. Scientia Pharmaceutica. 2022; 90(4):58. https://doi.org/10.3390/scipharm90040058
Chicago/Turabian StyleAzzam, Maha M., Abdelaziz M. Hussein, Basma H. Marghani, Nashwa M. Barakat, Mohsen M. M. Khedr, and Nabil Abu Heakel. 2022. "Retinoic Acid Potentiates the Therapeutic Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) against Cisplatin-Induced Hepatotoxicity in Rats" Scientia Pharmaceutica 90, no. 4: 58. https://doi.org/10.3390/scipharm90040058
APA StyleAzzam, M. M., Hussein, A. M., Marghani, B. H., Barakat, N. M., Khedr, M. M. M., & Heakel, N. A. (2022). Retinoic Acid Potentiates the Therapeutic Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) against Cisplatin-Induced Hepatotoxicity in Rats. Scientia Pharmaceutica, 90(4), 58. https://doi.org/10.3390/scipharm90040058








