Molecularly-Imprinted SERS: A Potential Method for Bioanalysis
Abstract
1. Introduction
2. Molecularly Imprinted Polymer (MIP)
2.1. Advantages and Limitations of MIP
2.2. Component of MIP
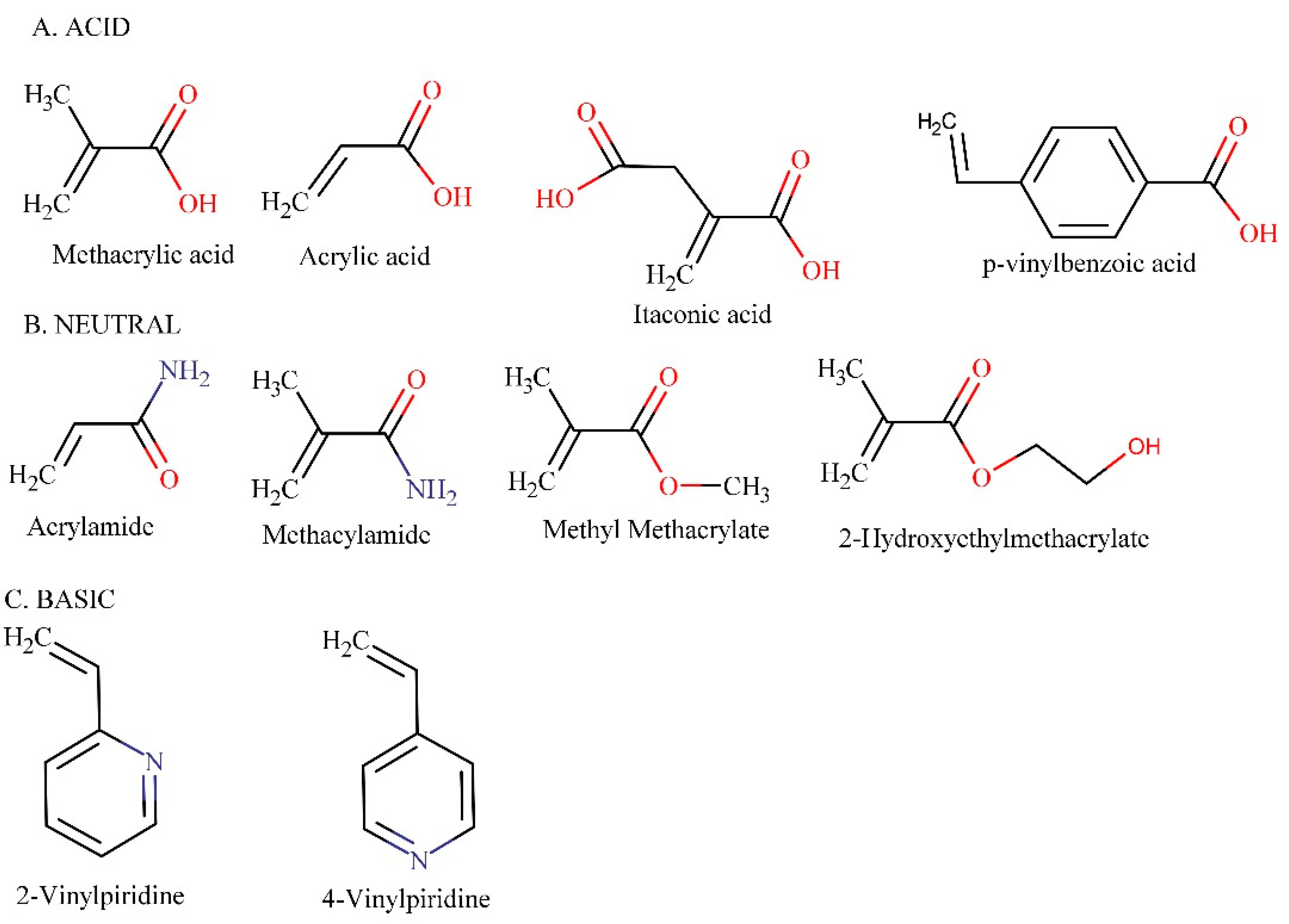
2.2.1. Functional Monomer
2.2.2. Templates
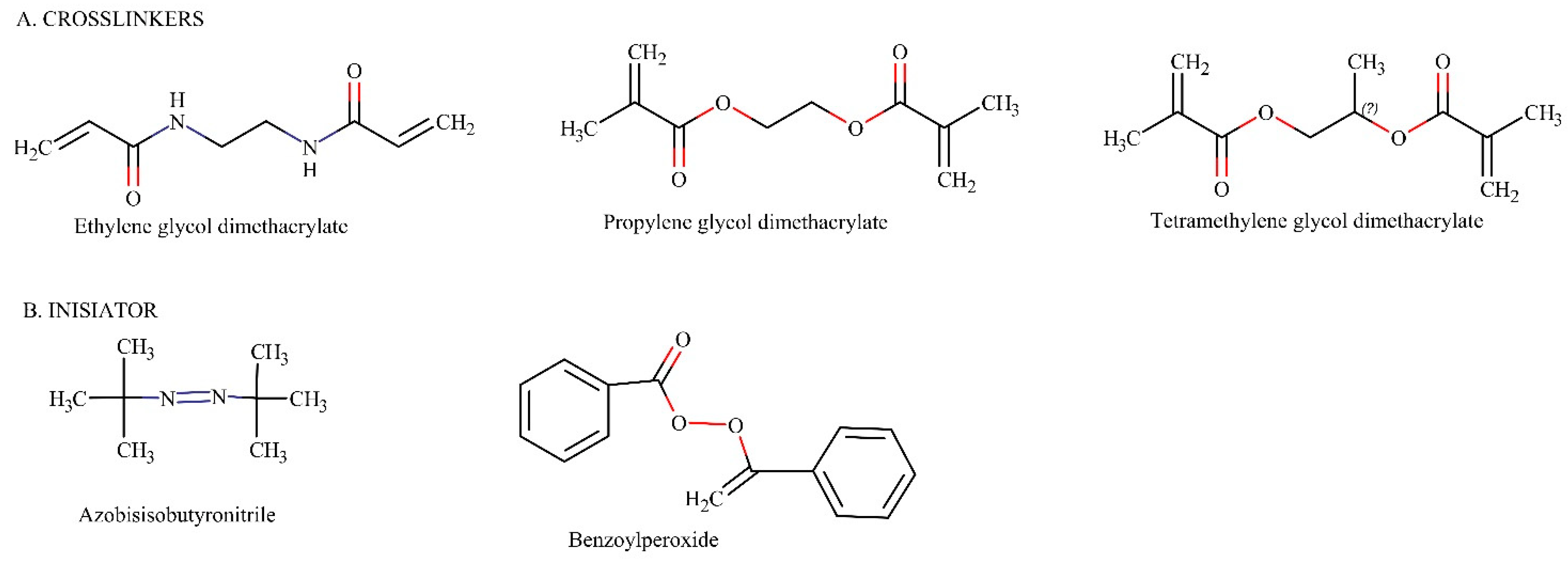
2.2.3. Crosslinkers
2.2.4. Initiator
2.3. Rational Study of MIP Synthesis
2.4. MIP Application in Analytical Chemistry
3. Surface-Enhanced Raman Spectroscopy
4. Molecularly-Imprinted SERS Methods
| No. | Chemical/Biological Compounds | Samples | Methods | Noble Metal | Functional Monomer (FM) | Template | Crosslinker | Rational Study | Analytical Performance | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Bitertanol | Food | MIPSERS | Au | MAA (methacrylic acid) | Triamedifon (dummy template) | Trimethylolpropane trimethacrylate (TRIM) | ND | LOD Cucumber: 0.041 mg/kg Peach: 0.029 mg/kg | [27] |
| 2. | Benzimidazole | Preliminary study | MIPSERS | Ag | MAM | Carbendazime (dummy) | EDGMA | ND | LOD: 1.0 × 10−8 mol/L | [28] |
| 3. | Caffeine | Wastewatere | MIPSERS | Ag | MAA | Theophylline (dummy) | EGDMA | ND | LOD: 100 ng/L | [50] |
| 4. | 2,6-dichlorophenol | Water | SGA MIP SERS | Au | MAA and AM | 2,6-dichlorophenol | EGDMA | ND | LOD: 200 nmol/L | [56] |
| 5. | Enrofloxacin hydrochloride | Water | AgMIM SERS | Ag | AM | Enrofloxacin hydrochloride | EGDMA | ND | LOD: 10−7 mol/L | [71] |
| 6. | Triazine fungicide | Rice and wheats | MIPSERS | Au | MAA | Prometryn and Simetryn | Trimethylopropane trimethacrylate (TRIM) | ND | Recoveries: 72.7–90.0% | [118] |
| 7. | Patulin | Fruits | MIPSERS | Au | 4-vinylpiridine (VP) | Patulin | 1,4-Diacryloylpiperazine (PDA) | ND | LOD: 5.67 × 10−12 M | [119] |
| 8. | Bisphenol A | Tap water | MIPSERS | Ag | 4-vinylpiridine (VP) | Bisphenol A | EDGMA | ND | LOD: 1 × 10−9 mol/L | [120] |
| 9. | Rhodamin 6G | Water | ZOAMIPSERS | Ag | AM (acrylamide) | Rhodamin 6G | Ethyleneglycol dimethacrylate (EDGMA) | ND | LOD: 10−13 mol/L | [121] |
| 10. | Carcinoembryonic antigen (CEA) | Serum | MIPSERS | Au | 4-vinylbenzeneboronic acid (VPBA) | Carcinoembryonic antigen (CEA) | EDGMA | ND | LOD: 0.1 ng/mL | [122] |
| 11. | λ -Cyhalotrin | Water | SGA MIP SERS | Ag | MAA and AM | Cyhalotrin | EDGMA | ND | LOD: 3.8 × 10−10 mol/L | [124] |
| 12. | Paracetamol | Waste water | MIPSERS | Au | MAA | Paracetamol | EDGMA | ND | LOD: 300 nM | [125] |
| 13. | Carbamate pesticides | Tap water | MIPSERS | Ag | Methylacrylamide (MAM) | Carbaryl and thiodicarb | EDGMA | DFT B3LYP level basis set 6–31G(d) | Recoveries Carbaryl: 86.0–89.7% Thiodicarb: 79.0–84.7% | [126] |
| 14. | Sulfamethazine | Meat | Ag-TiO2 MIP SERS | Ag | MAA and AM | Sulfamethazine | EDGMA | DFT to obtain molecular electrostatic potential (MEP) | LOD: 3.6 × 10−9 mol/L | [127] |
| 15. | Histamine | Liquor, vinegar, prawn | MIPSERS | Ag | MAA | Histamine dihydrochloride | EDGMA | ND | LOD: 3.088 × 10−9 mol/L | [128] |
| 16. | Tyrosine | Aqueous medium | PDA MIP SERS | Ag | AM | Tyrosine | EDGMA | ND | LOD: 10−9 mol/L | [129] |
| 17. | p-nitroaniline | Water | DG/Ag-MIP SERS | Ag | Methacrylamide | p-nitroaniline | N, N, N’, N’-Tetramethylethylenediamine (TEMED) | ND | LOD: 1.0 × 10−14 M | [130] |
| 18. | Antibiotics | Water | Ag/ESM SERS | Ag | AM | Spiramycin | EGDMA | ND | LOD: 0.027 nmol/L | [131] |
| 19. | Metformin HCl and Phenformin HCl | Hypoglycemic health product | MIP@Au-GO SERS | Au | MAA | Metformin HCl | EGDMA | ND | LOD: 0.1 mg/mL | [132] |
| 20. | Malachite green | Fish muscles | Au@AgNPs MIP SERS | Au and Ag | MAA | Abietic acid (dummy template) | EGDMA | Optimization: DFT M06-2X/6–31G** Binding energy: Basis set def2TZVP with or without zero-point energy correction (ZPEC) | LOD: 0.37–0.64 ng/g | [133] |
| 21. | Malachite green | Water and carp | MIP@Fe3O4 SERS | Ag | MAA | Malachite green | EGDMA | ND | LOD: Tap water: 1.50 pM Carp: 1.62 pM LOQ Tap water: 4.96 pM Carp: 5.38 pM | [134] |
| 22. | Propranolol | Complex samples | GO-MIP SERS | Ag | MAA | Propranolol | EGDMA | ND | LOD: 10−11 mol/L | [135] |
| 23. | 2,4-dichlorophenoxyacetic acid | Milk | MISPE SERS | Ag | 4-VP | 2,4-dichlorophenoxyacetic acid | EDGMA | ND | LOD: 0.006 ppm LOQ: 0.008 ppm | [136] |
| 24. | Chlorpyrifos | Apple juice | MIPSERS | Ag | MAA | Chlorpyrifos | EGDMA | ND | PLSR RMSEC: 0.0453 RMSECV: 0.1470 | [137] |
| 25. | Thiabendazole | Orange juice | MISPE SERS | Ag | MAA | Thiabendazole | Divinylbenzene | ND | LOD: 4 ppm | [138] |
| 26. | Atrazine | Apple juice | MIP SERS | Au | MAA | Atrazine | EGDMA | ND | LOD: L-AuNPs: 0.005 mg/L–0.01 mg/L M-AuNPs: 0.01 mg/L–0.05 mg/L S-AuNPs: 0.01 mg/L–0.05 mg/L | [139] |
| 27. | L-Phenylalanine | Serum | Au@MIP SERS | Au | Phenyltrimethoxysilane (PTMOS) | L-Phenylalanine | Tetraethyl orthosilicate (TEOS) | ND | LOD: 1.0 nmol/L | [140] |
| 28. | Bisphenol A | Polycarbonate plastic | Ag@MIP SERS | Ag | MAA | Bisphenol A | EGDMA | ND | LOD: 5 × 10−8 mol/L | [141] |
| 29. | Enrofloxacin hydrochloride | Water | Fe3O4@Ag@MIP SERS | Ag | Dopamine | Enrofloxacin hydrochloride | Dopamine | ND | LOD: 0.012 nmol/L | [142] |
| 30. | Enrofloxacin hydrochloride | Water | AGP MIM SERS | Ag | AM | Enrofloxacin hydrochloride | EGDMA | ND | LOD: 0.0078 nmol/L | [143] |
| 31. | Lysozyme | Clinical uses | AgMIP SERS | Ag | MAA and AM | Lysozyme | N,N-methylene acrylamide | DFT and MEP | LOD: 5 ng/mL | [144] |
| 32. | p-nitroaniline | Water | Ag@MIP SERS | Ag | Methylacrylamide | p-nitroaniline | EGDMA | ND | LOD: 10−12 M | [145] |
| 33. | PAH (polycyclic aromatic hydrocarbon) | Creek water and seawater | Au@MIP SERS | Au | MAA | Pyren and fluoranthene | Divinylvbenzene (DVB) | ND | LOD: 1 nM | [146] |
| 34. | Cloxacillin | Pig serum | MMIP SERS | ND | MAA | Cloxacillin | EGDMA | ND | LOD: 7.8 pmol | [147] |
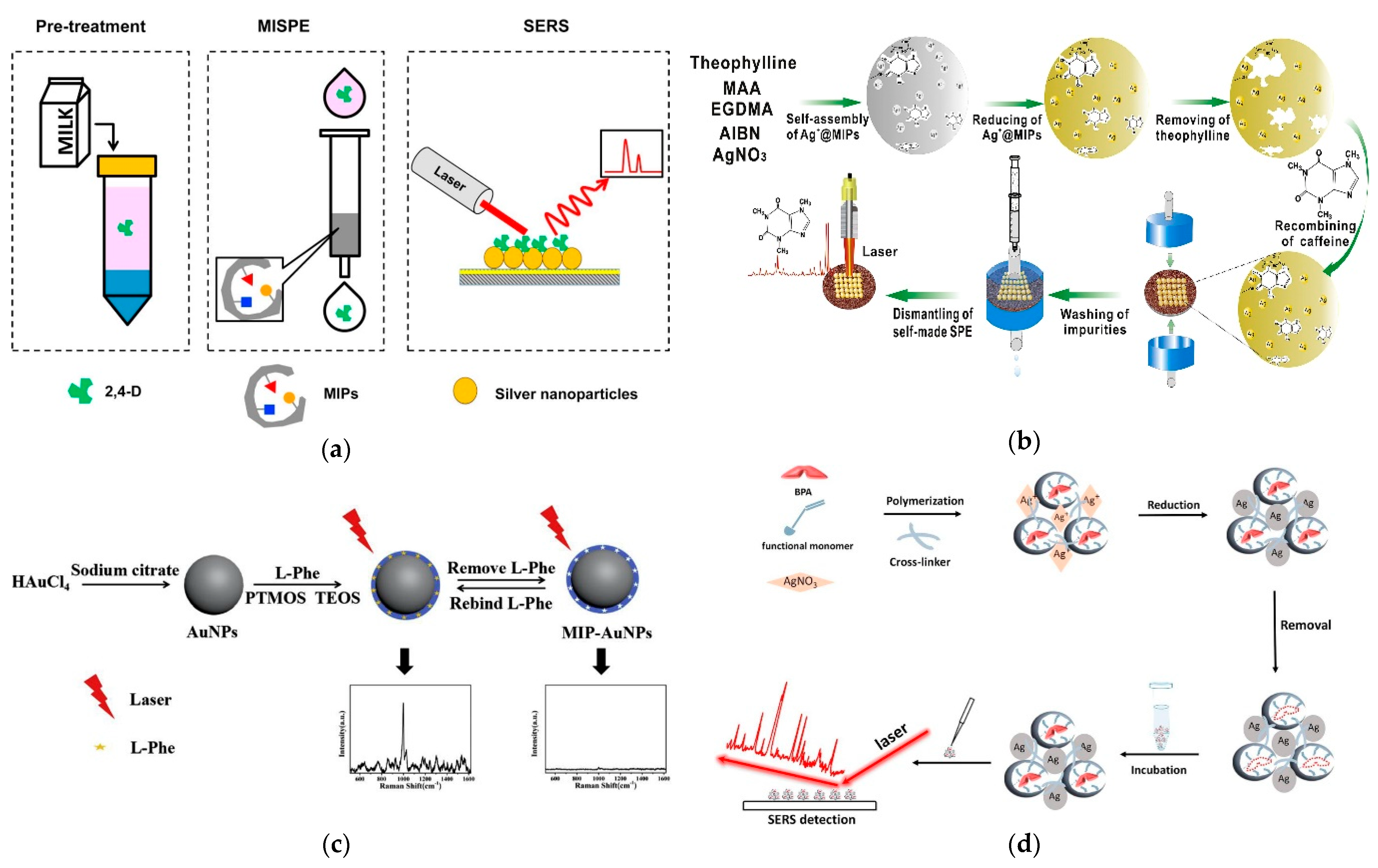
4.1. One Step MIP-SERS
4.2. Two Step MIP-SERS
5. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moein, M.M.; El Beqqali, A.; Abdel-Rehim, M. Bioanalytical method development and validation: Critical concepts and strategies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Sadeghi, T.; Ataie, Z.; Rahimi-Nasrabadi, M.; Eslami, N. Computational Design of a Selective Molecular Imprinted Polymer for Extraction of Pseudoephedrine from Plasma and Determination by HPLC. Anal. Chem. Lett. 2017, 7, 295–310. [Google Scholar] [CrossRef]
- Miranda, L.F.C.; Domingues, D.S.; Queiroz, M.E.C. Selective solid-phase extraction using molecularly imprinted polymers for analysis of venlafaxine, O-desmethylvenlafaxine, and N-desmethylvenlafaxine in plasma samples by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1458, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, M.; Giebułtowicz, J.; Luliński, P. Theoretical and experimental proof for selective response of imprinted sorbent—analysis of hordenine in human urine. J. Chromatogr. A 2020, 1613, 460677. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, S.; Pourmoslemi, S.; Larki Harchegani, A. Rapid determination of metanil yellow in turmeric using a molecularly imprinted polymer dispersive solid-phase extraction and visible light spectrophotometry. Food Chem. 2022, 380, 132120. [Google Scholar] [CrossRef]
- Han, F.; Zhou, D.B.; Song, W.; Hu, Y.Y.; Lv, Y.N.; Ding, L.; Zheng, P.; Jia, X.Y.; Zhang, L.; Deng, X.J. Computational design and synthesis of molecular imprinted polymers for selective solid phase extraction of sulfonylurea herbicides. J. Chromatogr. A 2021, 1651, 462321. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Qi, Y.; Zhang, Z.; Cong, S.; She, Y.; Cao, X. Synthesis of metal-organic framework @molecularly imprinted polymer adsorbents for solid phase extraction of organophosphorus pesticides from agricultural products. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1188, 123081. [Google Scholar] [CrossRef]
- Song, Y.P.; Zhang, L.; Wang, G.N.; Liu, J.X.; Liu, J.; Wang, J.P. Dual-dummy-template molecularly imprinted polymer combining ultra performance liquid chromatography for determination of fluoroquinolones and sulfonamides in pork and chicken muscle. Food Control 2017, 82, 233–242. [Google Scholar] [CrossRef]
- Song, Y.P.; Li, N.; Zhang, H.C.; Wang, G.N.; Liu, J.X.; Liu, J.; Wang, J.P. Dummy template molecularly imprinted polymer for solid phase extraction of phenothiazines in meat based on computational simulation. Food Chem. 2017, 233, 422–428. [Google Scholar] [CrossRef]
- Tarek, M.; Elzanfaly, E.S.; Amer, S.M.; Wagdy, H.A. Selective analysis of Nadifloxacin in human plasma samples using a molecularly imprinted polymer-based solid-phase extraction proceeded by UPLC-DAD analysis. Microchem. J. 2020, 158, 105162. [Google Scholar] [CrossRef]
- Prasad, B.B.; Rai, G. Molecular structure, vibrational spectra and quantum chemical MP2/DFT studies toward the rational design of hydroxyurea imprinted polymer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 400–411. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, X.; Gao, Y.; Li, Y.; Huang, W.; Chen, H.; Tang, X.; Tsunoda, M.; Li, J.; Zhang, Y.; et al. Synthesis of magnetic molecular imprinted polymers for solid-phase extraction coupled with gas chromatography-mass spectrometry for the determination of type II pyrethroid residues in human plasma. Microchem. J. 2021, 166, 106232. [Google Scholar] [CrossRef]
- Sheykhaghaei, G.; Hossainisadr, M.; Khanahmadzadeh, S.; Seyedsajadi, M.; Alipouramjad, A. Magnetic molecularly imprinted polymer nanoparticles for selective solid phase extraction and pre-concentration of Tizanidine in human urine. J. Chromatogr. B 2016, 1011, 1–5. [Google Scholar] [CrossRef]
- Wu, N.; Luo, Z.; Ge, Y.; Guo, P.; Du, K.; Tang, W.; Du, W.; Zeng, A.; Chang, C.; Fu, Q. A novel surface molecularly imprinted polymer as the solid-phase extraction adsorbent for the selective determination of ampicillin sodium in milk and blood samples. J. Pharm. Anal. 2016, 6, 157–164. [Google Scholar] [CrossRef]
- da Silva, P.H.R.; Diniz, M.L.V.; Pianetti, G.A.; da Costa César, I.; Ribeiro e Silva, M.E.S.; de Souza Freitas, R.F.; de Sousa, R.G.; Fernandes, C. Molecularly imprinted polymer for determination of lumefantrine in human plasma through chemometric-assisted solid-phase extraction and liquid chromatography. Talanta 2018, 184, 173–183. [Google Scholar] [CrossRef]
- Zhang, M.; He, J.; Shen, Y.; He, W.; Li, Y.; Zhao, D.; Zhang, S. Application of pseudo-template molecularly imprinted polymers by atom transfer radical polymerization to the solid-phase extraction of pyrethroids. Talanta 2018, 178, 1011–1016. [Google Scholar] [CrossRef]
- de Oliveira, H.L.; Pires, B.C.; Teixeira, L.S.; Dinali, L.A.F.; Simões, N.S.; Borges, W.D.S.; Borges, K.B. Novel restricted access material combined to molecularly imprinted polymer for selective magnetic solid-phase extraction of estrogens from human urine. Microchem. J. 2019, 149, 104043. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Tsalbouris, A.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Synthesis and application of molecularly imprinted polymers using sol–gel matrix imprinting technology for the efficient solid-phase extraction of BPA from water. Microchem. J. 2020, 157, 104965. [Google Scholar] [CrossRef]
- Arias, P.G.; Martínez-Pérez-Cejuela, H.; Combès, A.; Pichon, V.; Pereira, E.; Herrero-Martínez, J.M.; Bravo, M. Selective solid-phase extraction of organophosphorus pesticides and their oxon-derivatives from water samples using molecularly imprinted polymer followed by high-performance liquid chromatography with UV detection. J. Chromatogr. A 2020, 1626, 461346. [Google Scholar] [CrossRef]
- Yu, X.; Zeng, H.; Wan, J.; Cao, X. Computational design of a molecularly imprinted polymer compatible with an aqueous environment for solid phase extraction of chenodeoxycholic acid. J. Chromatogr. A 2020, 1609, 460490. [Google Scholar] [CrossRef]
- Cai, T.; Zhou, Y.; Liu, H.; Li, J.; Wang, X.; Zhao, S.; Gong, B. Preparation of monodisperse, restricted-access, media-molecularly imprinted polymers using bi-functional monomers for solid-phase extraction of sarafloxacin from complex samples. J. Chromatogr. A 2021, 1642, 462009. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Farajzadeh, M.A.; Afshar Mogaddam, M.R.; Nemati, M.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V. Molecularly imprinted polymer based-solid phase extraction combined with dispersive liquid–liquid microextraction using new deep eutectic solvent; selective extraction of valproic acid from exhaled breath condensate samples. Microchem. J. 2021, 161, 105772. [Google Scholar] [CrossRef]
- Yu, Q.; Gan, H.; Feng, N.; Li, Y.; Han, Y. Hydroxytyrosol magnetic molecularly imprinted polymers as the sorbent for solid-phase extraction for selective recognition of hydroxytyrosol from Chinese olive leaves. Mater. Today Commun. 2021, 29, 102992. [Google Scholar] [CrossRef]
- Hou, H.; Jin, Y.; Xu, K.; Sheng, L.; Huang, Y.; Zhao, R. Selective recognition of a cyclic peptide hormone in human plasma by hydrazone bond-oriented surface imprinted nanoparticles. Anal. Chim. Acta 2021, 1154, 338301. [Google Scholar] [CrossRef]
- Lai, H.; Yu, Z.; Li, G.; Zhang, Z. Advanced sample preparation techniques for rapid surface-enhanced Raman spectroscopy analysis of complex samples. J. Chromatogr. A 2022, 1675, 463181. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, F.; Jiang, Z.; Hong, S.; Zhang, C.; She, Y.; Jin, F.; Jin, M.; Wang, J. Rapid Analysis of Bitertanol in Agro-products Using Molecularly Imprinted Polymers-Surface-Enhanced Raman Spectroscopy. Food Anal. Methods 2018, 11, 1435–1443. [Google Scholar] [CrossRef]
- Ren, X.; Feng, X.; Jin, M.; Li, X. Dummy molecular imprinted polymers coated with silver microspheres via surface enhanced Raman scattering for sensitive detection of benzimidazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119321. [Google Scholar] [CrossRef]
- Bates, F.; Cela-Pérez, M.C.; Karim, K.; Piletsky, S.; López-Vilariño, J.M. Virtual Screening of Receptor Sites for Molecularly Imprinted Polymers. Macromol. Biosci. 2016, 16, 1170–1174. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chen, X.; Hu, S.; Gong, T.; Xian, Q. A novel molecularly imprinted polymer-solid phase extraction method coupled with high performance liquid chromatography tandem mass spectrometry for the determination of nitrosamines in water and beverage samples. Food Chem. 2019, 292, 267–274. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Fu, L.; Luo, L.; Chen, L. Rotational Paper-Based Microfluidic-Chip Device for Multiplexed and Simultaneous Fluorescence Detection of Phenolic Pollutants Based on a Molecular-Imprinting Technique. Anal. Chem. 2018, 90, 11827–11834. [Google Scholar] [CrossRef]
- Hashim, S.N.N.S.; Boysen, R.I.; Yang, Y.; Schwarz, L.J.; Danylec, B.; Hearn, M.T.W. Parallel enrichment of polyphenols and phytosterols from Pinot noir grape seeds with molecularly imprinted polymers and analysis by capillary high-performance liquid chromatography electrospray ionisation tandem mass spectrometry. Talanta 2020, 208, 120397. [Google Scholar] [CrossRef]
- Wang, L.; Yan, H.; Yang, C.; Li, Z.; Qiao, F. Synthesis of mimic molecularly imprinted ordered mesoporous silica adsorbent by thermally reversible semicovalent approach for pipette-tip solid-phase extraction-liquid chromatography fluorescence determination of estradiol in milk. J. Chromatogr. A 2016, 1456, 58–67. [Google Scholar] [CrossRef]
- Nadal, J.C.; Catalá-Icardo, M.; Borrull, F.; Herrero-Martínez, J.M.; Marcé, R.M.; Fontanals, N. Weak anion-exchange mixed-mode materials to selectively extract acidic compounds by stir bar sorptive extraction from environmental waters. J. Chromatogr. A 2022, 1663, 462748. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Cao, X. Synthesis of surface molecularly imprinting polymers for cordycepin and its application in separating cordycepin. Process Biochem. 2016, 51, 517–527. [Google Scholar] [CrossRef]
- Moura, S.L.; Fajardo, L.M.; Cunha, L.D.A.; Sotomayor, M.D.P.T.; Machado, F.B.C.; Ferrão, L.F.A.; Pividori, M.I. Theoretical and experimental study for the biomimetic recognition of levothyroxine hormone on magnetic molecularly imprinted polymer. Biosens. Bioelectron. 2018, 107, 203–210. [Google Scholar] [CrossRef]
- Roland, R.M.; Bhawani, S.A. Synthesis and Characterization of Molecular Imprinting Polymer Microspheres of Piperine: Extraction of Piperine from Spiked Urine. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Bezdekova, J.; Vlcnovska, M.; Zemankova, K.; Bacova, R.; Kolackova, M.; Lednicky, T.; Pribyl, J.; Richtera, L.; Vanickova, L.; Adam, V.; et al. Molecularly imprinted polymers and capillary electrophoresis for sensing phytoestrogens in milk. J. Dairy Sci. 2020, 103, 4941–4950. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Ran, R.-X.; Huang, Y.-P.; Liu, Z.-S. Synergistic effect of metal ions pivot and macromolecular crowding reagents on affinity of molecularly imprinted polymer. Eur. Polym. J. 2019, 120, 109242. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, G.; Wang, Z.; Ma, J.; Jia, Q. Design and synthesis of Fe3O4@Au@cyclodextrin-molecularly imprinted polymers labeled with SERS nanotags for ultrasensitive detection of transferrin. Sens. Actuators B Chem. 2022, 361, 131669. [Google Scholar] [CrossRef]
- Yu, X.; Liao, J.; Zeng, H.; Wan, J.; Cao, X. Synthesis of water-compatible noncovalent imprinted microspheres for acidic or basic biomolecules designed based on molecular dynamics. Polymer 2022, 257, 125253. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, J.; Li, Z.; Yan, Z.; Xu, G.; Li, H.; Guan, D. A molecularly imprinted polymer synthesized using β-cyclodextrin as the monomer for the efficient recognition of forchlorfenuron in fruits. Anal. Bioanal. Chem. 2017, 409, 5065–5072. [Google Scholar] [CrossRef] [PubMed]
- Boulanouar, S.; Combès, A.; Mezzache, S.; Pichon, V. Synthesis and application of molecularly imprinted silica for the selective extraction of some polar organophosphorus pesticides from almond oil. Anal. Chim. Acta 2018, 1018, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Khodayari, P. Magnetic molecularly imprinted polymer for the selective dispersive micro solid phase extraction of phenolphthalein in urine samples and herbal slimming capsules prior to HPLC-PDA analysis. Microchem. J. 2021, 160, 105712. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, X.; Zhao, D. Computer-aided design of molecularly imprinted polymers for simultaneous detection of clenbuterol and its metabolites. Polymers 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Cala, B.; Mizaikoff, B. Surrogate Imprinting Strategies: Molecular Imprints via Fragments and Dummies. ACS Appl. Polym. Mater. 2020, 2, 3714–3741. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent applications of molecularly imprinted sol-gel methodology in sample preparation. Molecules 2019, 24, 2889. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, J.; Zhang, Y.; Tian, Y.; Xu, P.; Zhang, X.; Li, Y.; Chen, J.; He, L. Application of magnetic hydroxyapatite surface-imprinted polymers in pretreatment for detection of zearalenone in cereal samples. J. Chromatogr. B 2022, 1201–1202, 123297. [Google Scholar] [CrossRef]
- Guo, Y.; Kang, L.; Chen, S.; Li, X. High performance surface-enhanced Raman scattering from molecular imprinting polymer capsulated silver spheres. Phys. Chem. Chem. Phys. 2015, 17, 21343–21347. [Google Scholar] [CrossRef]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Gao, F.; Feng, S.; Chen, Z.; Li-Chan, E.C.Y.; Grant, E.; Lu, X. Detection and Quantification of Chloramphenicol in Milk and Honey Using Molecularly Imprinted Polymers: Canadian Penny-Based SERS Nano-Biosensor. J. Food Sci. 2014, 79, N2542–N2549. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef]
- Piletsky, S. Molecular Imprinting of Polymers; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1587062194. [Google Scholar]
- Silva, L.M.; Foguel, M.V.; Sotomayor, M.d.P.T. Use of two functional monomers for a new approach to the synthesis of a magnetic molecularly imprinted polymer for ciprofloxacin. J. Mater. Res. Technol. 2021, 15, 511–523. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Qiao, Y.; Liu, L.; Wang, Q.; Che, G. High-sensitive molecularly imprinted sensor with multilayer nanocomposite for 2,6-dichlorophenol detection based on surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117784. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, K.; Xiao, D.; He, H. Computational-aided design of magnetic ultra-thin dummy molecularly imprinted polymer for selective extraction and determination of morphine from urine by high-performance liquid chromatography. J. Chromatogr. A 2016, 1473, 1–9. [Google Scholar] [CrossRef]
- Marć, M.; Panuszko, A.; Namieśnik, J.; Wieczorek, P.P. Preparation and characterization of dummy-template molecularly imprinted polymers as potential sorbents for the recognition of selected polybrominated diphenyl ethers. Anal. Chim. Acta 2018, 1030, 77–95. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, Y.; Jin, J.; Yang, J.; Li, F.; Shah, S.M.; Chen, J. Highly class-selective solid-phase extraction of bisphenols in milk, sediment and human urine samples using well-designed dummy molecularly imprinted polymers. J. Chromatogr. A 2014, 1360, 9–16. [Google Scholar] [CrossRef]
- Xiao, X.; Yan, K.; Xu, X.; Li, G. Rapid analysis of ractopamine in pig tissues by dummy-template imprinted solid-phase extraction coupling with surface-enhanced Raman spectroscopy. Talanta 2015, 138, 40–45. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.; Li, J.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Mdluli, P.S.; Chimuka, L. Experimental and theoretical study of molecular interactions between 2-vinyl pyridine and acidic pharmaceuticals used as multi-template molecules in molecularly imprinted polymer. React. Funct. Polym. 2016, 103, 33–43. [Google Scholar] [CrossRef]
- Kiełczyński, R.; Bryjak, M. Molecularly imprinted membranes for cinchona alkaloids separation. Sep. Purif. Technol. 2005, 41, 231–235. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Najafi, A.; Pino, V.; Anderson, J.L.; Ayala, J.H.; Afonso, A.M. Utilization of highly robust and selective crosslinked polymeric ionic liquid-based sorbent coatings in direct-immersion solid-phase microextraction and high-performance liquid chromatography for determining polar organic pollutants in waters. Talanta 2016, 158, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers11Electronic supplementary information (ESI) available: NMR, BET and elemental analysis. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef]
- Li, C.; Ngai, M.H.; Reddy, K.K.; Leong, S.C.Y.; Tong, Y.W.; Chai, C.L.L. A fluorescence-displacement assay using molecularly imprinted polymers for the visual, rapid, and sensitive detection of the algal metabolites, geosmin and 2-methylisoborneol. Anal. Chim. Acta 2019, 1066, 121–130. [Google Scholar] [CrossRef]
- Mehta, R.; van Beek, T.A.; Tetala, K.K.R. A micro-solid phase extraction device to prepare a molecularly imprinted porous monolith in a facile mode for fast protein separation. J. Chromatogr. A 2020, 1627, 461415. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, X.; Wan, J.; Cao, X. Synthesis of molecularly imprinted polymers based on boronate affinity for diol-containing macrolide antibiotics with hydrophobicity-balanced and pH-responsive cavities. J. Chromatogr. A 2021, 1642, 461969. [Google Scholar] [CrossRef]
- Panjan, P.; Monasterio, R.P.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Sesay, A.M.; Fernandez-Sanchez, J.F. Development of a folic acid molecularly imprinted polymer and its evaluation as a sorbent for dispersive solid-phase extraction by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2018, 1576, 26–33. [Google Scholar] [CrossRef]
- Fahim, A.M.; Magd, E.E.A. El Enhancement of Molecular imprinted polymer as organic fillers on bagasse cellulose fibers with biological evaluation and computational calculations. J. Mol. Struct. 2021, 1241, 130660. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Qiao, Y.; Wei, M.; Gao, L.; Wang, L.; Yan, Y.; Li, H. High-sensitive imprinted membranes based on surface-enhanced Raman scattering for selective detection of antibiotics in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117116. [Google Scholar] [CrossRef]
- Amin, S.; Damayanti, S.; Ibrahim, S. Interaction Study, Synthesis and Characterization of Molecular Imprinted Polymer Using Functional Monomer Methacrylate Acid and Dimethylamylamine as Template Molecule. J. Ilmu Kefarmasian Indones. 2018, 16, 12. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Soni, D.; Pratiwi, R.; Rahayu, D.; Megantara, S. Mutakin Synthesis of Diazepam-Imprinted Polymers with Two Functional Monomers in Chloroform Using a Bulk Polymerization Method. J. Chem. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V. Rational synthesis of pindolol imprinted polymer by non-covalent protocol based on computational approach. J. Mol. Model. 2013, 19, 3385–3396. [Google Scholar] [CrossRef]
- Krishnan, H.; Islam, K.M.S.; Hamzah, Z.; Ahmad, M.N. Rational computational design for the development of andrographolide molecularly imprinted polymer. AIP Conf. Proc. 2017, 1891, 020083. [Google Scholar] [CrossRef]
- Hammam, M.A.; Abdel-Halim, M.; Madbouly, A.; Wagdy, H.A.; El Nashar, R.M. Computational design of molecularly imprinted polymer for solid phase extraction of moxifloxacin hydrochloride from Avalox® tablets and spiked human urine samples. Microchem. J. 2019, 148, 51–56. [Google Scholar] [CrossRef]
- Okutucu, B.; Telefoncu, A. Optimization of serotonin imprinted polymers and recognition study from platelet rich plasma. Talanta 2008, 76, 1153–1158. [Google Scholar] [CrossRef]
- Ao, J.; Gu, J.; Yuan, T.; Li, D.; Ma, Y.; Shen, Z. Applying molecular modelling and experimental studies to develop molecularly imprinted polymer for domoic acid enrichment from both seawater and shellfish. Chemosphere 2018, 199, 98–106. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef]
- Fonseca, M.C.; Nascimento, C.S.; Borges, K.B. Theoretical investigation on functional monomer and solvent selection for molecular imprinting of tramadol. Chem. Phys. Lett. 2016, 645, 174–179. [Google Scholar] [CrossRef]
- Barros, L.A.; Custodio, R.; Rath, S. Design of a new molecularly imprinted polymer selective for hydrochlorothiazide based on theoretical predictions using Gibbs free energy. J. Braz. Chem. Soc. 2016, 27, 2300–2311. [Google Scholar] [CrossRef]
- Silva, C.F.; Menezes, L.F.; Pereira, A.C.; Nascimento, C.S. Molecularly Imprinted Polymer (MIP) for thiamethoxam: A theoretical and experimental study. J. Mol. Struct. 2021, 1231, 129980. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Kartasasmi, R.E.; Ibrahim, S. Synthesis and Application of Glibenclamide Imprinted Polymer for Solid Phase Extraction in Serum Samples Using Itaconic Acid as Functional Monomer. J. Appl. Sci. 2015, 15, 1288–1296. [Google Scholar] [CrossRef]
- Pereira, T.F.D.; da Silva, A.T.M.; Borges, K.B.; Nascimento, C.S. Carvedilol-Imprinted Polymer: Rational design and selectivity studies. J. Mol. Struct. 2019, 1177, 101–106. [Google Scholar] [CrossRef]
- Das, R.S.; Wankhade, A.V.; Kumar, A. Computationally designed ionic liquid based molecularly imprinted@ graphene oxide composite: Characterization and validation. J. Mol. Liq. 2021, 341, 116925. [Google Scholar] [CrossRef]
- Ganjeizadeh Rohani, F.; Mohadesi, A.; Ansari, M. A new diosgenin sensor based on molecularly imprinted polymer of para aminobenzoic acid selected by computer-aided design. J. Pharm. Biomed. Anal. 2019, 174, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Borges, K.B.; Do Nascimento, C.S. Rational design of a molecularly imprinted polymer for dinotefuran: Theoretical and experimental studies aimed at the development of an efficient adsorbent for microextraction by packed sorbent. Analyst 2018, 143, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Damayanti, S.; Ibrahim, S. Interaction binding study of dimethylamylamine with functional monomers to design a molecular imprinted polymer for doping analysis. J. Appl. Pharm. Sci. 2018, 8, 25–031. [Google Scholar] [CrossRef]
- González Fá, A.; Pignanelli, F.; López-Corral, I.; Faccio, R.; Juan, A.; Di Nezio, M.S. Detection of oxytetracycline in honey using SERS on silver nanoparticles. TrAC Trends Anal. Chem. 2019, 121, 115673. [Google Scholar] [CrossRef]
- Sobiech, M.; Zołek, T.; Luliński, P.; Maciejewska, D. Separation of octopamine racemate on (R,S)-2-amino-1-phenylethanol imprinted polymer—Experimental and computational studies. Talanta 2016, 146, 556–567. [Google Scholar] [CrossRef]
- Johnson, E.R.; Mackie, I.D.; DiLabio, G.A. Dispersion interactions in density-functional theory. J. Phys. Org. Chem. 2009, 22, 1127–1135. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, X.; Wan, J.; Cao, X. Rational design and synthesis of molecularly imprinted polymers (MIP) for purifying tylosin by seeded precipitation polymerization. Process Biochem. 2020, 94, 329–339. [Google Scholar] [CrossRef]
- Nagy-Szakolczai, A.; Sváb-Kovács, A.; Krezinger, A.; Tóth, B.; Nyulászi, L.; Horvai, G. The molecular imprinting effect of propranolol and dibenzylamine as model templates: Binding strength and selectivity. Anal. Chim. Acta 2020, 1125, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Rational development of molecular imprinted carbon paste electrode for Furazolidone detection: Theoretical and experimental approach. Sens. Actuators B Chem. 2021, 329, 129112. [Google Scholar] [CrossRef]
- Shoravi, S.; Olsson, G.D.; Karlsson, B.C.G.; Bexborn, F.; Abghoui, Y.; Hussain, J.; Wiklander, J.G.; Nicholls, I.A. In silico screening of molecular imprinting prepolymerization systems: Oseltamivir selective polymers through full-system molecular dynamics-based studies. Org. Biomol. Chem. 2016, 14, 4210–4219. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, N.; Ni, X.; Yu, Q.; Liu, H.; Huang, W.; Xu, W. Molecular dynamics simulations of molecularly imprinted polymer approaches to the preparation of selective materials to remove norfloxacin. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Bates, F.; Busato, M.; Piletska, E.; Whitcombe, M.J.; Karim, K.; Guerreiro, A.; del Valle, M.; Giorgetti, A.; Piletsky, S. Computational design of molecularly imprinted polymer for direct detection of melamine in milk. Sep. Sci. Technol. 2017, 52, 1441–1453. [Google Scholar] [CrossRef]
- Sheykhaghaei, G.; Sadr, M.H.; Khanahmadzadeh, S. Synthesis and characterization of core-shell magnetic molecularly imprinted polymer nanoparticles for selective extraction of tizanidine in human plasma. Bull. Mater. Sci. 2016, 39, 647–653. [Google Scholar] [CrossRef]
- Madrakian, T.; Fazl, F.; Ahmadi, M.; Afkhami, A. Efficient solid phase extraction of codeine from human urine samples using a novel magnetic molecularly imprinted nanoadsorbent and its spectrofluorometric determination. New J. Chem. 2016, 40, 122–129. [Google Scholar] [CrossRef]
- Ruiz-Córdova, G.A.; Villa, J.E.L.; Khan, S.; Picasso, G.; Del Pilar Taboada Sotomayor, M. Surface molecularly imprinted core-shell nanoparticles and reflectance spectroscopy for direct determination of tartrazine in soft drinks. Anal. Chim. Acta 2021, 1159, 338443. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Z.; Tang, Y.; Liu, X.; Zhang, X.; Liu, B.; Wang, X.; Draz, M.S.; Gao, X. Fluorometric determination of ciprofloxacin using molecularly imprinted polymer and polystyrene microparticles doped with europium(III)(DBM) 3 phen. Microchim. Acta 2019, 186, 334. [Google Scholar] [CrossRef]
- Elbelazi, A.; Canfarotta, F.; Czulak, J.; Whitcombe, M.J.; Piletsky, S.; Piletska, E. Development of a homogenous assay based on fluorescent imprinted nanoparticles for analysis of nitroaromatic compounds. Nano Res. 2019, 12, 3044–3050. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, J.; Zeng, Y.; Wang, H.; Yang, Y.; Zhai, Y.; Miao, D.; Li, L. Highly sensitive and selective detection of 4-nitroaniline in water by a novel fluorescent sensor based on molecularly imprinted poly(ionic liquid). Anal. Bioanal. Chem. 2020, 412, 5653–5661. [Google Scholar] [CrossRef]
- Üzek, R.; Sari, E.; Şenel, S.; Denizli, A.; Merkoçi, A. A nitrocellulose paper strip for fluorometric determination of bisphenol A using molecularly imprinted nanoparticles. Microchim. Acta 2019, 186, 218. [Google Scholar] [CrossRef]
- Toloza, C.A.T.; Almeida, J.M.S.; Khan, S.; dos Santos, Y.G.; da Silva, A.R.; Romani, E.C.; Larrude, D.G.; Freire, F.L.; Aucélio, R.Q. Gold nanoparticles coupled with graphene quantum dots in organized medium to quantify aminoglycoside anti-biotics in yellow fever vaccine after solid phase extraction using a selective imprinted polymer. J. Pharm. Biomed. Anal. 2018, 158, 480–493. [Google Scholar] [CrossRef]
- Bujak, R.; Gadzała-Kopciuch, R.; Nowaczyk, A.; Raczak-Gutknecht, J.; Kordalewska, M.; Struck-Lewicka, W.; Waszczuk-Jankowska, M.; Tomczak, E.; Kaliszan, M.; Buszewski, B.; et al. New sorbent materials for selective extraction of cocaine and benzoylecgonine from human urine samples. J. Pharm. Biomed. Anal. 2016, 120, 397–401. [Google Scholar] [CrossRef]
- Zuo, H.G.; Yang, H.; Zhu, J.X.; Guo, P.; Shi, L.; Zhan, C.R.; Ding, Y. Synthesis of Molecularly Imprinted Polymer on Surface of TiO2 Nanowires and Assessment of Malathion and its Metabolite in Environmental Water. J. Anal. Chem. 2019, 74, 1039–1055. [Google Scholar] [CrossRef]
- Wu, H.; Tian, Q.; Zheng, W.; Jiang, Y.; Xu, J.; Li, X.; Zhang, W.; Qiu, F. Non-enzymatic glucose sensor based on molecularly imprinted polymer: A theoretical, strategy fabrication and application. J. Solid State Electrochem. 2019, 23, 1379–1388. [Google Scholar] [CrossRef]
- Mars, A.; Mejri, A.; Hamzaoui, A.H.; Elfil, H. Molecularly imprinted curcumin nanoparticles decorated paper for electrochemical and fluorescence dual-mode sensing of bisphenol A. Microchim. Acta 2021, 188, 1–11. [Google Scholar] [CrossRef]
- Nadim, A.H.; Abd El-Aal, M.A.; Al-Ghobashy, M.A.; El-Saharty, Y.S. Facile imprinted polymer for label-free highly selective potentiometric sensing of proteins: Case of recombinant human erythropoietin. Anal. Bioanal. Chem. 2021, 413, 3611–3623. [Google Scholar] [CrossRef]
- Kumar, D.R.; Dhakal, G.; Nguyen, V.Q.; Shim, J.J. Molecularly imprinted hornlike polymer@electrochemically reduced graphene oxide electrode for the highly selective determination of an antiemetic drug. Anal. Chim. Acta 2021, 1141, 71–82. [Google Scholar] [CrossRef]
- Altintas, Z.; Abdin, M.J.; Tothill, A.M.; Karim, K.; Tothill, I.E. Ultrasensitive detection of endotoxins using computationally designed nanoMIPs. Anal. Chim. Acta 2016, 935, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.; Liu, M.; Deng, P.; Tang, S.; Jiang, J.; Feng, H.; Qian, D.; He, L. Ag/N-doped reduced graphene oxide incorporated with molecularly imprinted polymer: An advanced electrochemical sensing platform for salbutamol determination. Biosens. Bioelectron. 2017, 90, 210–216. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Yang, S.; An, Y.; Zhang, F.; He, P. A novel electrochemical biosensor with molecularly imprinted polymers and aptamer-based sandwich assay for determining amyloid-β oligomer. J. Electroanal. Chem. 2020, 862, 114017. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Tavares, A.P.M.; Sales, M.G.F. In-situ generated molecularly imprinted material for chloramphenicol electrochemical sensing in waters down to the nanomolar level. Sens. Actuators B Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
- Feng, S.; Lu, X. Molecularly imprinted polymers integrated with surface enhanced Raman spectroscopy: Innovative chemosensors in food science. Lipid Technol. 2015, 27, 14–17. [Google Scholar] [CrossRef]
- Yan, M.; She, Y.; Cao, X.; Ma, J.; Chen, G.; Hong, S.; Shao, Y.; Abd EI-Aty, A.M.; Wang, M.; Wang, J. A molecularly imprinted polymer with integrated gold nanoparticles for surface enhanced Raman scattering based detection of the triazine herbicides, prometryn and simetryn. Microchim. Acta 2019, 186, 143. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Li, G.; Xu, X.; Zhu, L.; Chen, X.; Wang, J. Surface-Imprinted Gold Nanoparticle-Based Surface-Enhanced Raman Scattering for Sensitive and Specific Detection of Patulin in Food Samples. Food Anal. Methods 2019, 12, 1648–1657. [Google Scholar] [CrossRef]
- Ren, X.; Cheshari, E.C.; Qi, J.; Li, X. Silver microspheres coated with a molecularly imprinted polymer as a SERS substrate for sensitive detection of bisphenol A. Microchim. Acta 2018, 185, 242. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Wang, X.; Jiang, J.; Xu, Y.; Liu, X.; Yan, Y.; Li, C. Preparation of a self-cleanable molecularly imprinted sensor based on surface-enhanced Raman spectroscopy for selective detection of R6G. Anal. Bioanal. Chem. 2017, 409, 4627–4635. [Google Scholar] [CrossRef]
- Feng, J. A boronate-modified molecularly imprinted polymer labeled with a SERS-tag for use in an antibody-free immunoassay for the carcinoembryonic antigen. Microchim. Acta 2019, 186, 774. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Zhou, N.; Wang, X.; Deng, D.; Luo, L.; Chen, L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Wang, Z.; Wang, Y.; Dai, J.; Gao, L.; Wei, M.; Yan, Y.; Li, C. A polydopamine-based molecularly imprinted polymer on nanoparticles of type SiO2@rGO@Ag for the detection of λ-cyhalothrin via SERS. Microchim. Acta 2018, 185, 193. [Google Scholar] [CrossRef]
- Decorbie, N.; Tijunelyte, I.; Gam-Derouich, S.; Solard, J.; Lamouri, A.; Decorse, P.; Felidj, N.; Gauchotte-Lindsay, C.; Rinnert, E.; Mangeney, C.; et al. Sensing Polymer/Paracetamol Interaction with an Independent Component Analysis-Based SERS-MIP Nanosensor. Plasmonics 2020, 15, 1533–1539. [Google Scholar] [CrossRef]
- Cheshari, E.C. Core–shell Ag-dual template molecularly imprinted composite for detection of carbamate pesticide residues. Chem. Pap. 2021, 75, 3679–3693. [Google Scholar] [CrossRef]
- Ren, X.; Yang, L.; Li, Y.; Li, X. Design and synthesis of a sandwiched silver microsphere/TiO2 nanoparticles/molecular imprinted polymers structure for suppressing background noise interference in high sensitivity surface-enhanced Raman scattering detection. Appl. Surf. Sci. 2021, 544, 148879. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. A surface-imprinted surface-enhanced Raman scattering sensor for histamine detection based on dual semiconductors and Ag nanoparticles. Food Chem. 2022, 369, 130971. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, D.; Wang, J.; Zhang, J.; Jiang, W.; Zhou, T.; Liu, C.; Che, G. Synthesis of hydrophilic SERS-imprinted membrane based on graft polymerization for selective detection of L-tyrosine. Sens. Actuators B Chem. 2021, 340, 129955. [Google Scholar] [CrossRef]
- Chen, S. High-performance detection of p-nitroaniline on defect-graphene SERS substrate utilizing molecular imprinting technique. Microchem. J. 2021, 168, 106536. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Li, H.; Li, Y.; Li, L.; Shao, J.; Li, Y.; Wang, J.; Xue, Y.; Zhang, J.; et al. Synthesis of SERS imprinted membrane based on Ag/ESM with different morphologies for selective detection of antibiotics in aqueous sample. Opt. Mater. 2021, 121, 111581. [Google Scholar] [CrossRef]
- Lu, R.; Qi, Z.; Wang, S.; Tian, X.; Xu, X. Rapid detection of illegal biguanides in hypoglycemic health products using molecular imprinting combined with SERS technology. Microchem. J. 2021, 169, 106523. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Kang, Y.; Miao, J.; Lai, K. Selective recognition and determination of malachite green in fish muscles via surface-enhanced Raman scattering coupled with molecularly imprinted polymers. Food Control 2021, 130, 108367. [Google Scholar] [CrossRef]
- Ekmen, E. Surface molecularly-imprinted magnetic nanoparticles coupled with SERS sensing platform for selective detection of malachite green. Sens. Actuators B Chem. 2020, 325, 128787. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, J.; Zhang, L.; Chao, C.; Guo, J.; Cheng, Y.; Zhu, Y.; Xu, G. Ultrasensitive SERS detection of propranolol based on sandwich nanostructure of molecular imprinting polymers. Sens. Actuators B Chem. 2018, 255, 110–116. [Google Scholar] [CrossRef]
- Hua, M.Z.; Feng, S.; Wang, S.; Lu, X. Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers–surface-enhanced Raman spectroscopy. Food Chem. 2018, 258, 254–259. [Google Scholar] [CrossRef]
- Feng, S. Development of molecularly imprinted polymers-surface-enhanced Raman spectroscopy/colorimetric dual sensor for determination of chlorpyrifos in apple juice. Sens. Actuators B Chem. 2017, 241, 750–757. [Google Scholar] [CrossRef]
- Feng, J.; Hu, Y.; Grant, E.; Lu, X. Determination of thiabendazole in orange juice using an MISPE-SERS chemosensor. Food Chem. 2018, 239, 816–822. [Google Scholar] [CrossRef]
- Zhao, B.; Feng, S.; Hu, Y.; Wang, S.; Lu, X. Rapid determination of atrazine in apple juice using molecularly imprinted polymers coupled with gold nanoparticles-colorimetric/SERS dual chemosensor. Food Chem. 2019, 276, 366–375. [Google Scholar] [CrossRef]
- Zhou, J.; Sheth, S.; Zhou, H.; Song, Q. Highly selective detection of L-Phenylalanine by molecularly imprinted polymers coated Au nanoparticles via surface-enhanced Raman scattering. Talanta 2020, 211, 120745. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, R.; Liao, S.; Miao, Y.; Zhang, B.; Wang, F.; Yang, H. In situ reduced silver nanoparticles embedded molecularly imprinted reusable sensor for selective and sensitive SERS detection of Bisphenol A. Appl. Surf. Sci. 2018, 457, 323–331. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Jiang, W.; Zhou, T.; He, J.; Luan, Y.; Shang, Y.; Liu, C.; Che, G. Magnetically assisted imprinted sensor for selective detection antibiotics in river based on surface-enhanced Raman scattering. Opt. Mater. 2020, 108, 110200. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Wang, D.; Wang, J.; Jiang, W.; Zhou, T.; Liu, C.; Che, G. Synthesization of flexible SERS imprinted sensor based on Ag/GO composites and selective detection of antibiotic in aqueous sample. Adv. Powder Technol. 2021, 32, 3405–3411. [Google Scholar] [CrossRef]
- Ren, X.; Yang, L.; Li, Y.; Cheshari, E.C.; Li, X. The integration of molecular imprinting and surface-enhanced Raman scattering for highly sensitive detection of lysozyme biomarker aided by density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, K.; Ha, Y.; Chen, S.; Chen, W.; Sun, C.; Dai, Z.; Shi, X. Silver Molecularly Imprinting Polymer for the Determination of p-Nitroaniline by Surface Enhanced Raman Scattering. Anal. Lett. 2019, 52, 1888–1899. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Ashley, J.; Wu, K.; Hansen, M.F.; Schmidt, M.S.; Boisen, A.; Sun, Y. Quantitative detection of trace level cloxacillin in food samples using magnetic molecularly imprinted polymer extraction and surface-Enhanced raman spectroscopy nanopillars. Anal. Chem. 2017, 89, 11484–11490. [Google Scholar] [CrossRef]
- Nguyen, R.; Goodell, J.C.; Shankarappa, P.S.; Zimmerman, S.; Yin, T.; Peer, C.J.; Figg, W.D. Development and validation of a simple, selective, and sensitive LC-MS/MS assay for the quantification of remdesivir in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1171, 122641. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisnuwardhani, H.A.; Ibrahim, S.; Mukti, R.R.; Damayanti, S. Molecularly-Imprinted SERS: A Potential Method for Bioanalysis. Sci. Pharm. 2022, 90, 54. https://doi.org/10.3390/scipharm90030054
Wisnuwardhani HA, Ibrahim S, Mukti RR, Damayanti S. Molecularly-Imprinted SERS: A Potential Method for Bioanalysis. Scientia Pharmaceutica. 2022; 90(3):54. https://doi.org/10.3390/scipharm90030054
Chicago/Turabian StyleWisnuwardhani, Hilda Aprilia, Slamet Ibrahim, Rino R. Mukti, and Sophi Damayanti. 2022. "Molecularly-Imprinted SERS: A Potential Method for Bioanalysis" Scientia Pharmaceutica 90, no. 3: 54. https://doi.org/10.3390/scipharm90030054
APA StyleWisnuwardhani, H. A., Ibrahim, S., Mukti, R. R., & Damayanti, S. (2022). Molecularly-Imprinted SERS: A Potential Method for Bioanalysis. Scientia Pharmaceutica, 90(3), 54. https://doi.org/10.3390/scipharm90030054






