Abstract
Taste is the most crucial organoleptic parameter affecting patient compliance in the case of drugs with poor palatability. Taste masking is a major challenge for the development of orally ingested active pharmaceutical constituents in the pharmaceutical industry. Numerous conventional taste-masking techniques have been extensively studied. In parallel, affecting the drug solubility or release is a major concern of conventional taste-masking techniques. Recently, many nanocarrier systems have been introduced, claiming the advantage of effective taste masking without affecting either the drug solubility or its release. In this review, we will present new techniques for taste masking, including taste-masking techniques utilizing nanocarrier systems such as liposomes, polymeric and solid lipid nanoparticles, polymeric micelles, submicron lipid emulsions, and nanogels. We will chiefly highlight the composition of these systems and their applications in designing oral therapeutic delivery systems successful in masking the taste of bitter molecules.
1. Introduction
Oral administration, among the multiple routes used to deliver medicines, is the most readily accepted route and used extensively as it offers a lot of advantages such as being easy, self-administrable, and painless which increase patient acceptance and compliance [1]. Organoleptic properties are considered crucial criteria for the development of an oral solid dosage form as they could affect consumer liking and compliance [2]. Drugs of poor or unaccepted palatability have to be formulated focusing mainly on enhancing the palatability to the maximum to mitigate the bad impact of poor palatability on patient compliance and the whole acceptance of the oral dosage form [3,4]. The taste of oral drugs which pass and reside on the tasting sensory buds in the mouth is highly critical [5]. As the majority of the active pharmaceutical ingredients (APIs) show unaccepted palatability or extensive bitterness, taste acceptance is of high concern, especially from the point of formulation development. Methods and techniques utilized for taste improvement have to be involved in formulation development [5].
The conventional techniques have been studied extensively. Using flavors and sweeteners is reported as the simplest technique [6,7]. Another common technique is the coating of drugs using a suitable polymer [8]; the coating acts as a physical barrier to the drug particles, thereby minimizing interaction between the drug and taste buds [9]. Ion exchange resins are an interesting option used to develop cost-effective, rapid, and simple techniques of taste masking; these resins are crosslinked water-insoluble polymers which contain salt-forming groups in multiple places on the chain of the polymer [10]. Microencapsulation is a process by which very tiny droplets or particles of liquid or solid material are surrounded or coated with a continuous film of a polymeric material which could act as a barrier between the drug and the sensory places [11]. Various prodrugs of nalbuphine, naloxone, naltrexone, oxymorphone, butorphanol, and levallorphan, in which the 3-phenolic hydroxyl group was esterified, lack the bitter taste [12,13]. An ondansetron-magnesium aluminum silicate adsorption system masked the bitter taste of ondansetron HCl, utilizing the excipient, which can delay the reach of the drug to the taste buds [14]. To mask the bitterness, a specific bitterness inhibitor would be most useful. Lipoprotein, made of phosphatidic acid and beta-lactoglobulin, selectively suppresses the taste responses to bitter substances. Inhibitors work by inhibiting human taste sensation to bitter stimuli in the taste buds [15]. Masking the bitter taste of tablets by a water-insoluble gel formed by sodium alginate and bivalent metal was studied. Artemether is a drug used to treat malaria with an intensely bitter taste. Its taste masking was done by solid dispersion with mono amino glycyrrhizinate pentahydrate by solvent evaporation method [16].
This review is a collective of the taste-masking approaches utilizing nanocarrier systems, highlighting the composition of these systems and their application in designing oral therapeutic delivery systems for bitter-tasting molecules. A study selection flow diagram and characteristics table are shown in Figure 1 and Table 1. In addition, a diagram showing the number of studies used in this review per year is included in Figure 2.
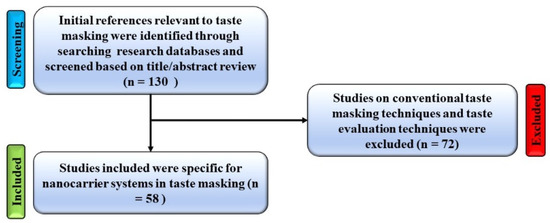
Figure 1.
Study selection flow diagram.

Table 1.
Summary table of study characteristics.
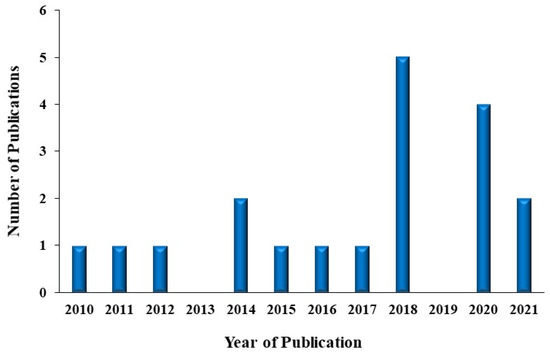
Figure 2.
Number of publications for nanocarrier systems in taste masking per year.
2. Nanocarrier Systems and Taste Masking
Nanocarrier systems can be in the form of liposomes, nanoparticles, nanosuspensions, nanomicelles, or nanoemulsions [36]. Nanocarriers can be also divided, according to their physical state, into either hard or soft forms, meanwhile, the intermediate between both forms is existing [37]. An example of the hard form is nanoparticles (whether lipid or polymeric), which are characterized by lacking elasticity and flexibility. Soft forms such as polymeric micelles, liposomes, and nanoemulsions have the ability to deform and reform. Soft forms could cross tissue extracellular spaces and capillary beds; such ability comes from their flexibility. On the other hand, the hard forms may block capillaries [37]. Different nanocarrier systems are illustrated in Figure 3. Table 2 summarizes the various nanocarrier systems formulated to mask the taste of different drugs, highlighting the materials used to prepare these systems. Table 3 summarizes the advantages, disadvantages, and characteristics of nanocarrier systems used in taste masking.
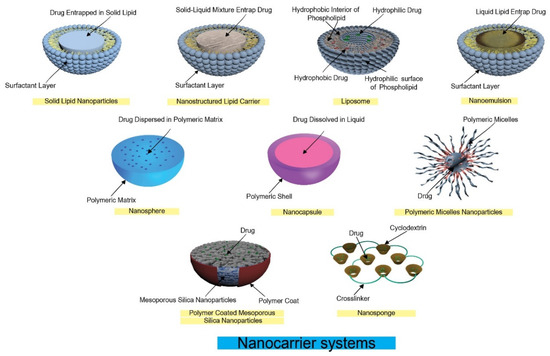
Figure 3.
Different nanocarrier systems used for taste masking.

Table 2.
Nanocarrier systems formulated to mask the taste of different drugs.

Table 3.
Advantages, disadvantages, and characteristics of nanocarrier systems in taste masking.
2.1. Liposomes
As they could entrap either hydrophobic or hydrophilic molecules, its biodegradability, and biocompatibility, liposomes have received attention in the area of drug delivery [38,39]. Liposome composition is based mainly on phospholipids and other additional lipids like cholesterol. Liposomes could enclose or entrap an aqueous payload inside the formed vesicle; meanwhile, they also have the ability to entrap hydrophobic materials within the lipid bilayer [40]. Liposomes deliver drugs with no need for either active or passive uptake by target cells for the nano-vehicle, based on the interaction between the lipid compositions of liposomes with the lipids of the cell membrane [41]. Based on the payload required to be entrapped and the pattern of release, liposomes could be customized by determining the suitable phospholipids and/or preparation method. The preparation method has a significantly high impact on the entrapment efficiency of the drug during the preparation. The main determinant factors for selecting the technique of preparation are ease of scaling the process up, particle size required, polydispersity, and physicochemical characteristics of entrapped drugs [42]. Preparation techniques can be divided into three main types: solvent dispersion, detergent removal, and mechanical dispersion.
On the industrial level, liposome development is still facing different challenges, including inapplicable upscaling, low physical stability, and drug leakage [43].
Liposomes and Taste Masking
Wei-Lun Tang et al. [17] developed mefloquine liposomes that cover the taste of the highly bitter antimalarial drug to formulate a pediatric liquid dosage form. The extremely bad taste of mefloquine was covered by the liposomal entrapment; the researcher used a solvent-assisted loading technique through ammonium sulfate gradient to load mefloquine, and the lipids used in liposomes preparation were 1, 2-distearoyl-sn-glycero-3-phosphocholine and cholesterol.
Ying Zhu et al. [18] studied the impact of formulating loratadine liposomes composed of phospholipids and cholesterol by thin hydration on taste masking, dissolution, and bioavailability of the drug. They reported that the liposomes acted as physical barriers for the entrapped drug and had the ability to enhance the bitter taste of loratadine.
Caffeine as a bitter drug was prepared as chitosan-coated nanoliposomes, also called chitosomes. The liposomes were composed of lecithin, cholesterol, and polysorbate 80, and coated with chitosan polymer; preparation was done by thin hydration method. Chitosome of caffeine had an acceptable taste compared to the caffeine alone. The caffeine chitosome showed better stability than liposomes [19].
2.2. Polymeric Nanoparticles
The polymeric nanoparticles are constructed of polymers that are biocompatible and biodegradable in nature, with sizes between 10–1000 nm where the drug becomes either encapsulated, dissolved, entrapped, or attached to the nanoparticle matrix. The method by which the nanoparticle system is prepared determines whether the formed nanoparticles are nanospheres or nanocapsules. When the drug is entrapped into the cavity and surrounded by a membrane of unique polymer, the system is called nanocapsules. On the other hand, the physical and uniform dispersion of a drug into the matrix of polymer is called nanospheres [44,45].
Polymeric Nanoparticles and Taste Masking
Naik et al. [20] developed a taste masked ibuprofen oral powder using polymeric nanoparticles technique by spray drying, using methacrylic acid-methyl methacrylate copolymer (1:1) (Eudragit® L100) as a pH-dependent polymer insoluble in lower pH medium, in addition to polyvinylpyrrolidone K30 (PVP K30) and β cyclodextrin. The researchers had a dual aim to improve the solubility and palatability of ibuprofen, where they stated that the ratio of PVP K30, β cyclodextrin, and methacrylic acid-methyl methacrylate (1:1) copolymer to the drug should be crucially balanced to fulfill the aim of the study.
Krieser et al. [21] developed saquinavir nanoparticles to enhance the taste of pediatric oral solution and improve stability using the polymers methacrylic acid-methyl methacrylate (1:2) copolymer (Eudragit® RS 100), pullulan, and the triglyceride capric/caprylic by interfacial polymer technique. The prepared saquinavir nanoparticles exhibited a masked bitter taste.
2.3. Solid Lipid Nanoparticles (SLNs)
SLNs are physically solid in nature, either at body or room temperature. It is a colloidal system composed of lipids stabilized by using a surfactant [46]. SLNs gather the benefits from emulsions, polymeric nanoparticles, and liposomes, avoiding some defects from these systems [47,48]. SLNs are composed of a phospholipid coat covering a core matrix which is solid and lipophilic. SLNs entrap lipophilic molecules in higher quantity than liposomes due to their lipophilic core matrix, surrounded by phospholipid that attaches to the lipophilic core by its lipophilic part [49].
SLNs release drugs in a prolonged manner in addition to their ability to protect the entrapped molecule. SLNs are less liable to coalescence and aggregation which makes them more stable. They are biodegradable with nontoxic composition, in addition to the cost-effectiveness of their production and ease of scaling up, ranking them superior to other nanocarrier systems to prepare and use in taste-masking of bitter molecules [47].
The main limitations of SLNs are low loading capacity, drug expulsion post-polymorphic transition during storage, and the higher water content of the dispersions (70–99%) [50].
Solid Lipid Nanoparticles and Taste Masking
Zhang et al. [22] prepared reversed SLNs of atomoxetine hydrochloride for taste masking. Plain liposomes were prepared, then mixed with an equal volume of atomoxetine hydrochloride solution followed by lyophilization. The drug and phospholipids dried powder was dispersed in medium-chain triglycerides and stirred until the suspension was clear.
SLNs with enteric coating were prepared by Chao Li et al. [23] for masking the unacceptable taste of enrofloxacin as well as improving drug stability and oral bioavailability, using combined techniques of hot homogenization and ultrasonic for emulsification. Octadecanoic acid was selected as a lipid matrix, polyvinyl alcohol as the emulsifier, and polyacrylic resin as an enteric coating material. The study concluded that enteric coating SLNs of enrofloxacin improved its palatability.
Dandagi et al. [24] developed quinine sulphate loaded SLNs to mask the taste of the antimalarial drug; glyceryl monostearate was selected as the lipid with the surfactants polysorbate 80, poloxamer 407, and poloxamer 188.
2.4. Nanostructured Lipid Carriers (NLCs)
As a result of SLNs limitations, Muller et al. [51] developed NLCs. Development of NLCs was done by replacing part of solid lipids in the case of SLNs with a liquid lipid leading to the formation of drug incorporated in the liquid lipid matrix. Because of the superior composition characteristics over SLNs, NLCs are potential carriers because of their higher loading capacity.
Nanostructured Lipid Carriers and Taste Masking
Akhoond Zardini et al. [25] developed NLCs containing lycopene with dual improved solubility and enhanced taste. NLCs were prepared using combined techniques of ultrasonication and high shear homogenization. Glyceryl monostearate and glyceryl distearate were used as solid lipids, Caprylic/Capric triglyceride was used as the liquid lipid, and Tween 80 and lecithin as co-surfactants. The resulting lycopene NLCs showed no taste when added to orange juice.
2.5. Polymeric Micelles
Unimers are the building units of polymeric micelles. Unimers are composed of lipophilic blocks and hydrophilic blocks co-polymerized in either di-block or tri-block forms, resulting in synthetic amphiphilic copolymers. When unimers are self-assembled spontaneously, they form polymeric micelles. The core of the polymeric micelles is usually lipophilic, while their shell is hydrophilic [52,53,54]. The hydrophilic shell of polymeric micelles prevents the micelles from aggregation, facilitating dispersion in aqueous media. The size of micelles ranges from 10 to 100 nm. Following the rule “like dissolves like”, the lipophilic core entraps the lipophilic molecules. Poly(ethylene oxide) is one of the most familiar hydrophilic blocks, that may also be known as poly(ethylene glycol) (PEG); the subunit monomer that is considered the building unit of such hydrophilic block is –CH2–CH2–O–, the length of the block ranging from 1 to 15 kDa [55,56].
Polymeric Micelles and Taste Masking
Li et al. [26] proposed amphiphilic block copolymers as micelle forming agents that might have potential for taste masking in aqueous solution. Four water-soluble bitter drugs were used in the study, specifically Berberine hydrochloride, Quinine sulfate, Gentiopicroside, and Matrine, to test the capability of amphiphilic block copolymers, namely, mPEG2000-PLLA2000, mPEG2000-PCL2000, mPEG2000-PLGA50/502000, and PLLA2000-PEG2000-PLLA2000, to mask the bitter taste.
2.6. Reverse Micelles Nanoparticles
In the reverse micelles system, the aqueous droplet in the nanosized range is stabilized into the organic liquid with the help of a surfactant. The size of the reverse micelles nanoparticles is affected by the aqueous content of the systems; the higher the water, the higher the particle size of reverse micelles [57].
Reverse Micelles Nanoparticles and Taste Masking
Huang et al. [27], prepared reverse micelles for taste masking of azithromycin using the freeze-drying method, where the phospholipid drug mixture was dispersed in medium-chain triglycerides and lyophilized. The drug is entrapped with the lecithin micelles in medium-chain triglycerides, which might be considered a physical barrier that shields or separate the drug from taste sensors. The study concluded that the bitter taste of azithromycin was successfully masked by the reverse micelles.
2.7. Submicron Lipid Emulsions
Pharmaceutical and cosmetic industries extensively deploy emulsions as delivery carriers, due to their multiple benefits [58,59]. Emulsions are usually formed by the aid of emulsifiers to disperse an immiscible liquid in the form of fine droplets into another one. The dispersed droplets are the internal phase and the other liquid is the external or continuous phase. The surfactant and co-surfactant are responsible for emulsion stability, preventing the dispersed droplets from separation or coalescence. Emulsions are categorized into two types, water in oil (w/o) and oil in water (o/w). In the case of (w/o), water is the internal or dispersed phase, while oil is the external or continuous phase, and the reverse for (o/w) [60,61]. Microemulsions and nanoemulsions which have higher stability get special interest from the pharmaceutical and cosmetic manufacturers [62] as such types of emulsions show better bioavailability [63] and can be designed for passive drug targeting.
The droplet size is the main difference between macro and microemulsions; in the case of macro type the droplet size is above 100 nm, while in the case of micro type the droplet size ranges from 5 to 100 nm. The smaller the particle the size, the more stable the emulsion [28]. Microemulsion can be recognized by its translucent appearance and low viscosity, while the macro type is opaque and has a higher viscosity.
Submicron Lipid Emulsion and Taste Masking
Monteagudo et al. [28] optimized the lipid-based formulation self-emulsifying drug delivery system of phenobarbital for the improvement of its taste, stability, and solubility. The selected systems are composed of surfactant, oil phase, co-surfactant, and water (20:4:20:56 and 20:4:35:41). Polyoxyl 40 hydrogenated castor oil (Cremophor® RH40) and caprylocaproyl polyoxyl-8-glycerides (Labrasol®) were used as surfactants. Isopropyl myristate, glycerol monocaprylocaprate (Capmul® MCM L), caprylic/capric triglyceride (Captex® 355), propylene glycol caprylate (Imwitor® 408), and propylene glycol dicaprylate/dicaprate (Miglyol® 840) were used as the oil phase. Diethylene glycol monoethyl ether (Transcutol® P), propylene glycol, PEG 400, and glycerol were used as co-surfactants. The study concluded that the selected formulations improved the solubility, stability, and taste of phenobarbital.
Hasan et al. [29] developed a microemulsion to emulsify the oily flavoring agent and use this microemulsion to cover the bad taste of liquid paracetamol. In their study, Hasan et al. prepared a self microemulsifying drug delivery system (SMEDDS) of peppermint oil flavor using the following materials: medium-chain triglyceride (Crodamol® GTCC), propylene glycol dicaprylate/caprate (Crodamol® PC), PEG 6 caprylic/capric glycerides (Glycerox® 767HC), and PEG 40 hydrogenated castor oil (Croduret® 40ss), in addition to polyoxyl 40 hydrogenated castor oil and oleic acid. Formulations of medium-chain triglyceride: PEG 6 caprylic/capric glycerides: PEG 40 hydrogenated castor oil at ratios of (0:80:20), (6:54:40), or (10:40:50) developed peppermint oil SMEDDS which had a potential taste-masking ability for paracetamol.
2.8. Nanogels
Upon placing polymer networks—cross-linked either chemically or physically—in a good solvent, they swell into particles within the nanosized range forming ‘nanogels’. “Nanogel” (NanoGel™) describes the polynucleotides delivery through polymer networks of cross-linked non-ionic polymer and poly-ionic polymer [64]. The progress in polymer science aims to develop nanosystems with smart properties that guarantee effective treatment using nanogels. The nanogels could target a specific site to deliver the included drug, in a sustainable and controlled manner [65].
Nanogels and Taste Masking
Unfortunately, until now, no taste masking has been adopted using nanogels based on our literature review.
2.9. Nanosponges (NSs)
NSs could be described as colloidal structures that encapsulate different molecules [66]. NSs are lipophilic in nature, but they have the ability to spread in water, thus they help in masking the taste of unaccepted flavors by changing the physical state of a compound from liquid to solid. This helps decrease the contact of the drug with the sensory sites on the tongue [67].
NSs could be prepared by the interaction of the cross-linking agent with cyclodextrins. Caldera et al. [68] in their review categorized NSs into four generations. The first one includes urethane, carbonate, ether, and ester. In the second generation, there are polymers with specific properties, for example, fluorescence or charged side chains. The third generation contains stimuli-responsive NSs. In this type, NSs behavior may change as per changes in environment; for example, change in pH, change in temperature, or change in oxidative or reducing factors. The fourth one has high selectivity to the specific included drug.
Nanosponges and Taste Masking
Omar et al. [30], formulated cyclodextrin-based NSs of griseofulvin to enhance its taste and bioavailability. Different plain β-cyclodextrin NSs (NS1, NS2, and NS3) have been formulated using a cross-linking reaction of β-cyclodextrin that is assisted by ultrasonication, and cross-linker diphenyl carbonate (DPC).
2.10. Inclusion Complex Formation
Different cyclodextrin complexes could assemble in the form of nanoscale aggregates in aqueous solutions [69]. Previous work concluded that β-cyclodextrin is the recommended type for inclusion complexation. Different processes include physical mixing technique by mixing the drug with the cyclodextrin, as well as wet kneading of drug with the cyclodextrin leading to inclusion complex [69]. Cyclodextrin has alcoholic hydroxyl moieties from the outside; such exterior moieties make the exterior part of cyclodextrin hydrophilic, and the central cavity is covered with carbon and oxygen atoms coming from the glucose residues. This nature of cyclodextrin gives it the ability to engulf molecules as a guest in the cavities of cyclodextrin, which is known as the inclusion complex. The inclusion complex could enhance the taste of unaccepted bitter active constituent by hiding the bitter active molecule inside the cyclodextrin cavity, in addition to improving the stability of the included molecule by preventing its degradation, and moreover acting as a solubilizing agent [31].
Inclusion Complex Formation and Taste Masking
Ranitidine hydrochloride was subjected to inclusion complex with hydroxypropyl beta-cyclodextrin (HP-β-CD), where a higher HP-β-CD to ranitidine hydrochloride ratio indicated a higher efficiency of taste masking [31].
Cetirizine and beta-cyclodextrin (β-CD) inclusion complex was evaluated from the point of taste masking ability of β-CD [32].
Shah et al. [33] developed an inclusion complex of artemether (ARM) with β-CD. ARM-β-CD was developed to enhance the palatability of the bitter ARM, in addition to improving solubility; the final product was a pediatric suspension containing the ARM-β-CD inclusion complex. The ARM-β-CD was prepared by two methods, physical mixture and kneading; both techniques gave the complex. ARM:β-CD complex in a ratio of 1:2 was the best drug to dextrin quantity from the point of palatability as per taste scoring done.
2.11. PH-Responsive Co-Ordination Polymer Coated Mesoporous Silica Nanoparticles (MSNs)
The developed drug delivery system was simple and efficient in oral taste masking. The system works by regulating the release of the bitter drug through a pH change in the gastrointestinal tract. In this system, a pH-sensitive metal-organic coordination polymer (CP), which was Fe-4, 4′-bipyridine (Fe-bipy) complex, works as a taste-masker [34].
PH-Responsive Co-Ordination Polymer Coated MSNs
The pH-sensitive Fe-bipy was grafted onto the MSNs containing the model bitter drug mequindox (MEQ) in its mesopores through metal-organic coordination cross-linking, resulting in the CP-coated nanodrug [29].
In artificial saliva (pH 6.6), the Fe-bipy CP effectively prevents the leaking of the loaded guest molecule MEQ from the MSN-NH2-MEQ-Fe-bipy. On the other hand, in artificial gastric fluid (pH 1.0), the coordination bonds of the Fe-4,4′-bipyridine complex were broken, leading to the release of MEQ molecules from MSN-NH2-MEQ-Fe-bipy [34].
2.12. Nanohybrid System
In this technique the drug inorganic-clay nanohybrid, which is prepared by intercalating the drug to the clay interlayer space, is coated with a polymer resulting in an improved taste-masked system with enhanced drug release [35].
Nanohybrid System and Taste Masking
Sildenafil (SDN) was intercalated to montmorillonite (MMT) as the inorganic clay, to form the nanohybrid system. The intercalation was followed by coating the SDN-MMT nanohybrid with polyvinylacetal diethylaminoacetate (AEA) as the basic polymer. The AEA-coated SDN-MMT nanohybrid showed low dissolution release compared to the marketed Viagra® Tablet at neutral pH, the low dissolution resulting in less exposure of the drug to the mouth taste sensors and enhanced taste in the buccal cavity [35].
3. Conclusions
The emerging new techniques of nanotechnology have shown promising potential for taste masking of bitter drugs. The mechanism for taste masking of such techniques is based on the physical barrier formed by the nanocarrier systems, which entrap the bitter drug, minimizing or abolishing the contact of the drug with the sensory sites on the tongue. We have observed the limited work of applying nanocarrier systems for taste masking, despite the effective results as per the gathered data within this review. We propose that limited work based on nanocarrier systems might be caused by the complexity of techniques, the need of expensive tools, the inapplicability of scaling up for such technique, or the long process which might make scaling up not commercially accepted. Nanocarrier systems have the surplus advantages of improving the drug solubility and stability, in addition to taste masking compared to the conventional approaches, enabling nanocarrier systems to achieve solutions for many challenges facing formulators. This means nanocarrier systems have potential for solving the problem of taste acceptance, especially for any emerging drug that has a promising role in disease treatment but meanwhile is suffering from low solubility and low stability. On the other hand, the manufacturing processes of nanocarrier systems are costly, sophisticated, and time-consuming.
4. Future Perspective
Nanocarrier systems for taste masking application have not yet received enough focus from the manufacturers of pharmaceutical products. Further applications of nanocarrier systems and further research work are required to make use of these techniques on various bitter taste molecules.
Author Contributions
Conceptualization, N.E.H.N. and A.N.E.; methodology, N.E.H.N.; data curation, N.E.H.N.; writing—original draft preparation, N.E.H.N.; writing—review and editing, A.N.E. and A.R.F.; visualization, N.E.H.N. and A.R.F.; supervision, A.N.E. and A.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| API | Active Pharmaceutical Ingredient |
| AEA | Polyvinylacetal diethylaminoacetate |
| β-CD | Beta-cyclodextrin |
| CP | Coordination polymer |
| Fe-bipy | Fe-4, 4′-bipyridine |
| HP-β-CD | Hydroxypropyl beta-cyclodextrin |
| MEQ | Mequindox |
| MMT | Montmorillonite |
| MSNs | Mesoporous silica nanoparticles |
| PCL | poly (ε-caprolactone) |
| PEG | Polyethylene Glycol |
| PLGA | Poly Lactic-co-Glycolic Acid |
| PLLA | poly (l-lactic acid) |
| PVP | Polyvinyl Pyrrolidone |
| SLNs | Solid lipid nanoparticles |
| NLC | Nanostructured lipid carriers |
| NS | Nanosponge |
| SMEDDS | Self microemulsifying drug delivery system |
References
- Bernkop-Schnurch, A. Nanocarrier systems for oral drug delivery: Do we really need them? Eur. J. Pharm. Sci. 2013, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.S.; Lakshmi, P.K. Electronic tongue: An analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef]
- Douroumis, D. Orally disintegrating dosage forms, and taste-masking technologies; 2010. Expert Opin. Drug Deliv. 2011, 8, 665–675. [Google Scholar] [CrossRef]
- Beck, T.K.; Jensen, S.; Bjoern, G.K.; Kidmose, U. The masking effect of sucrose on perception of bitter compounds in Brassica vegetables. J. Sens. Stud. 2014, 29, 190–200. [Google Scholar] [CrossRef]
- Lankpord, B.L.; Becker, C.H. The use of some imitation flavors for masking distasteful drugs. II. Quinine hydrochloride. J. Am. Pharm. Assoc. 1951, 40, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin. Drug Deliv. 2007, 4, 417–426. [Google Scholar] [CrossRef]
- Bansal, A.; Kreig, B.; Sharma, N.; McGinnis, J.; Bhatia, I.; Paz, C. Taste masking of granulated acetaminophen by water insoluble ethylcellulose coating. Folia Med. 2021, 63, 97–104. [Google Scholar] [CrossRef]
- Borodkin, S.; Sundberg, D.P. Polycarboxylic acid ion-exchange resin adsorbates for taste coverage in chewable tablets. J. Pharm. Sci. 1971, 60, 1523–1527. [Google Scholar] [CrossRef]
- Al-Omran, M.F.; Al-Suwayeh, S.A.; El-Helw, A.M.; Saleh, S.I. Taste masking of diclofenac sodium using microencapsulation. J. Microencapsul. 2002, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R. Computationally designed prodrugs for masking the bitter taste of drugs. J. Drug Des. 2012, 1, 106. [Google Scholar] [CrossRef]
- Hussain, M.A.; Aungst, B.J.; Koval, C.A.; Shefter, E. Improved buccal delivery of opioid analgesics and antagonists with bitterless prodrugs. Pharm. Res. 1988, 5, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Kharb, V.; Saharan, V.A.; Kharb, V.; Jadhav, H.; Purohit, S. Formulation and characterization of taste masked ondansetron magnesium aluminum silicate adsorption systems. Drug Dev. Ind. Pharm. 2016, 42, 1291–1299. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Kashiwayanagi, M.; Kurihara, K. Specific inhibitor for bitter taste: Inhibition of frog taste nerve responses and human taste sensation to bitter stimuli. Brain Res. Protoc. 1997, 1, 292–298. [Google Scholar] [CrossRef]
- Shah, P.P.; Mashru, R.C. Development and evaluation of artemether taste masked rapid disintegrating tablets with improved dissolution using solid dispersion technique. AAPS PharmSciTech 2008, 9, 494–500. [Google Scholar] [CrossRef][Green Version]
- Tang, W.L.; Tang, W.H.; Chen, W.C.; Diako, C.; Ross, C.F.; Li, S.D. Development of a Rapidly Dissolvable Oral Pediatric Formulation for Mefloquine Using Liposomes. Mol. Pharm. 2017, 14, 1969–1979. [Google Scholar] [CrossRef]
- Zhu, Y.; You, X.; Huang, K.; Raza, F.; Lu, X.; Chen, Y.; Dhinakar, A.; Zhang, Y.; Kang, Y.; Wu, J.; et al. Effect of taste masking technology on fast dissolving oral film: Dissolution rate and bioavailability. Nanotechnology 2018, 29, 304001. [Google Scholar] [CrossRef]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan–coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Naik, J.; Rajput, R.; Singh, M.K. Development and Evaluation of Ibuprofen Loaded Hydrophilic Biocompatible Polymeric Nanoparticles for the Taste Masking and Solubility Enhancement. BioNanoScience 2021, 11, 21–31. [Google Scholar] [CrossRef]
- Krieser, K.; Emanuelli, J.; Daudt, R.M.; Bilatto, S.; Willig, J.B.; Guterres, S.S.; Pohlmann, A.R.; Buffon, A.; Correa, D.S.; Külkamp–Guerreiro, I.C. Taste-masked nanoparticles containing Saquinavir for pediatric oral administration. Mater. Sci. Eng. C 2020, 117, 111315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Wang, T.; Li, H.; Huang, R.; Zhang, Z.; Wang, Y.; Quan, D. Taste masking of water-soluble drug by solid lipid microspheres: A child-friendly system established by reversed lipid-based nanoparticle technique. J. Pharm. Pharmacol. 2020, 72, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Chen, D.; Xu, W.; Tao, Y.; Pan, Y.; Meng, K.; Abu Bakr Shabbir, M.; Liu, Q.; Huang, L.; et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int. J. Nanomed. 2019, 14, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Dandagi, P.M.; Rath, S.P.; Gadad, A.P.; Mastiholimath, V.S. Taste masked quinine sulphate loaded solid lipid nanoparticles for flexible pediatric dosing. Indian J. Pharm. Educ. Res. 2014, 48, 93–99. [Google Scholar] [CrossRef]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
- Li, P.; Tian, Y.; Ke, X.M.; Tan, Q.C.; Han, X.; Ma, H.Y.; Pei, J.; Lin, J.Z.; Xu, R.C.; Han, L.; et al. Amphiphilic Block Copolymers: A Novel Substance for Bitter–Masking in Aqueous Solutions. Mol. Pharm. 2020, 17, 1586–1595. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Wang, T.; Shen, L.; Zhang, Z.; Wang, Y.; Quan, D. Creation of an assessment system for measuring the bitterness of azithromycin–containing reverse micelles. Asian J. Pharm. Sci. 2018, 13, 343–352. [Google Scholar] [CrossRef]
- Monteagudo, E.; Langenheim, M.; Salerno, C.; Buontempo, F.; Bregni, C.; Carlucci, A. Pharmaceutical optimization of lipid-based dosage forms for the improvement of taste–masking, chemical stability and solubilizing capacity of phenobarbital. Drug Dev. Ind. Pharm. 2014, 40, 783–792. [Google Scholar] [CrossRef]
- Hasan, N.M.; Al–aram, M.S.; Al–wadie, M.S.; Althobaiti, F.A.; Al–Malki, M.J. Flavored self microemulsifying lipid formulations for masking the organoleptic taste of pharmaceutical actives. J. Appl. Pharm. Sci. 2015, 5, 127–134. [Google Scholar] [CrossRef][Green Version]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Chay, S.K.; Keating, A.V.; James, C.; Aliev, A.E.; Haider, S.; Craig, D.Q. Evaluation of the taste-masking effects of (2–hydroxypropyl)–β–cyclodextrin on ranitidine hydrochloride; a combined biosensor, spectroscopic and molecular modelling assessment. RSC Adv. 2018, 8, 3564–3573. [Google Scholar] [CrossRef]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Mashru, R.C. Palatable reconstitutable dry suspension of artemether for flexible pediatric dosing using cyclodextrin inclusion complexation. Pharm. Dev. Technol. 2010, 15, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.M.; Wang, L.; Yuan, H.Q.; Wang, X.Y.; Mei, T.X.; Qu, M.R. Taste masking of a drug by pH–responsive coordination polymer-coated mesoporous silica nanoparticles. RSC Adv. 2016, 6, 109453–109459. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, G.; Oh, Y.J.; Park, J.W.; Choy, Y.B.; Park, M.C.; Yoon, Y.J.; Lee, H.J.; Chang, H.C.; Choy, J.H. A nanohybrid system for taste masking of sildenafil. Int. J. Nanomed. 2012, 7, 1635–1649. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano–Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef]
- Alexander, T.; Florence, J.S. Modern Pharmaceutics, Volume 2: Applications and Advances, 5th ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, p. 560. [Google Scholar]
- Chen, L.-J.; Yang, C.-X.; Yan, X.-P. Liposome–Coated Persistent Luminescence Nanoparticles as Luminescence Trackable Drug Carrier for Chemotherapy. Anal. Chem. 2017, 89, 6936–6939. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Lee, Y.W.; Scaletti, F.; Yu, R.; Rotello, V.M. Intracellular delivery of proteins by nanocarriers. Nanomedicine 2017, 12, 941–952. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei–Sadabady, R.; Davaran, S.; Woo Joo, S.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati–Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Khalil, H.; Wang, R.; Lu, T.; Zhao, W.; Zhang, Y.; Chen, Y.; Chen, T. Fusion between fluid liposomes and intact bacteria: Study of driving parameters and in vitro bactericidal efficacy. Int. J. Nanomed. 2016, 11, 4025–4036. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Mansha, M.; Khan, I.; Ullah, N.; Qurashi, A. Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole–containing conjugated polymers. Int. J. Hydrog. Energy 2017, 42, 10952–10961. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, R.S.; Hebbar, H.U. Reverse micelles for nanoparticle synthesis and biomolecule separation. In Nanoscience in Food and Agriculture 4; Springer: Cham, Switzerland, 2017; pp. 181–211. [Google Scholar]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable lipid emulsions–Advancements, opportunities and challenges. AAPS PharmSciTech 2010, 11, 1526–1540. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent Water-in-Oil Dispersions: The Oleopathic Hydro-Micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Tang, X. Chemical stability of teniposide in aqueous and parenteral lipid emulsions. Drug Dev. Ind. Pharm. 2009, 35, 508–513. [Google Scholar] [CrossRef]
- Simovic, S.; Hui, H.; Song, Y.; Davey, A.K.; Rades, T.; Prestidge, C.A. An oral delivery system for indomethicin engineered from cationic lipid emulsions and silica nanoparticles. J. Control. Release 2010, 143, 367–373. [Google Scholar] [CrossRef]
- Ades, A.; Carvalho, J.P.; Graziani, S.R.; Amancio, R.F.; Souen, J.S.; Pinotti, J.A.; Maranhão, R.C. Uptake of a cholesterol-rich emulsion by neoplastic ovarian tissues. Gynecol. Oncol. 2001, 82, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers. In Multifunctional Pharmaceutical Nanocarriers; Torchilin, V., Ed.; Springer: New York, NY, USA, 2008; pp. 67–80. [Google Scholar]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano–Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).