Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth
Abstract
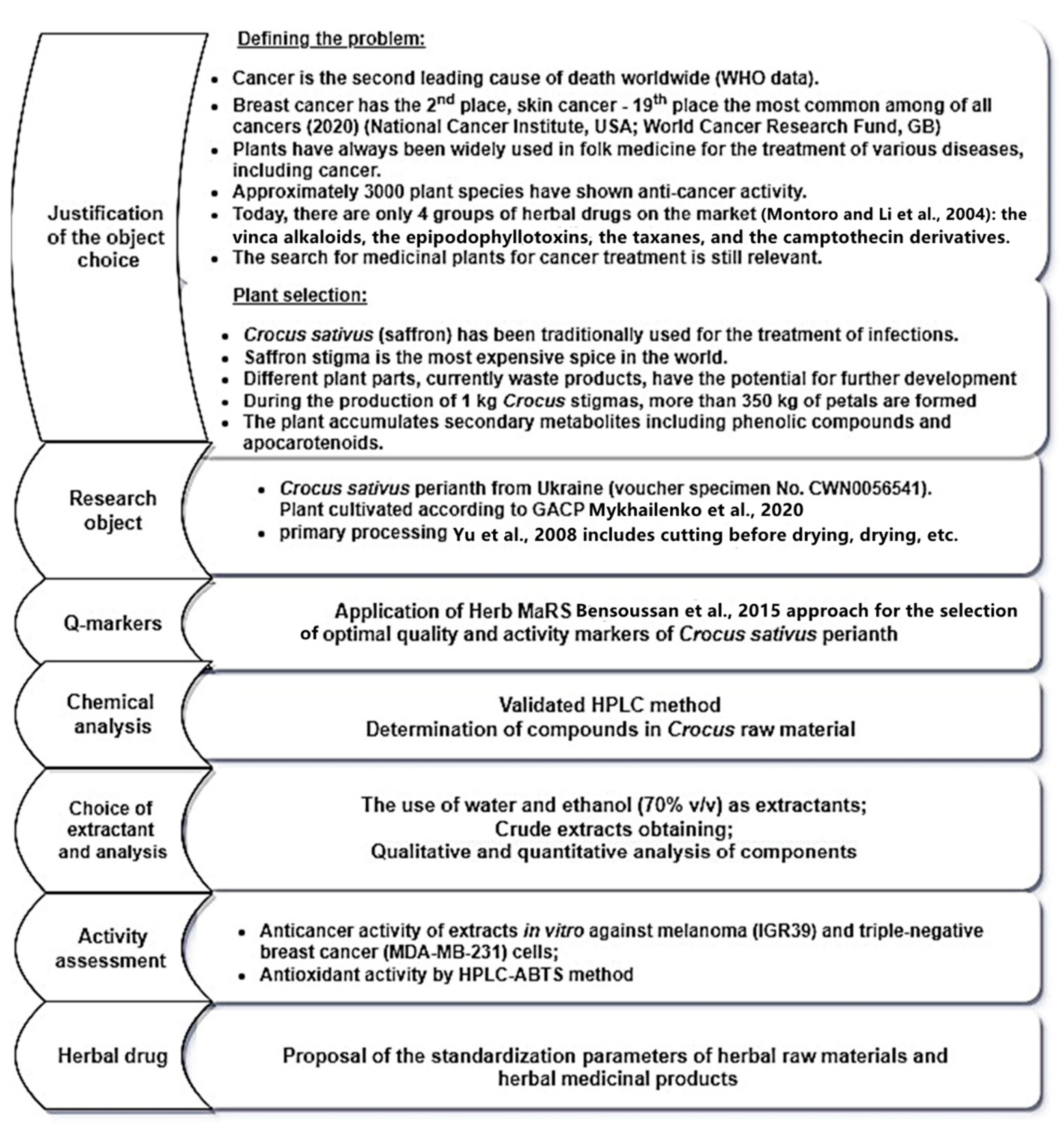
:1. Introduction
2. Results
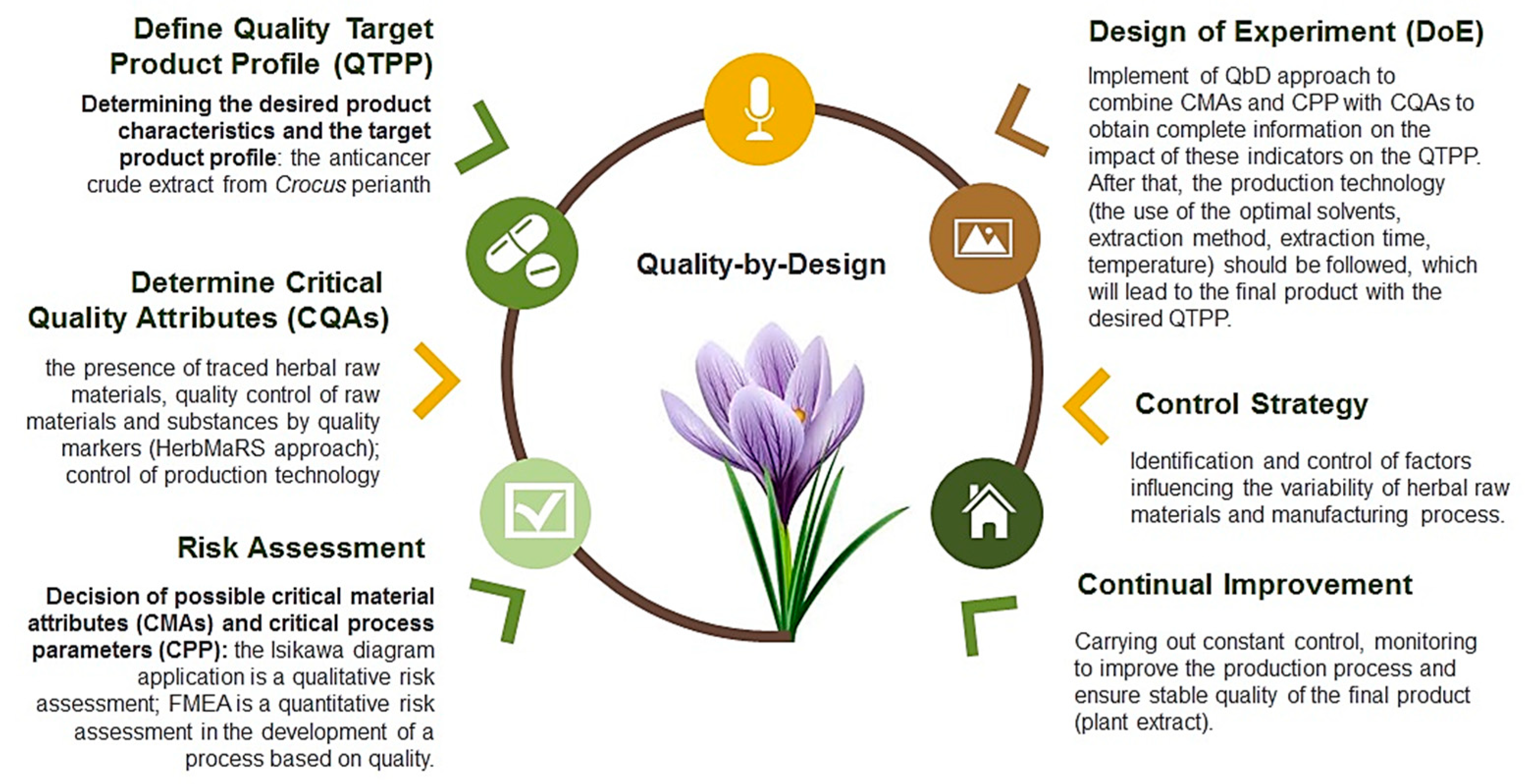
2.1. Used of QbD Approach
- ➢
- determination of the desired characteristics or the Quality Target Product Profile (QTPP);
- ➢
- identification of potentially Critical Quality Attributes of a drug (CQAs);
- ➢
- determination of possible Critical Process Parameters (CPPs) and the pharmaceutical substance characteristics and excipients (Critical Material Attributes (CMAs));
- ➢
- development and implementation of Design of Experiment (DoE), its optimization, the definition of control strategies, and improvements.
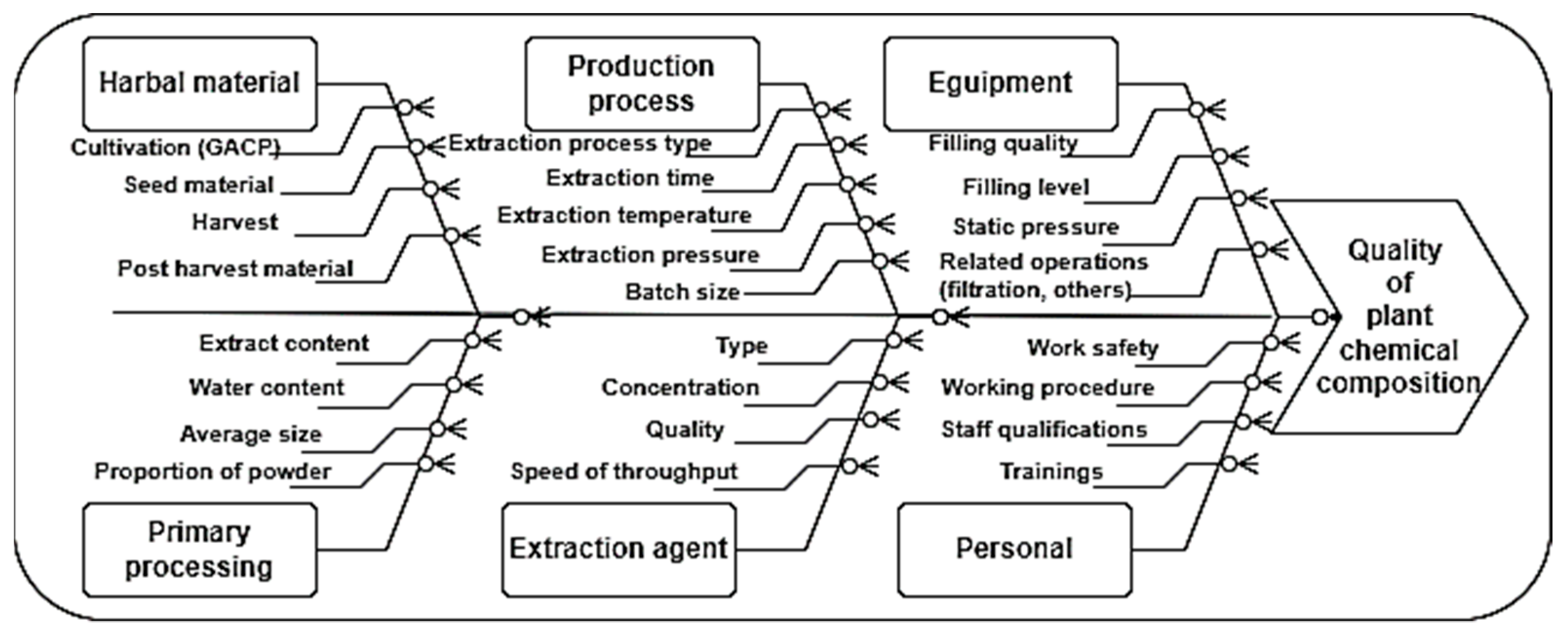
2.2. Risk Assessment of Crocus Perianth Extracts Production
2.3. Design of Experiment
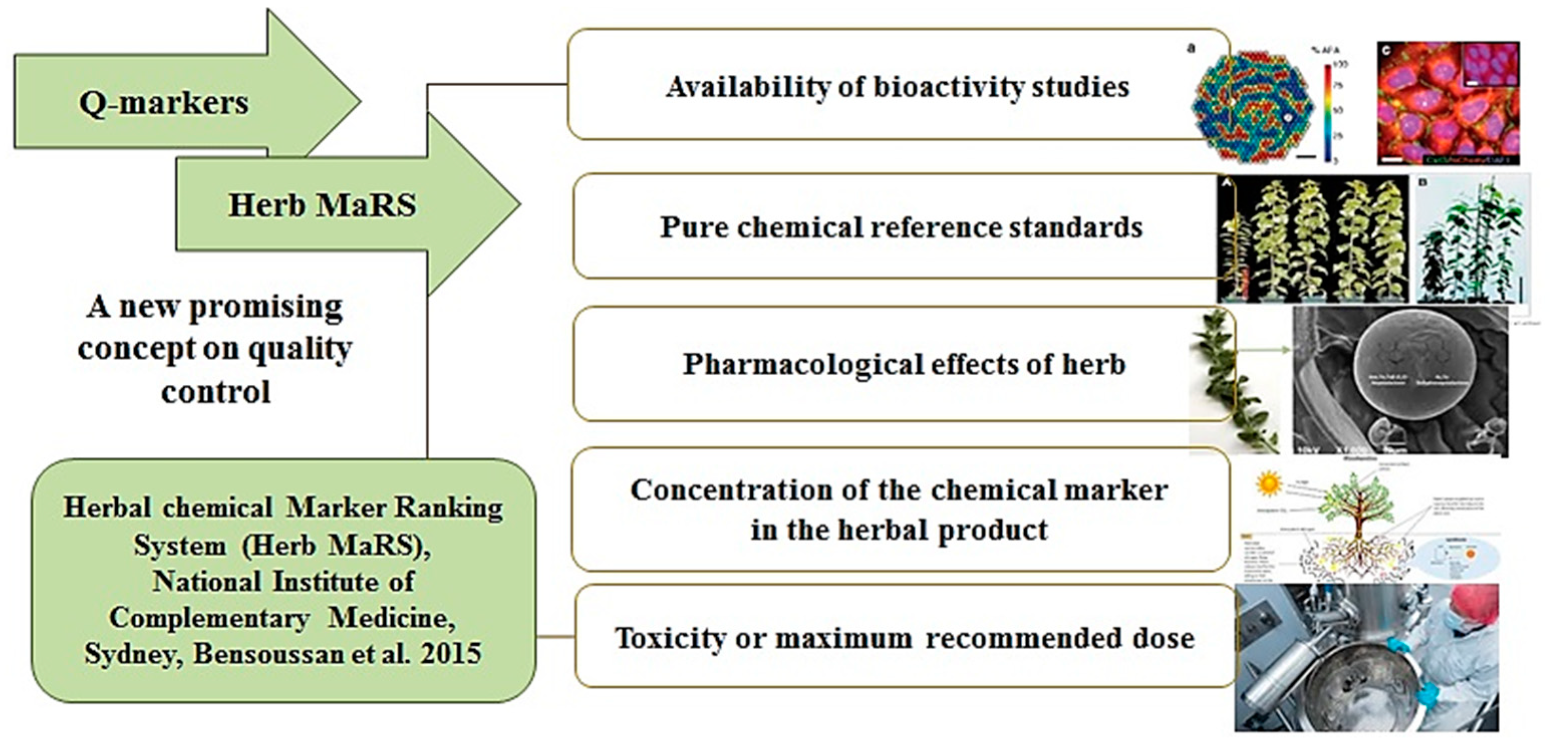
2.4. Selection of Crocus Perianth Q-Markers with the Herb MaRS-Approach
2.4.1. Traditional Saffron Used
2.4.2. Current Pharmacology Application of Compounds
2.5. Assessment of C. sativus Perianth and Its Crude Extract Chemical Composition
2.5.1. Perianth Raw Material
2.5.2. Perianth Extracts
2.6. Assessment of Crocus Perianth Crude Extract Bioactivity
2.6.1. Antioxidant Activity
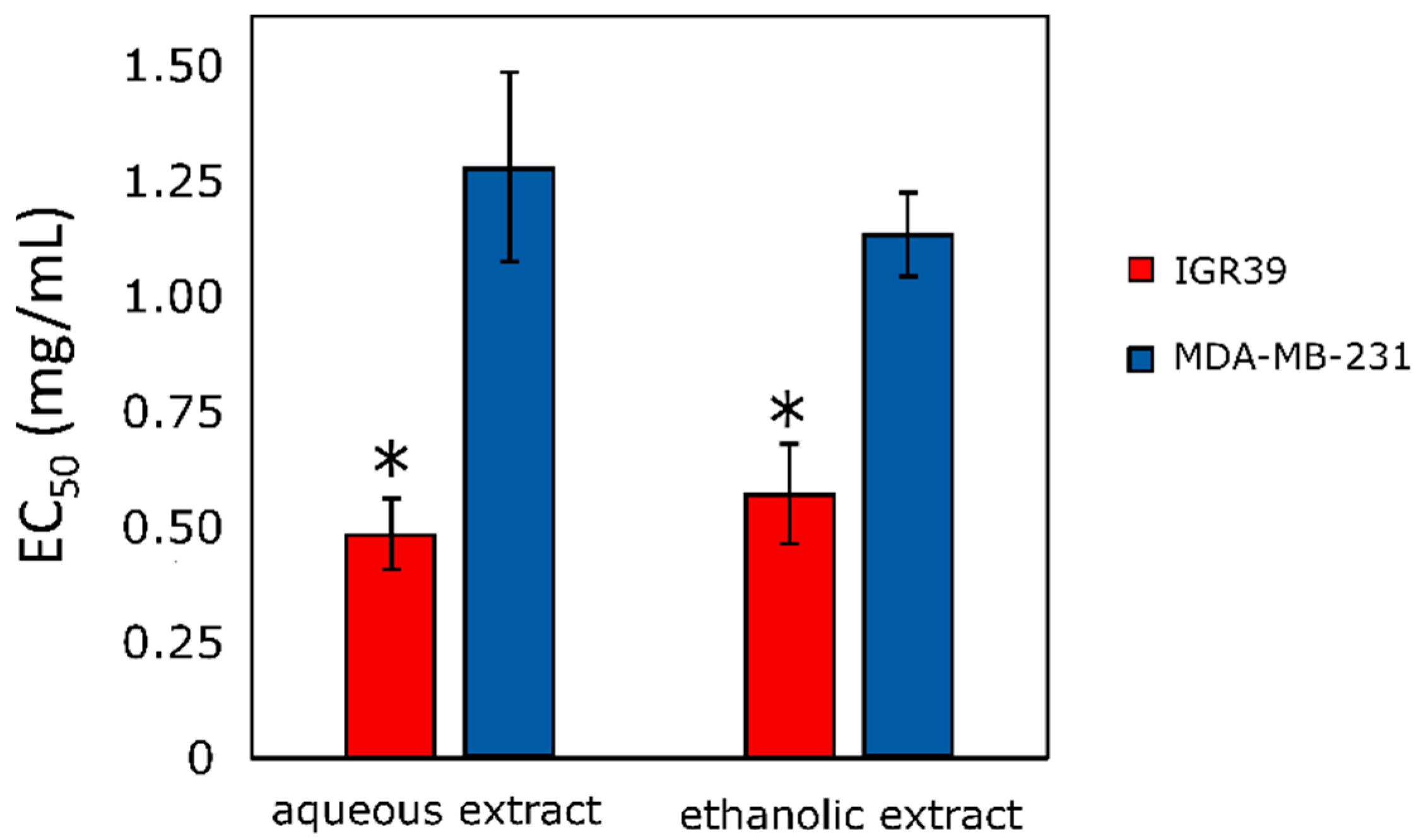
2.6.2. Cytotoxic Activity of Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Quality Characterization of Crocus Perianth
3.3. Extraction Procedure of Crocus Perianth for Bioassay
3.4. Condition of HPLC and HPLC-ABTS Analysis
3.5. In Vitro Assessment of Cytotoxic Activity
3.6. Experimental Design
3.7. Risk Analysis
3.8. Herb MaRS Approach
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| CPP | critical process parameters |
| CQAs | critical quality attributes |
| DoE | Design of experiment |
| EMA | European Medicines Agency |
| FDA | United States Food and Drug Administration |
| FMEA | Failure Modes and Effects Analysis |
| GACP | Good Agricultural and Collection Practices |
| GAP | Good Agricultural Practice |
| GHP | Good Handling Practice |
| GMP | Good Manufacturing Practice |
| HACCP | Hazard Analysis and Critical Control Points System |
| Herb MaRS | Herbal Chemical Marker Ranking System |
| HPLC-DAD | high-performance liquid chromatography coupled with diode array detector |
| ICH | International Council for the Harmonization of Specifications for Pharmaceutical Products for Human Use |
| QbD | Quality by Design |
| Q-marker | quality marker |
| QTPP | quality target product profile |
| WHO | World Health Organization |
References
- Jimenez-Garcia, S.; Vazquez-Cruz, M.; Guevara-González, R.G.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A. Current approaches for enhanced expression of secondary metabolites as bioactive compounds in plants for agronomic and human health purposes. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Araújo, R.; Martínez-Vázquez, D.G.; Charles-Rodríguez, A.V.; Rangel-Ortega, S.; Robledo-Olivo, A. Bioactive Compounds from agricultural residues, their obtaining techniques, and the antimicrobial effect as postharvest additives. Int. J. Food Sci. 2021, 2021, 9936722. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.R.; Rasul Suleria, H.A. Human health benefits of plant bioactive compounds. In Potentials and Prospects, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2021; p. 396. [Google Scholar]
- European Medicines Agency. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products; EMA/HMPC/201116/2005 Rev. 2; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- European Medicines Agency (EMA). ICH Guideline Q8 (R2) on Pharmaceutical Development. Step 5; EMA/CHMP/ICH/167068/2004; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- EMA. EMA-FDA Pilot Program for Parallel Assessment of Quality-by-Design Applications: Lessons Learnt and Q&A Resulting from the First Parallel Assessment; EMA/430501/2013; Human Medicines Development and Evaluation; European Medicines Agency and U.S. Food and Drug Administration: Silver Spring, MD, USA, 2013. [Google Scholar]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Salinas, M.E.M.R.; Alonso, G.L. Bioactivity and bioavailability of the major metabolites of Crocus sativus L. flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgarpanah, J.; Darabi-Mahboub, E.; Mahboubi, A.; Mehrab, R.; Hakemivala, M. In-vitro evaluation of Crocus sativus L. petals and stamens as natural antibacterial agents against food-borne bacterial strains. Iran. J. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) flower water extract disrupts the cecal microbiome, brush border membrane functionality, and morphology in vivo (gallus gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Luciani, L.; Tuberoso, C.I.; Congiu, F.; Pizza, C. Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J. Food Sci. 2012, 77, 893–900. [Google Scholar] [CrossRef]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable processing of floral bio-residues of saffron (Crocus sativus L.) for valuable biorefinery products. Plants 2021, 10, 523. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Santana-Méridas, O.; Polissiou, M.; Vioque, J.; Astraka, K.; Alaizd, M.; Herraiz-Peñalvera, D.; Tarantilis, P.A.; Girón-Calle, J. Polyphenol composition and in vitro antiproliferative effect of corm, tepal and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2). J. Funct. Foods 2016, 24, 18–25. [Google Scholar] [CrossRef] [Green Version]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; N’Da, C.I.; Gidaro, M.C.; Costa, G.; et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Desenko, V.; Ivanauskas, L.; Georgiyants, V. Standard operating procedure of Ukrainian saffron cultivation according with Good Agricultural and Collection Practices to assure quality and traceability. Ind. Crops Prod. 2020, 151, 112376–112387. [Google Scholar] [CrossRef]
- Shanaida, M.; Golembiovska, O.; Hudz, N.; Wieczorek, P.P. Phenolic compounds of herbal infusions obtained from some species of the Lamiaceae family. Curr. Issues Pharm. Med. Sci. 2018, 31, 194–199. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Overview of Comments Received on Draft ‘Reflection Paper on Markers Used for Quantitative and Qualitative Analysis of Herbal Medicinal Products and Traditional Herbal Medicinal Products’; EMEA/HMPC/253629/2007; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bensoussan, A.; Lee, S.; Murray, C.; Bourchier, S.; van der Kooy, F.; Pearson, J.; Liu, J.; Chang, D.; Khoo, C. Choosing chemical markers for quality assurance of complex herbal medicines: Development and application of the Herb MaRS criteria. Clin. Pharmacol. Ther. 2015, 97, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Guideline on the Evaluation of Anticancer Medicinal Products in Man; EMA/CHMP/205/95 Rev.5; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- European Medicines Agency. Questions and Answers on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products—Revision 6; EMA/HMPC/41500/2010 Rev.6; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- European Medicines Agency. Guideline on Specifications Test Procedures and Acceptance Criteria for Herbal Substances Herbal Preparations and Herbal Medicinal Products Traditional Herbal Medicinal Products—Revision 2; EMA/HMPC/162241/2005 Rev. 2; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Schmidt, A.; Strube, J. Distinct and quantitative validation method for predictive process modeling with examples of liquid-liquid extraction processes of complex feed mixtures. Processes 2019, 7, 298. [Google Scholar] [CrossRef] [Green Version]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal Plants: Influence of Environmental Factors on the Content of Secondary Metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 15, pp. 259–277. [Google Scholar]
- EMEA. Guideline on Good Agricultural and Collection Practice (GACP) for Starting Materials of Herbal Origin, London, European Medicines for Human Use; EMEA/HMPC/246816/2005; EMEA: London, UK, 2006. [Google Scholar]
- Uhlenbrock, L.; Sixt, M.; Strube, J. Quality-by-Design (QbD) process evaluation for phytopharmaceuticals on the example of 10-deacetylbaccatin III from yew. Resour. Effic. Technol. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q9 on Quality Risk Management; EMA/CHMP/ICH/24235/2006; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Savchenko, L.; Pidpruzhnykov, Y.; Ivanauskas, L.; Lukošius, A.; Georgiyants, V. Risk assessment for compounding ointments quality by Ishikawa diagram construction. Farmacia 2021, 69, 688–696. [Google Scholar] [CrossRef]
- Fahmy, R.; Kona, R.; Dandu, R.; Xie, W.; Claycamp, G.; Hoag, S.W. Quality by design I: Application of failure mode effect analysis (FMEA) and Plackett-Burman design of experiments in the identification of “main factors” in the formulation and process design space for roller-compacted ciprofloxacin hydrochloride immediate-release tablets. AAPS Pharm. Sci. Technol. 2012, 13, 1243–1254. [Google Scholar]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Sagbo, I.J.; Otang-Mbeng, W. Plants used for the traditional management of cancer in the eastern cape province of South Africa: A review of ethnobotanical surveys, ethnopharmacological studies and active phytochemicals. Molecules 2021, 26, 4639. [Google Scholar] [CrossRef]
- Bolhassani, A. Chapter 10—Bioactive components of saffron and their pharmacological properties. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 289–311. [Google Scholar]
- Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. The genus Crocus L.: A review of ethnobotanical uses, phytochemistry and pharmacology. Ind. Crops Prod. 2021, 171, 113923–113948. [Google Scholar] [CrossRef]
- Montoro, P.; Tuberoso, C.I.G.; Maldini, M.; Cabras, P.; Pizza, C. Qualitative profile and quantitative determination of flavonoids from Crocus sativus L. petals by LC-MS/M. Nat. Prod. Commun. 2008, 3, 2013–2016. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Trapero, A.; Ahrazem, O.; Rubio-Moraga, A.; Jimeno, M.L.; Gómez, M.D.; Gómez-Gómez, L. Characterization of a glucosyltransferase enzyme involved in the formation of kaempferol and quercetin sophorosides in Crocus sativus. Plant Physiol. 2012, 159, 1335–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignolini, P.; Heimler, D.; Pinelli, P.; Ieri, F.; Sciullo, A.; Romani, A. Characterization of by-products of saffron (Crocus sativus L.) production. Nat. Prod. Commun. 2008, 3, 1959–1962. [Google Scholar] [CrossRef] [Green Version]
- Singab, A.N.B.; Ayoub, I.M.; El-Shazly, M.; Korinek, M.; Wu, T.-Y.; Cheng, Y.-B.; Wu, Y.-C. Shedding the light on Iridaceae: Ethnobotany, phytochemistry and biological activity. Ind. Crops Prod. 2016, 92, 308–335. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Petrikaitė, V.; Korinek, M.; El-Shazly, M.; Chen, B.-H.; Yen, C.-H.; Hsieh, C.-F.; Bezruk, I.; Dabrišiūtė, A.; Ivanauskas, L.; et al. Bio-guided bioactive profiling and HPLC-DAD fingerprinting of Ukrainian saffron (Crocus sativus stigma): Moving from Correlation toward Causation. BMC Complement. Med. Ther. 2021, 21, 203. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Lesyk, R.; Georgiyants, V. Characterization of phytochemical components of Crocus sativus leaves using HPLC-MS/MS and GC-MS: A new potential by-product. Sci. Pharm. 2021, 89, 28. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.M.; Tsatsakis, A. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed. 2015, 5, 376–391. [Google Scholar]
- Nassiri-Asl, M.; Hosseinzadeh, H. Neuropharmacology effects of saffron (Crocus sativus) and its active constituents. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Academic Press: Cambridge, MA, USA, 2015; Volume 3, pp. 29–39. [Google Scholar]
- Mir, M.A.; Ganai, S.A.; Mansoor, S.; Jan, S.; Mani, P.; Masoodi, K.Z.; Amin, H.; Rehman, M.U.; Ahmad, P. Isolation, purification and characterization of naturally derived crocetin beta-D-glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER-alpha/HDAC2. Saudi J. Biol. Sci. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anti Cancer Agents Med. Chem. 2019, 19, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, J.; Xiao, M. Picrocrocin exhibits growth inhibitory effects against SKMEL- 2 human malignant melanoma cells by targeting JAK/ STAT5 signaling pathway, cell cycle arrest and mitochondrial mediated apoptosis. J. BUON 2018, 23, 1163–1168. [Google Scholar] [PubMed]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017, 69, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2020, 26, 86. [Google Scholar] [CrossRef]
- Chryssanthi, D.G.; Lamari, F.N.; Iatrou, G.; Pylara, A.; Karamanos, N.K.; Cordopatis, P. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007, 27, 357–362. [Google Scholar]
- Chen, S.S.; Gu, Y.; Lu, F.; Qian, D.P.; Dong, T.T.; Ding, Z.H.; Zhao, S.; Yu, Z.H. Antiangiogenic effect of crocin on breast cancer cell MDA-MB-231. J. Thorac. Dis. 2019, 11, 4464–4473. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, A.; Serio, M.; Canuti, L.; Canini, A. Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am. J. Plant Sci. 2012, 3, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Chryssanthi, D.G.; Dedes, P.G.; Karamanos, N.K.; Cordopatis, P.; Lamari, F.N. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011, 77, 146–151. [Google Scholar] [CrossRef]
- Escribano, J.; Alonso, G.L.; Coca-Prados, M.; Fernandez, J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef]
- Razavi, B.M.; Amanloo, M.A.; Imenshahidi, M.; Hosseinzadeh, H. The relaxant activity of safranal in isolated rat aortas is mediated predominantly via an endothelium-independent mechanism: Vasodilatory mechanism of safranal. J. Pharmacopunct. 2016, 19, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, S.; Madan, K. The role of safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review. Heliyon 2021, 7, e06117. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, C.; Seçme, M.; Bağcı, G.; Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. 2015, 36, 9437–9446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Park, S.U. A recent overview on the biological and pharmacological activities of ferulic acid. Excli J. 2019, 18, 132–138. [Google Scholar]
- Son, B.K.; Choi, Y.S.; Sohn, Y.W. Anticancer effect of ferulic acid on cultured human skin melanoma cells. J. Exp. Biomed. Sci. 2006, 12, 457–461. [Google Scholar]
- Rajendra, P.N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Mei, S.; Ma, H.; Chen, X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems—A novel research direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Ghosal, S.; Chattopadhyay, U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 1996, 42, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Protective role of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 2008, 31, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Ganapathy, E.; Sakthisekaran, D. Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 137–142. [Google Scholar] [CrossRef]
- Gundogdu, G.; Dodurga, Y.; Elmas, L.; Tasci, S.Y.; Karaoglan, E.S. Investigation of the anticancer mechanism of isoorientin isolated from Eremurus Spectabilis leaves via cell cycle pathways in HT-29 human colorectal adenocarcinoma cells. Eurasian J. Med. 2018, 50, 168–172. [Google Scholar] [CrossRef]
- Ye, T.; Su, J.; Huang, C.; Yu, D.; Dai, S.; Huang, X.; Chen, B.; Zhou, M. Isoorientin induces apoptosis, decreases invasiveness, and downregulates VEGF secretion by activating AMPK signaling in pancreatic cancer cells. OncoTargets Ther. 2016, 9, 7481–7492. [Google Scholar] [CrossRef] [Green Version]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of Pueraria tuberosa. Oxid. Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Ku, S.K.; Lee, I.C.; Bae, J.S. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflamm. Res. 2012, 61, 217–224. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Drewa, G.; Schachtschabel, D.O.; Pałgan, K.; Grzanka, A.; Sujkowska, R. The influence of rutin on the weight, metastasis and melanin content of B16 melanotic melanoma in C57BL/6 mice. Neoplasma 1998, 45, 266–271. [Google Scholar]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid. Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D. Rutin exerts cytotoxic and senescence-inducing properties in human melanoma cells. Toxics 2021, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Kamal, A.M.; Haggag, E.G.; El Sayed, K.A. Rutin as a novel c-met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer 2017, 69, 1256–1271. [Google Scholar] [CrossRef]
- Chen, F.; Chen, X.; Yang, D.; Che, X.; Wang, J.; Li, X.; Zhang, Z.; Wang, Q.; Zheng, W.; Wang, L.; et al. Isoquercitrin inhibits bladder cancer progression in vivo and in vitro by regulating the PI3K/Akt and PKC signaling pathways. Oncol. Rep. 2016, 36, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Orfali Gd Duarte, A.C.; Bonadio, V.; Martinez, N.P.; de Araújo, M.E.; Priviero, F.B.; Carvalho, P.O.; Priolli, D.G. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef]
- Chen, Q.; Li, P.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2014, 33, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.; Mazzio, E.; Soliman, K.F.A. Whole transcriptomic analysis of apigenin on TNFα immuno-activated MDA-MB-231 breast cancer cells. Cancer Genom. Proteom. 2019, 16, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7-a comparative study. Cell Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, R80–R88. [Google Scholar] [CrossRef] [Green Version]
- Ghițu, A.; Schwiebs, A.; Radeke, H.H.; Avram, S.; Zupko, I.; Bor, A.; Pavel, I.Z.; Dehelean, C.A.; Oprean, F.; Bojin, C.; et al. A comprehensive assessment of apigenin as an antiproliferative, proapoptotic, antiangiogenic and immunomodulatory phytocompound. Nutrients 2019, 11, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. Excli J. 2017, 16, 795–807. [Google Scholar] [PubMed]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin inhibits cell migration and invasion in human osteosarcoma cells. Cell Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Liu, M.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 Cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Lin, C.L.; Lin, J.K. 1,2,3,4,6-penta-O-galloyl-β-Dglucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J. Agric. Food Chem. 2011, 59, 6765–6775. [Google Scholar] [CrossRef]
- Yanqiu, H.; Linjuan, C.; Jin, W.; Hao, H.; Shi, Y.; Xue, G.; Ren, H. The effects of quercetin and kaempferol on multidrug resistance and the expression of related genes in human erythroleukemic K562/A cells. Afr. J. Biotechnol. 2011, 10, 13399–13406. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.Y.; Xie, J.; Wang, Z.L.; Wen, Y.L.; Zhao, C.C.; Tao, L.; Li, L.F.; Tian, Y.; Sheng, J. Astragalin inhibits the proliferation and migration of human colon cancer HCT116 cells by regulating the NF-κB sSignaling pathway. Front. Pharmacol. 2021, 12, 639256. [Google Scholar]
- Wang, Z.; Lv, J.; Li, X.; Lin, Q. The flavonoid Astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem. Biol. Drug Des. 2021, 98, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Y.; Jiang, X.; Zhang, H.; Gao, Z.; Li, Y.; Fu, R.; Li, L.; Li, J.; Cui, H.; et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019, 38, 225. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, Y.; Li, J.; Huang, S.; Gao, R. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci. Rep. 2020, 40, BSR20192826. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.U.; Nasim, S.; Baig, I.; Jalil, S.; Orhan, I.; Sener, B.; Choudhary, M.I. Anti-inflammatory isoflavonoids from the rhizomes of Iris germanica. J. Ethnopharmacol. 2003, 86, 177–180. [Google Scholar] [CrossRef]
- Jun, H.J.; Hoang, M.H.; Lee, J.W.; Yaoyao, J.; Lee, J.H.; Lee, D.H.; Lee, H.J.; Seo, W.D.; Hwang, B.Y.; Lee, S.J. Iristectorigenin B isolated from Belamcanda chinensis is a liver X receptor modulator that increases ABCA1 and ABCG1 expression in macrophage RAW 264.7 cells. Biotechnol. Lett. 2012, 34, 2213–2221. [Google Scholar] [CrossRef]
- Lim, H.; Park, B.K.; Shin, S.Y.; Kwon, Y.S.; Kim, H.P. Methyl caffeate and some plant constituents inhibit age-related inflammation: Effects on senescence-associated secretory phenotype (SASP) formation. Arch. Pharm. Res. 2017, 40, 524–535. [Google Scholar] [CrossRef]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, S.B.; Jung, S.H.; Cha, K.H.; Park, W.D.; Sohn, Y.C.; Nho, C.W. Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway. Mol. Cells 2009, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.; Yang, J.; Chai, H.; Li, Y.; Yang, J.; Jia, Z.; Wang, Z. Tectoridin, an isoflavone glycoside from the flower of Pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology 2010, 276, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.Z.; Bathaie, S.Z. Historical uses of saffron: Identifying potential new avenues for modern research. Avicenna J. Phytomed. 2011, 1, 57–66. [Google Scholar]
- WHO. Monographs on Selected Medicinal Plants; World Health Organization: Barcelona, Spain, 2007; Volume 3, pp. 126–135. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Extraction, characterization, stability and biological activity of flavonoids isolated from Chamomile flowers. Mol. Cell Pharmacol. 2009, 1, 138–153. [Google Scholar] [CrossRef]
- Xiao, J. Review dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar]
- Marksa, M.; Radušienė, J.; Jakštas, V.; Ivanauskas, L.; Marksienė, R. Development of an HPLC post-column antioxidant assay for Solidago canadensis radical scavengers. Nat. Prod. Res. 2015, 30, 536–543. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology; Q2 (R1); ICH Secretariat: Geneva, Switzerland, 2005; p. 17. [Google Scholar]
| Risk Area, CCP/QCP | Failure Mode | Potential Cause or Route of Failure | Detection or Control Method | Risk Analysis | Correction Action | |||
|---|---|---|---|---|---|---|---|---|
| S | P | D | RPN | |||||
| Herbal raw material QPT |
| Poor cultivation management, inappropriate soil and irrigation; physical properties; long duration of handling and transporting, warm and humid condition; poor personal hygiene in collection; wrong handling by personnel. | Compliance with GACP principles of all cultivation processes; certified suppliers with HACCP program; documenting; visual inspection; botanical identification; soil analysis; metal detector; microbiological analysis. | 5 | 4 | 4 | 80 | Rejection, sorting, instructions to supplier |
| 4 | 3 | 3 | 36 | |||||
| 5 | 4 | 4 | 80 | |||||
| 5 | 4 | 5 | 60 | |||||
| Primary processing CCP |
| Improper control of temperature and time of drying. operator error, poor development; material variation. | Calibration of thermometer and timer, maintenance program; personnel training; visual control; monitoring; microbiological analysis; chemical analysis. | 5 | 4 | 5 | 100 | Re-dry, sorting out; repair and replace damaged equipment |
| 5 | 3 | 5 | 75 | |||||
| 4 | 3 | 2 | 24 | |||||
| 4 | 3 | 2 | 24 | |||||
| Extraction agent CCP |
| Uniformity of the extractant concentration; content of extractive compounds; ethanol concentration; operator’s error. | Alcoholometry; monitoring; chemical analysis; calibration; temperature control. | 5 | 4 | 4 | 80 | Instruction to operator |
| 5 | 4 | 5 | 100 | |||||
| 5 | 4 | 5 | 100 | |||||
| 5 | 3 | 3 | 45 | |||||
| Production process CCP |
| Poor monitoring; operator’s error, equipment failure; machine failure, poor development; improper control of temperature and time, improper sealing of system; uniformity of the raw material. | Calibration of thermometer and timer, maintenance program, personnel training; monitoring; visual control; temperature control. | 5 | 4 | 3 | 60 | Repair and replace damaged equipment |
| 5 | 4 | 3 | 60 | |||||
| 5 | 4 | 4 | 80 | |||||
| 5 | 4 | 3 | 60 | |||||
| 5 | 4 | 3 | 60 | |||||
| Equipment QPT |
| Defective devices for extraction, evaporation and drying. | All equipment should be easily cleaned to minimize contamination; calibration of equipment, personnel training; monitoring; visual control; temperature control. | 4 | 2 | 1 | 8 | Repair and replace damaged equipment |
| 3 | 2 | 1 | 6 | |||||
| 3 | 2 | 1 | 6 | |||||
| 3 | 2 | 1 | 6 | |||||
| Personal QPT |
| Human error; poor personnel hygiene, wrong handling by personnel. | All persons having contact with raw materials should observe a strict level of personal hygiene; personnel training; monitoring; visual control. | 4 | 2 | 1 | 8 | Training, instruction to operator |
| 4 | 3 | 2 | 24 | |||||
| 5 | 3 | 2 | 30 | |||||
| 4 | 3 | 2 | 24 | |||||
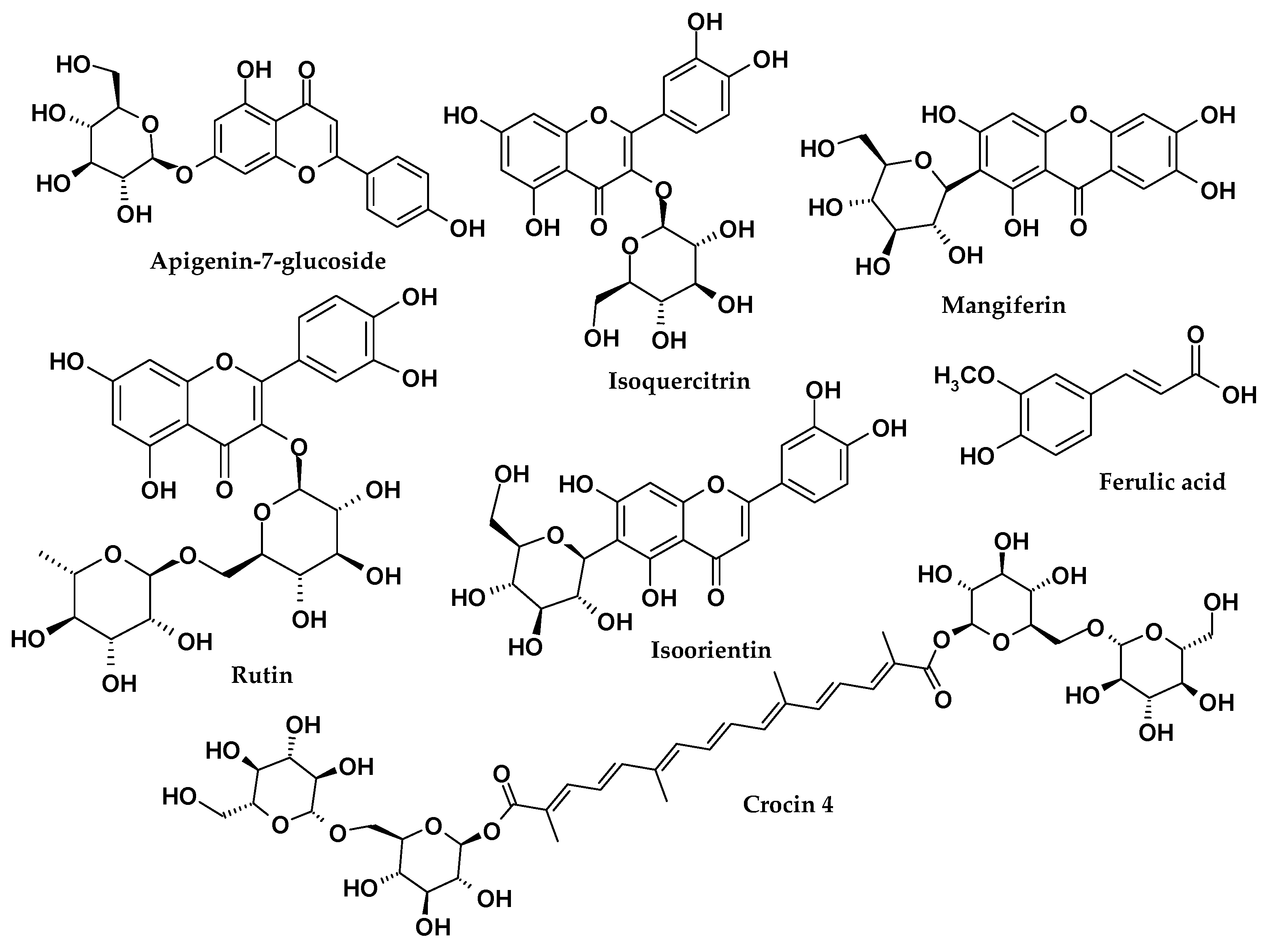
| Compound | Activity | Herb MaRS Ranking * | Reference |
|---|---|---|---|
| All crocins | Anticancer, cytotoxic, antioxidant, neuroprotective, retinal damage protection, antidepressant, anti-Alzheimer, hypolipidemic, anti-inflammatory. | 5 | [41,42,43,44,45,46,47,48,49,50,51] |
| Picrocrocin | Anticancer, antineoplastic, antioxidant. | 4 | [45,52] |
| Safranal | Antitussive, anticonvulsant, antioxidant, antianxiety, antidepressant, antinociceptive, anti-ischemia. | 3 | [53,54] |
| Ferulic acid | Anticancer, anti-inflammatory, antioxidant, antibacterial, antidiabetic. | 5 | [55,56,57,58] |
| Caffeic acid | Anticancer, antioxidant, anti-inflammatory. | 5 | [59,60] |
| Mangiferin | Anticancer, antiviral, anti-inflammatory, antidiabetic, antitumor, lipometabolism regulating, cardioprotective, antihyperuricemic, neuroprotective, antioxidant, antipyretic, analgesic, antibacterial, immunomodulatory. | 5 | [61,62,63,64,65,66,67] |
| Isoorientin | Anticancer, anti-inflammatory, QS inhibitor, antinociceptive, gastroprotective. | 5 | [68,69,70] |
| Kaempferol-3-O-sophoroside | Antiinflammatory, antitumor, antioxidative, antiallergic, antidiabetic. | 4 | [71] |
| Rutin | Anticancer, anti-inflammatory, QS inhibitor, antibacterial, antiprotozoal, antitumor, antiallergic, antiviral, cytoprotective, vasoactive, hypolipidaemic, antiplatelet, antispasmodic, antihypertensive. | 5 | [72,73,74,75,76,77] |
| Isoquercitrin | Anticancer, antioxidant, antiproliferative, anti-inflammatory, anti-hypertensive, antidiabetic. | 5 | [78,79,80] |
| Apigenin | Anticancer, antiallergic, anti-inflammatory, antioxidant, antimutagenic, anticarcinogenic. | 5 | [81,82,83,84] |
| Apigenin-7-O-glucoside | Cytotoxic effect, antifungal, anticancer, antiproliferative. | 5 | [85,86,87] |
| Quercitin and its derivatives | Anticancer, antiviral, antiprotozoal, antimicrobial, antiallergic, anti-inflammatory. | 5 | [88,89,90,91] |
| Kaempferol and its derivatives | Antioxidant, anti-inflammatory, antimicrobial, anticancer, cardioprotective, neuroprotective, antidiabetic. | 5 | [92,93,94,95,96] |
| Astragalin | Anticancer, anti-inflammatory, antioxidant, neuroprotective. | 4 | [97,98] |
| Isoramnetin | Anticancer, cardiovascular, cerebrovascular protection, anti-inflammatory, antioxidant. | 4 | [99,100] |
| Nigricin | High anti-inflammatory activity. | 3 | [101] |
| Iristectorigenin B | Liver X receptor modulator, anti-inflammatory, antioxidant. | 4 | [102,103] |
| Tectoridin | Anticancer, anti-inflammatory, antioxidant, hepatoprotectivy, hypoglycemic, antiallergic, anaphylaxis inhibitory. | 5 | [104,105,106] |
| Compound | Calibration Curve a | Correlation Coefficient r2 (n = 6) | Linear Range (μg/mL) | RSD, % | LoD b (ng/mL) | LoQ c (ng/mL) | |
|---|---|---|---|---|---|---|---|
| 1 | Mangiferin | f(x) = 29,263.5x + 13,863.9 | 0.999795 | 0.28–145.00 | 1.32 | 310 | 940 |
| 2 | Isoorientin | f(x) = 26,559.9x + 2849.65 | 0.999996 | 0.73–92.85 | 1.41 | 8 | 24 |
| 3 | Ferulic acid | f(x) = 54,955.4x − 638.345 | 0.999959 | 0.44–56.50 | 1.60 | 30 | 80 |
| 4 | Rutin | f(x) = 16,072.5x + 1499.73 | 0.999879 | 0.16–20.24 | 1.07 | 96 | 290 |
| 5 | Isoquercitrin | f(x) = 24,139.7x + 3904.44 | 0.999894 | 0.35–44.56 | 1.02 | 73 | 220 |
| 6 | Crocin | f(x) = 3789.03x + 220.836 | 0.999588 | 1.15–147.20 | 1.28 | 100 | 300 |
| 7 | Tectoridin | f(x) = 76,104.4x + 114,152 | 0.999580 | 0.51–260.00 | 0.55 | 130 | 400 |
| 8 | Astragalin | f(x) = 20,536.0x + 1618.68 | 0.999987 | 0.37–47.70 | 1.01 | 90 | 270 |
| 9 | Apigenin-7-glucoside | f(x) = 38,477.5x + 4025.41 | 0.999925 | 0.25–32.00 | 0.82 | 53 | 160 |
| 10 | Quercetin | f(x) = 39,349.5x + 1454.47 | 0.999850 | 0.16–20.08 | 0.67 | 31 | 90 |
| 11 | Kaempferol | f(x) = 29,888.8x + 1814.27 | 0.999924 | 0.14–18.32 | 0.90 | 37 | 110 |
| 12 | Iristectorigenin B | f(x) = 109,562x + 68,062.7 | 0.999681 | 0.23–120.00 | 0.85 | 50 | 150 |
| 13 | Nigricin | f(x) = 89,415.4x + 103,288 | 0.999404 | 0.35–181.00 | 0.30 | 40 | 130 |
| 14 | Safranal | f(x) = 39,230.1x – 11,887.2 | 0.999529 | 1.33–42.56 | 1.35 | 120 | 360 |
| 15 | Caffeic acid | f(x) = 57,646.8x − 3853.48 | 0.999922 | 0.72–91.92 | 1.56 | 20 | 60 |
| 16 | Apigenin | f(x) = 50,138.3x + 5722.97 | 0.999889 | 0.2–25.76 | 0.53 | 25 | 80 |
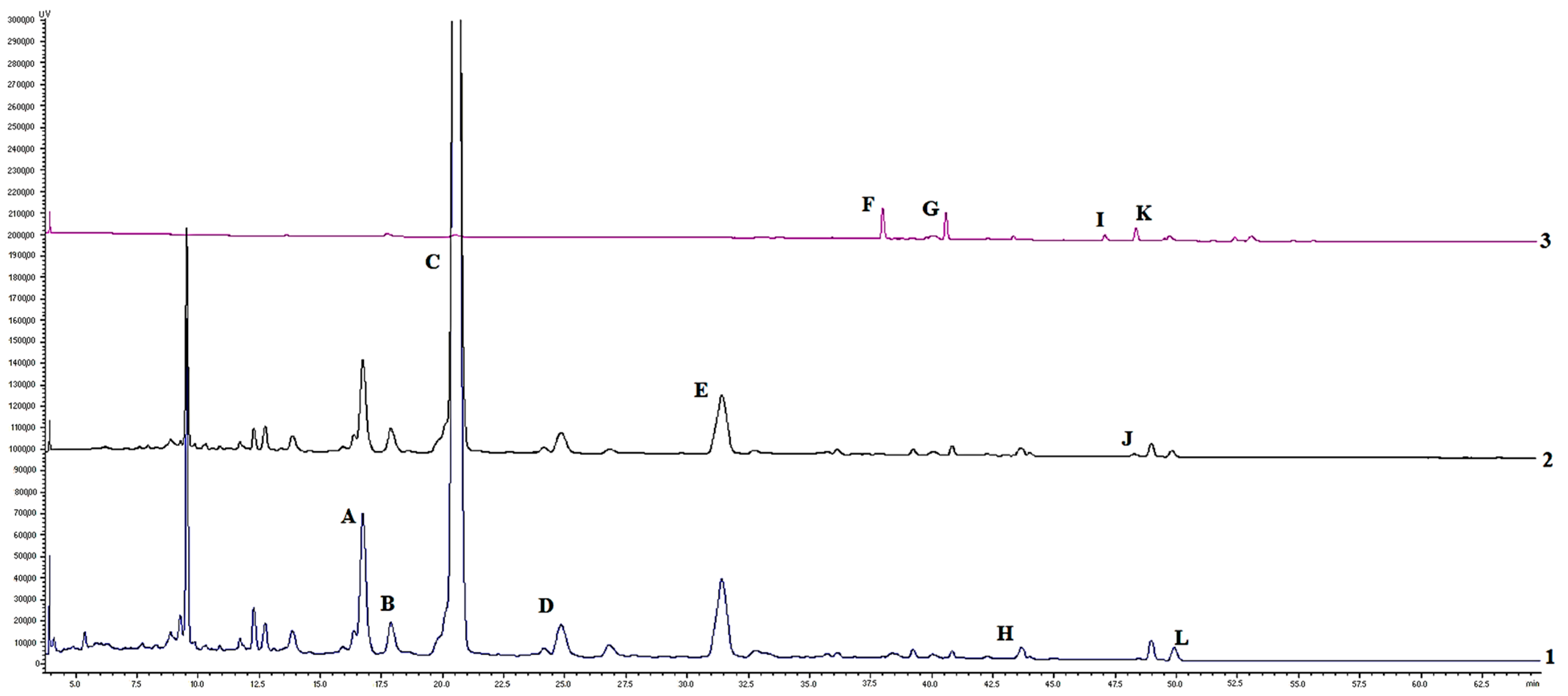
| Compound | Retention Time, min/λ, nm | Raw Material | Crocus Perianth Extracts | |
|---|---|---|---|---|
| Water | Hydroethanolic | |||
| Mangiferin | 13.83/270 | 1.060 ± 0.751 | 1.091 ± 0.014 | 0.885 ± 0.010 |
| Isoorientin | 16.74/310 | 7.389 ± 0.369 | 0.668 ± 0.112 | - |
| Kaempherol-3-O-sophoroside | 17.94/310 | 2.329 ± 0.114 | - | - |
| Rutin | 20.46/310 | 160.45 ± 7.805 | 81.157 ± 0.580 | 65.785 ± 1.089 |
| Ferulic acid | 22.74/310 | 0.025 ± 0.08 | 0.045 ± 0.004 | 0.247 ± 0.003 |
| Isoquercitrin | 24.76/350 | 2.704 ± 0.104 | 1.785 ± 0.004 | 1.322 ± 0.026 |
| Tectoridin | 31.34/270 | 2.230 ± 0.093 | 1.423 ± 0.003 | 0.921 ± 0.070 |
| Apigenin-7-O-glucoside | 31.41/340 | 8.114 ± 0.387 | 2.587 ± 1.587 | - |
| trans-Crocin 4 | 38.00/440 | 2.662 ± 0.113 | 3.788 ± 0.015 | 0.203 ± 0.003 |
| trans-Crocin 2 | 40.59/440 | 2.299 ± 0.109 | - | - |
| Quercetin | 43.63/310 | 0.481 ± 0.019 | 0.229 ± 0.540 | 0.253 ± 0.003 |
| cis-Crocin 4 | 47.07/440 | 0.591 ± 0.028 | - | - |
| cis-Crocin 3 | 48.36/440 | 1.356 ± 0.057 | 0.809 ± 0.361 | - |
| Nigricin | 48.94/270 | 0.117 ± 0.015 | 0.052 ± 1.137 | 0.099 ± 0.022 |
| Iristectorigenin B | 49.15/270 | 0.142 ± 0.05 | 0.139 ± 0.012 | 0.142 ± 0.006 |
| Kaempferol | 49.43/310 | 0.916 ± 0.031 | 0.820 ± 0.003 | 1.018 ± 0.021 |
| Compound | Retention Time | Water Extract | Hydroethanolic Extract |
|---|---|---|---|
| Mangiferin | 15.34 | 15.76 ± 0.28 | 128.13 ± 2.25 |
| Isoorientin | 18.67 | 5.51 ± 0.10 | - |
| Rutin | 22.42 | 7.78 ± 0.14 | 3.77 ± 0.07 |
| Ferulic acid | 23.77 | 9.12 ± 0.16 | 110.15 ± 1.94 |
| Tectoridin | 29.90 | 15.16 ± 0.27 | 11.60 ± 0.20 |
| Quercetin | 44.37 | 10.05 ± 0.18 | 121.11 ± 2.13 |
| Apigenin-7-O-glucoside | 46.82 | 6.13 ± 0.11 | - |
| Iristectorigenin B | 51.71 | 13.47 ± 0.24 | 23.06 ± 0.41 |
| Nigricin | 52.88 | 5.66 ± 0.10 | 3.04 ± 0.05 |
| Total | 88.64 ± 1.56 | 400.86 ± 7.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mykhailenko, O.; Ivanauskas, L.; Bezruk, I.; Petrikaitė, V.; Georgiyants, V. Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth. Sci. Pharm. 2022, 90, 19. https://doi.org/10.3390/scipharm90010019
Mykhailenko O, Ivanauskas L, Bezruk I, Petrikaitė V, Georgiyants V. Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth. Scientia Pharmaceutica. 2022; 90(1):19. https://doi.org/10.3390/scipharm90010019
Chicago/Turabian StyleMykhailenko, Olha, Liudas Ivanauskas, Ivan Bezruk, Vilma Petrikaitė, and Victoriya Georgiyants. 2022. "Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth" Scientia Pharmaceutica 90, no. 1: 19. https://doi.org/10.3390/scipharm90010019
APA StyleMykhailenko, O., Ivanauskas, L., Bezruk, I., Petrikaitė, V., & Georgiyants, V. (2022). Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth. Scientia Pharmaceutica, 90(1), 19. https://doi.org/10.3390/scipharm90010019








