The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Simulation of Linalool/CD Inclusion Complex Formation
2.2. Phase Solubility Study of Linalool/CD Inclusion Complexes
2.3. Linalool/CD Inclusion Complex Formation
2.4. Quantitative Analysis of Linalool/CD Inclusion Complexes by High Performance Liquid Chromatography (HPLC)
2.5. Characterization of Linalool/CD Inclusion Complexes by Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Characterization of Linalool/CD Inclusion Complexes by Thermogravimetric Analysis (TGA)
2.7. Characterization of Linalool/CD Inclusion Complexes by Differential Scanning Calorimetry (DSC)
2.8. Enantiomeric Analysis of Linalool/CD Inclusion Complexes by Gas Chromatography with Flame Ionization Detection (GC-FID)
2.9. Preservation Study of Linalool/CD Inclusion Complexes
3. Results
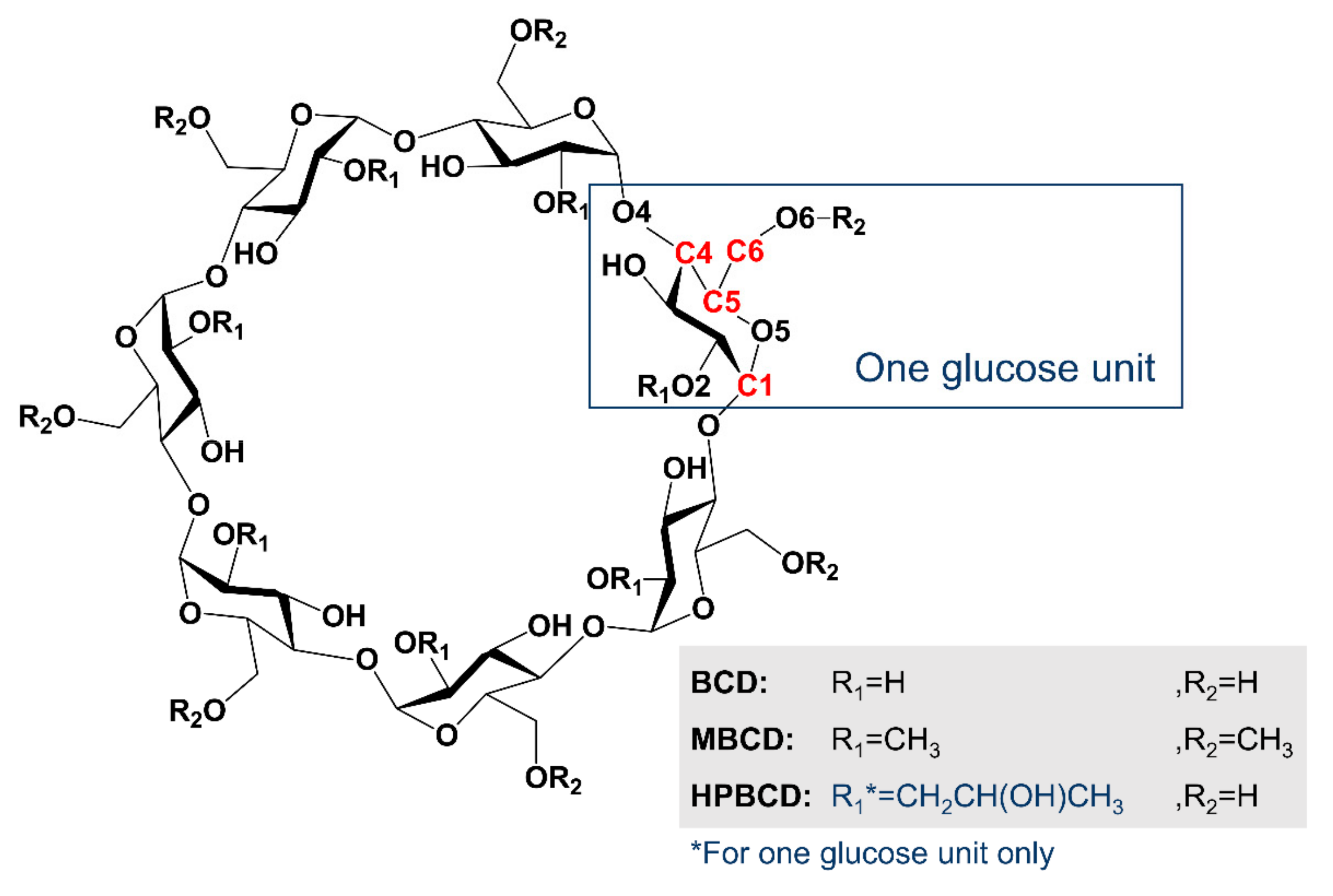
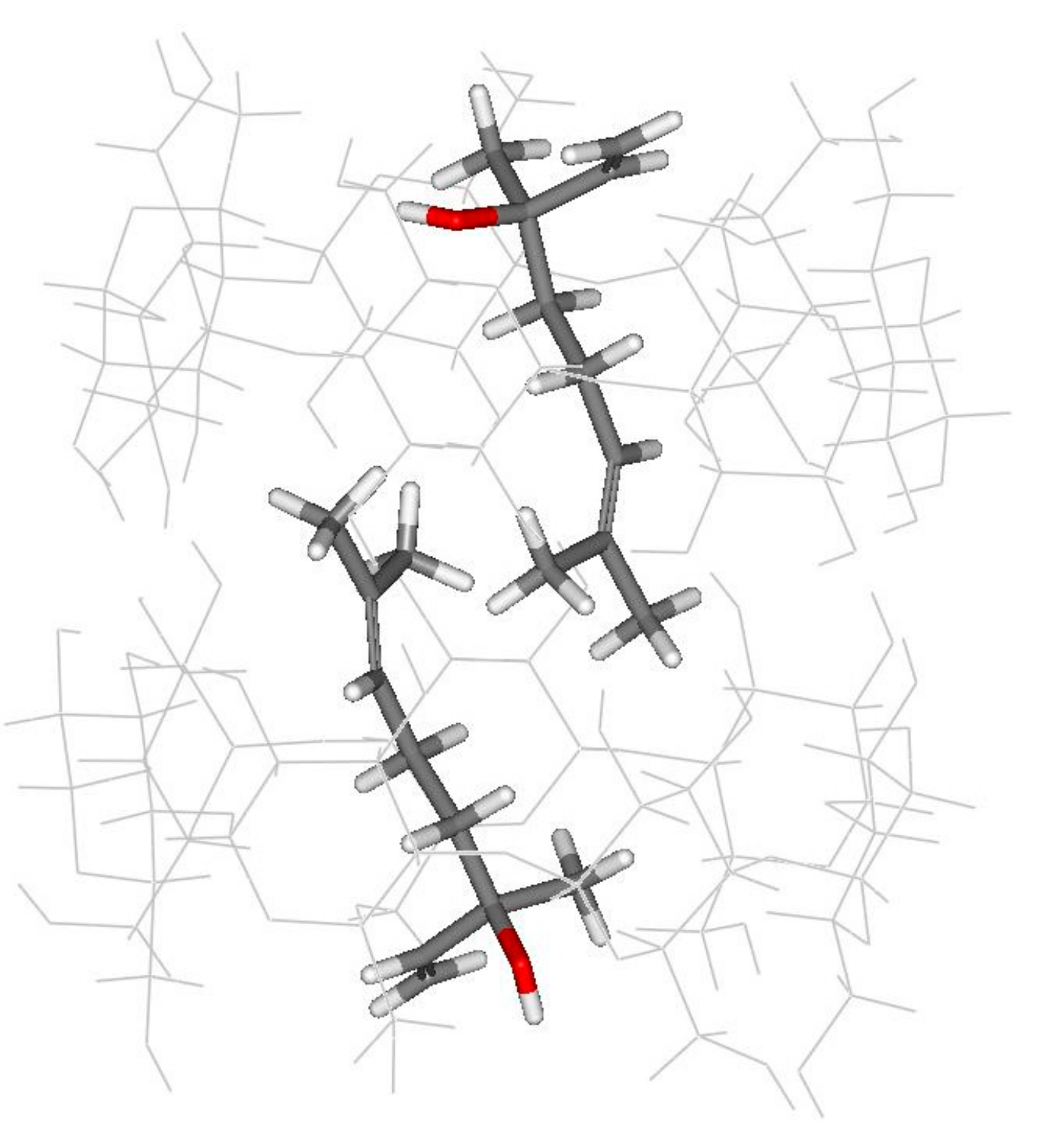
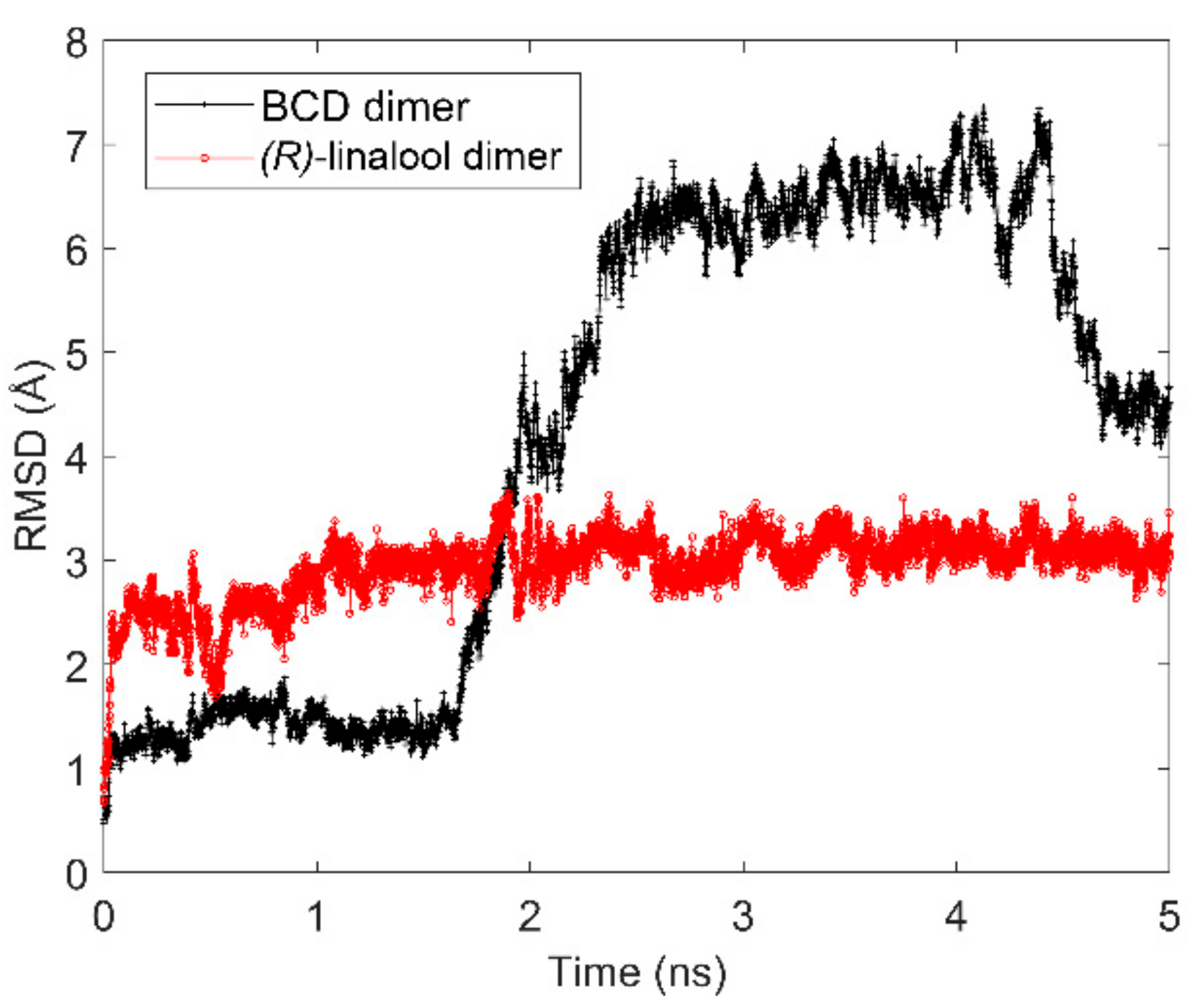
3.1. Computational Simulation of Linalool/BCD Inclusion Complex Formation
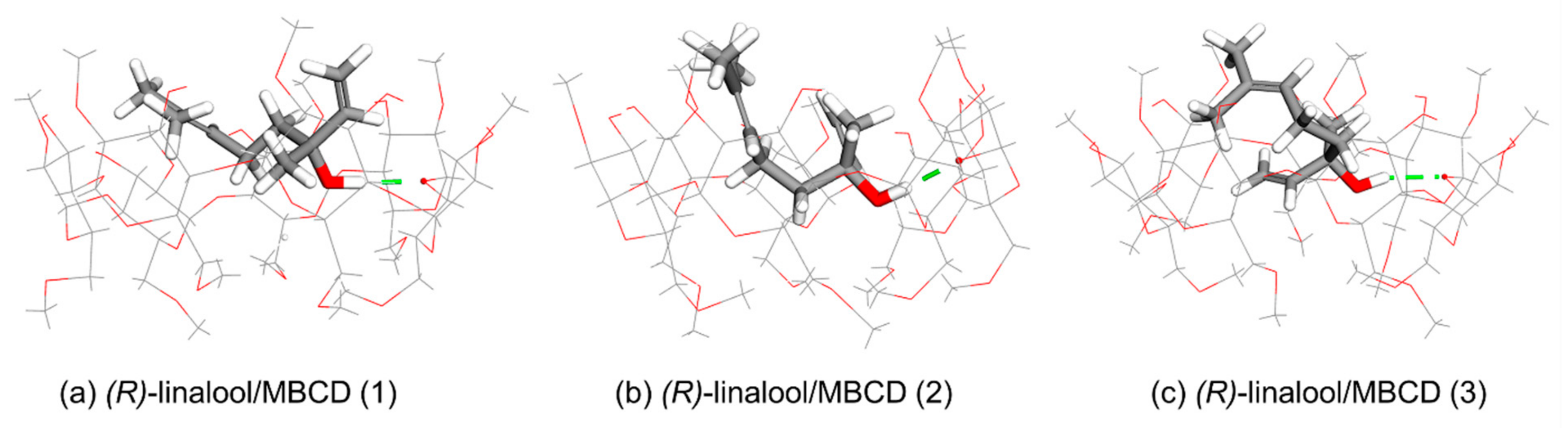
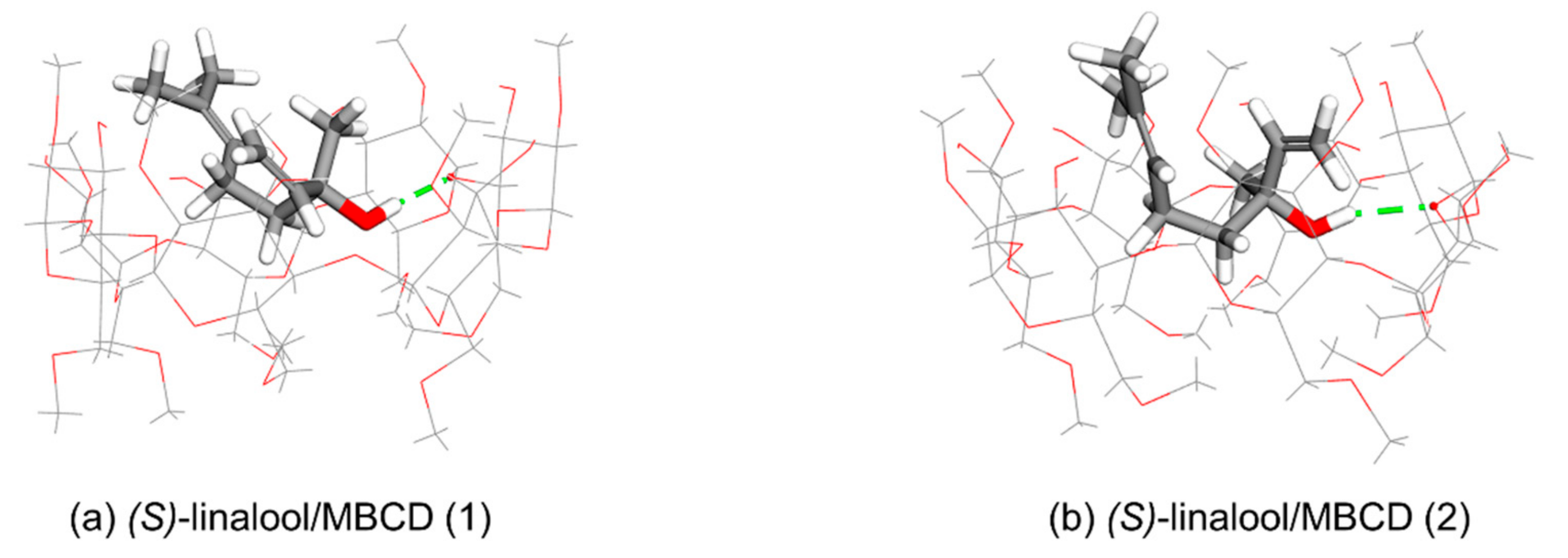
3.2. Computational Simulation of Linalool/MBCD Inclusion Complex
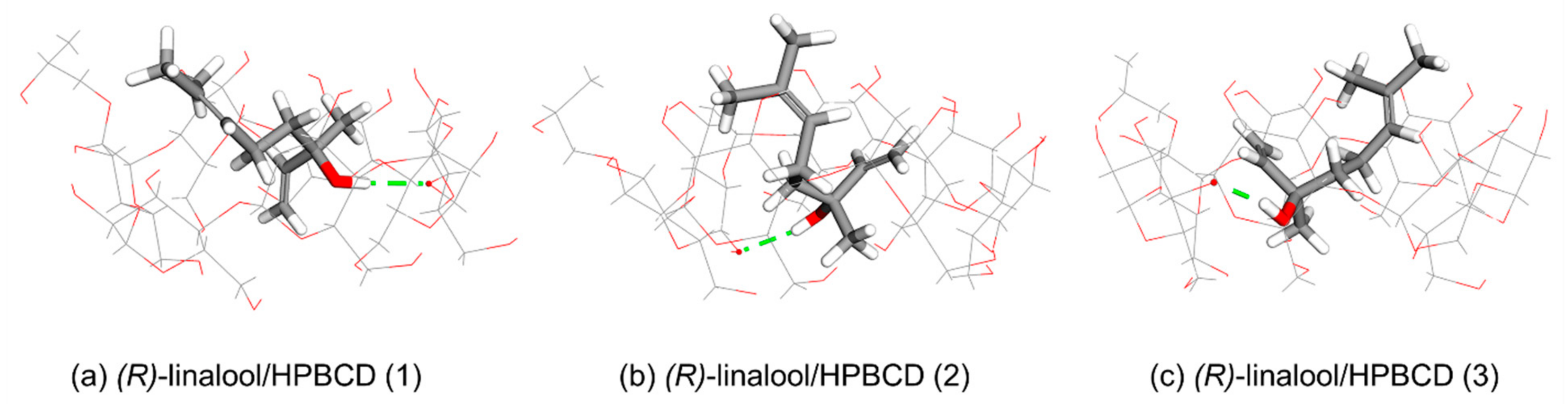
3.3. Computational Simulation of Linalool/HPBCD Inclusion Complex
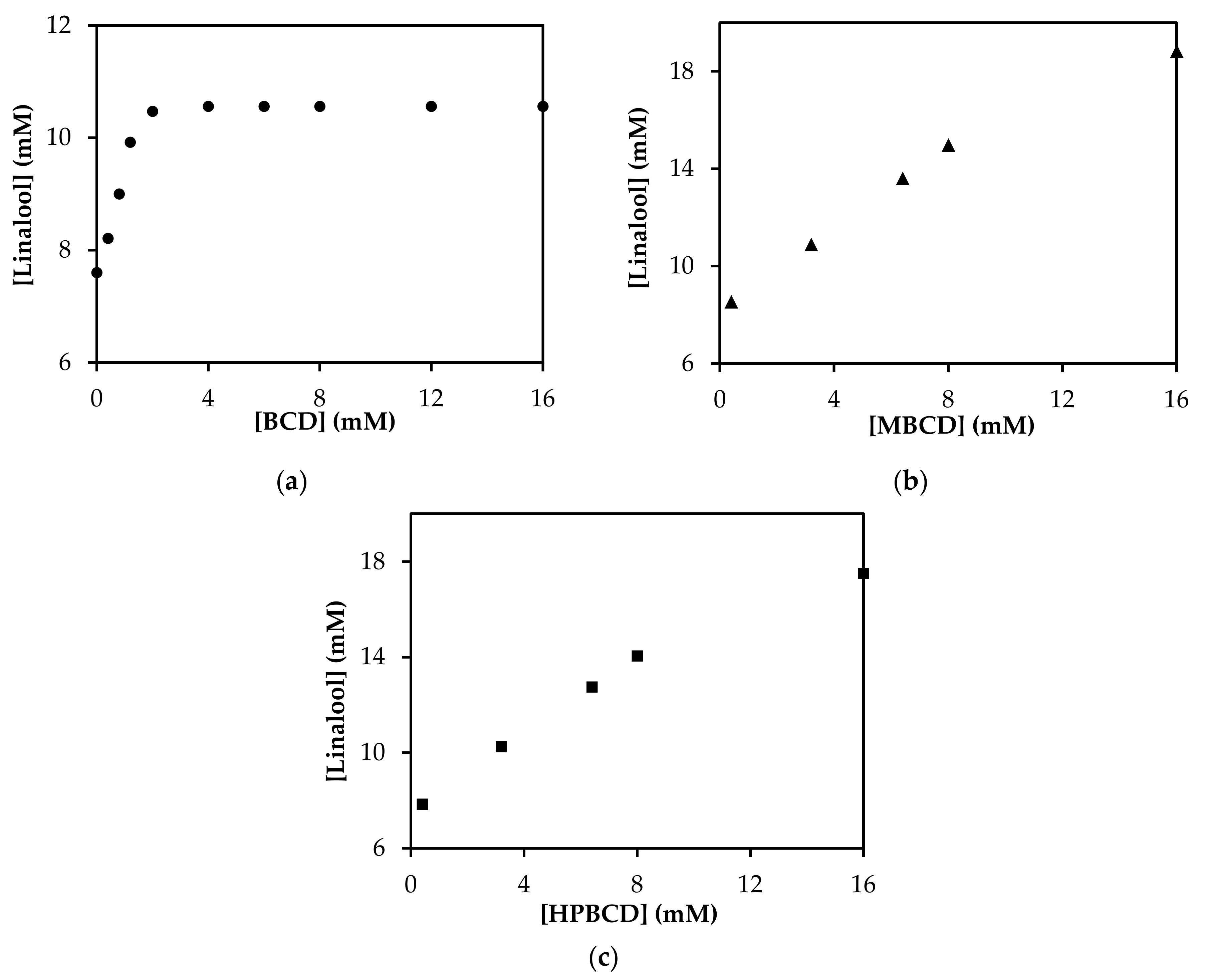
3.4. Phase Solubility Study of Linalool/CD Inclusion Complexes
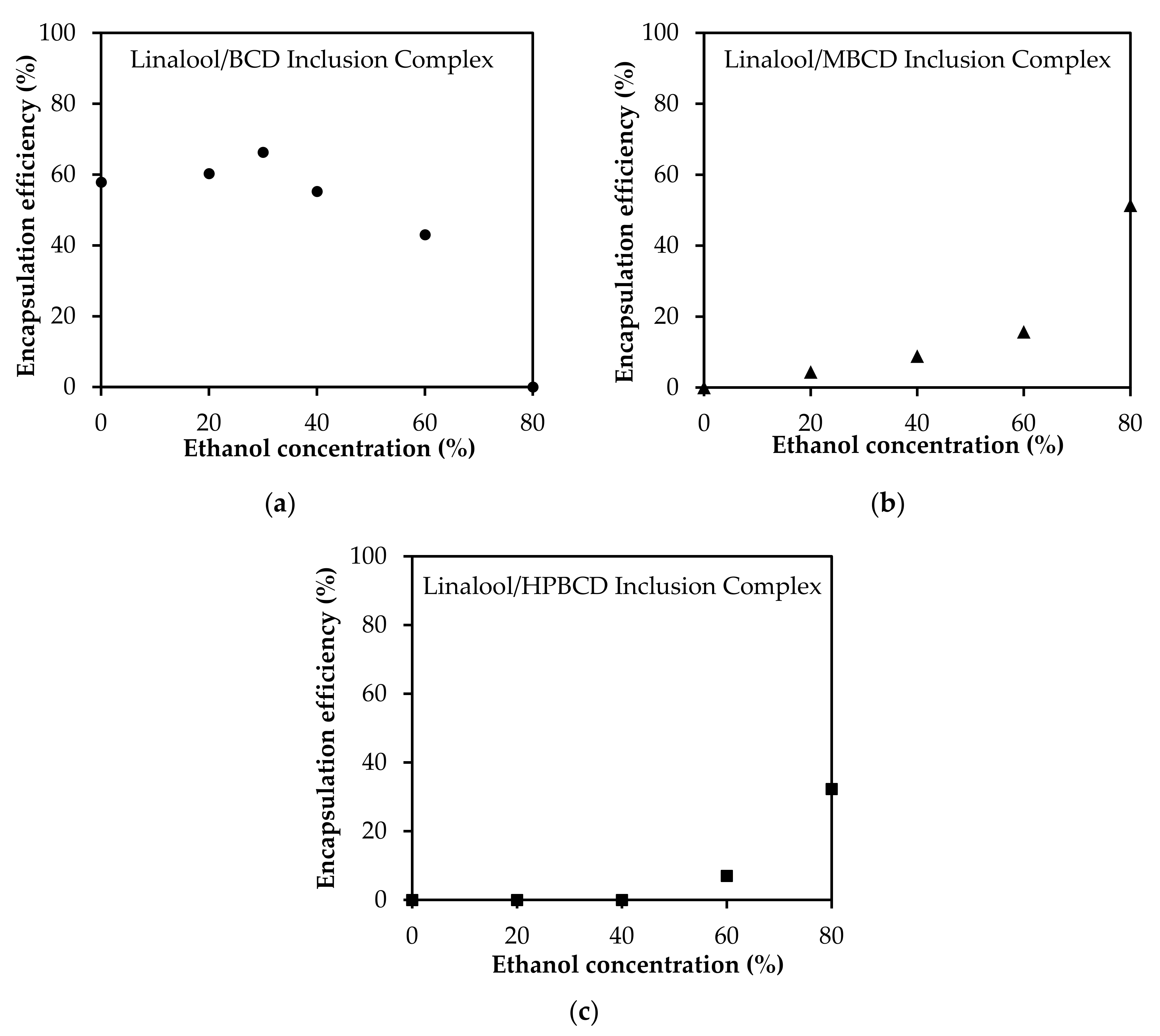
3.5. Solvent Effect on the Encapsulation Efficiency of Linalool/CD Inclusion Complexes
3.6. Characterization of Linalool/CD Inclusion Complexes by FTIR
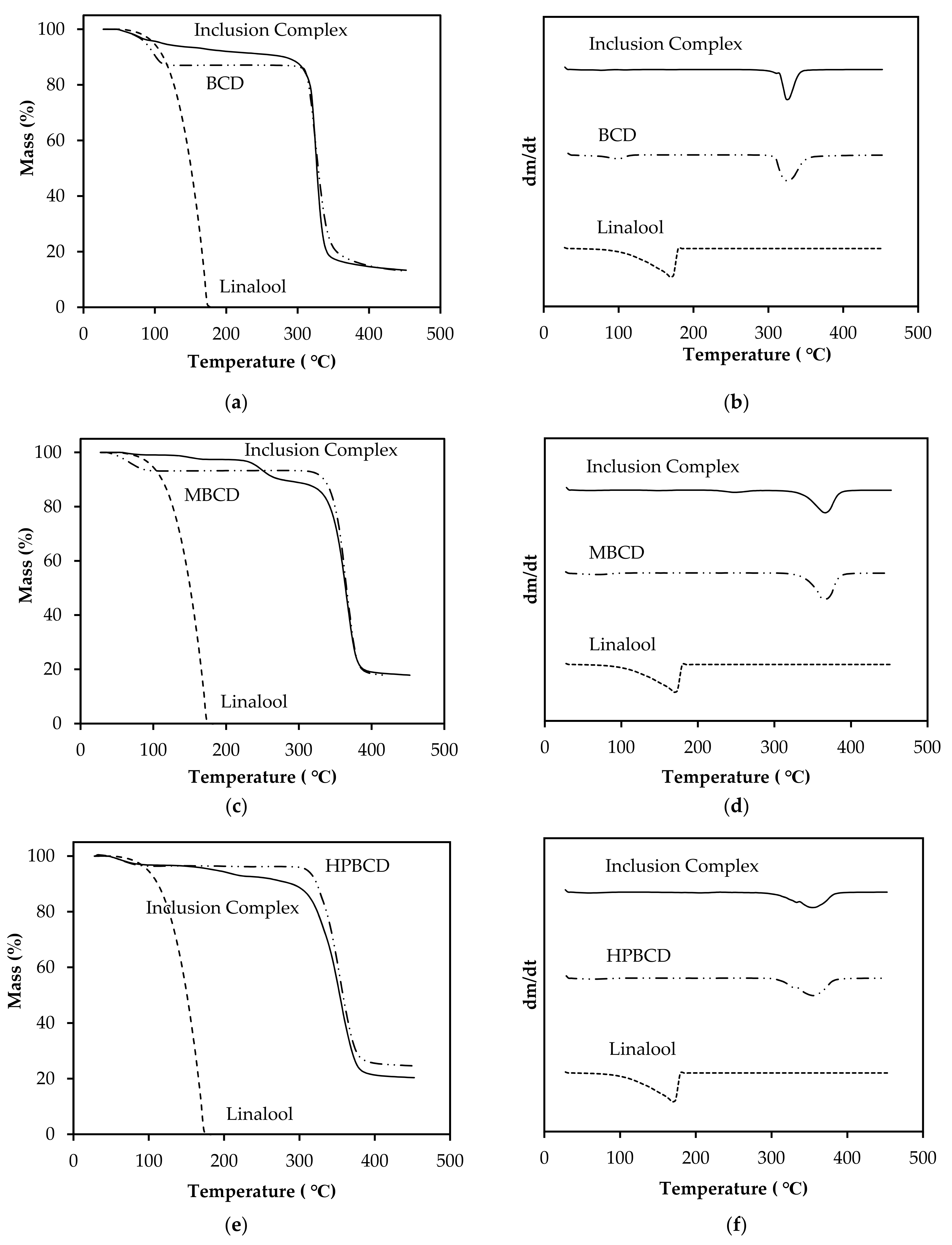
3.7. Characterization of Linalool/CD Inclusion Complexes by TGA
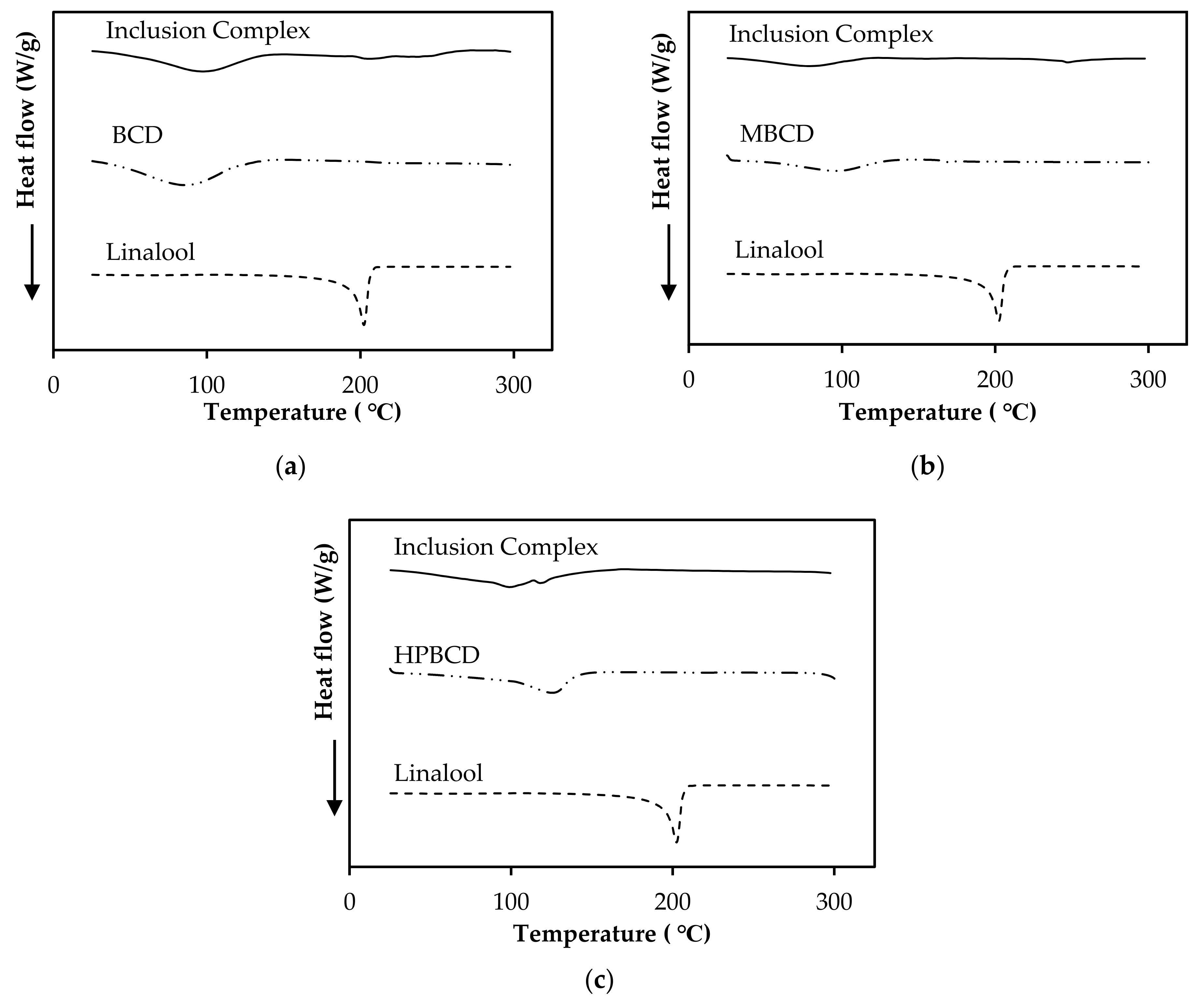
3.8. Characterization of Linalool/CD Inclusion Complexes by DSC
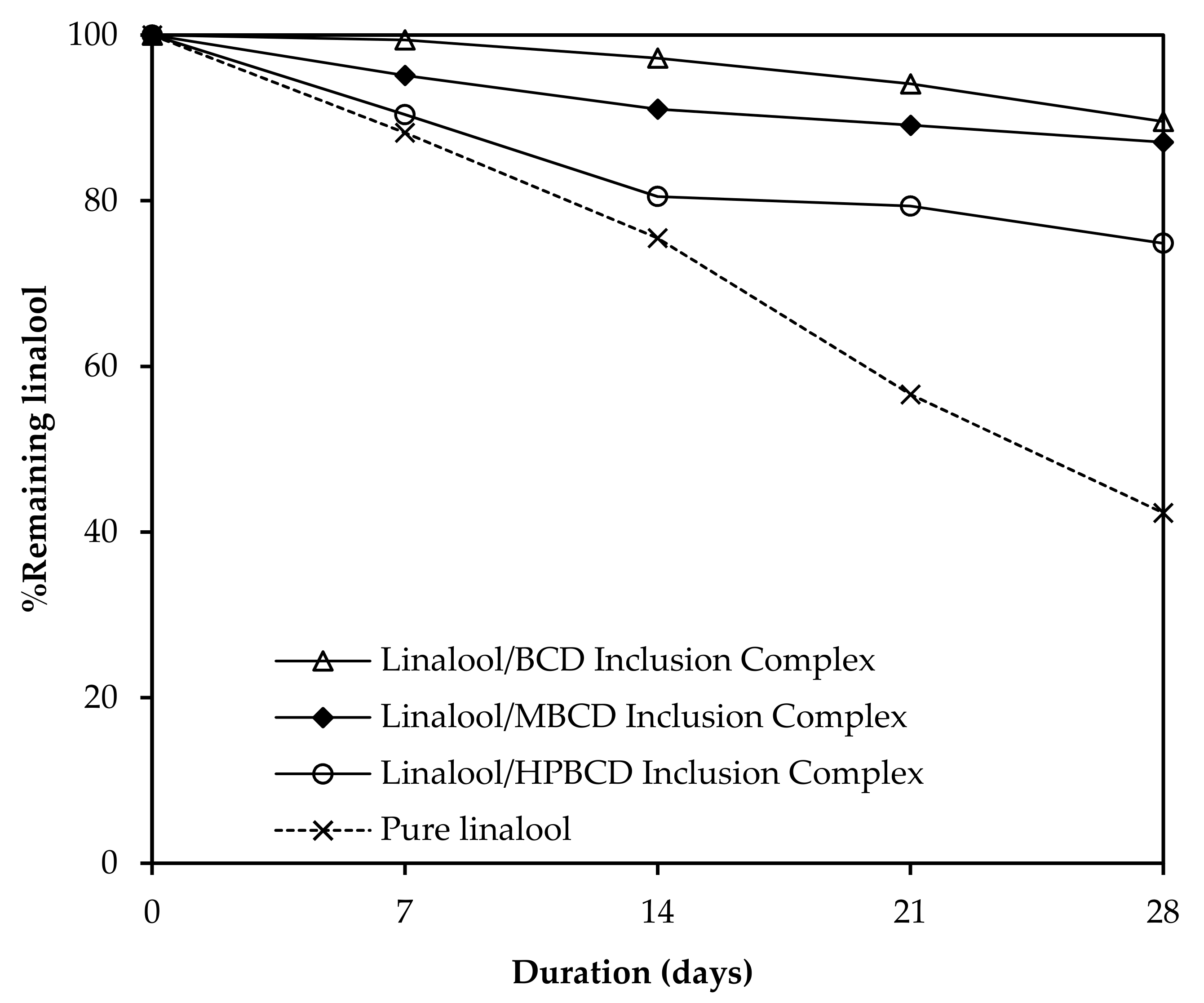
3.9. Preservation Study of Linalool/CD Inclusion Complexes
3.10. Enantiomeric Selection of Linalool/CD Inclusion Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mughal, M.H. Linalool: A Mechanistic Treatise. J. Nutr. Food Technol. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Lara, C.S.; Barata, L.E.S.; Sampaio, P.d.T.B.; Eberlin, M.N.; Fidelis, C.H.d.V. Linalool Enantiomeric Distribution in Rosewood-reminiscent Populations in Central Amazon. J. Essent. Oil Res. 2018, 30, 464–469. [Google Scholar] [CrossRef]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical Isomers and Odor Thresholds of Volatile Constituents in Citrus Sudachi. J. Food Sci. Technol. Int. 1997, 3, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A Review on a Key Odorant Molecule with Valuable Biological Properties. Flavour Frag. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Bonnländer, B.; Cappuccio, R.; Liverani, F.S.; Winterhalter, P.J.F. Analysis of Enantiomeric Linalool Ratio in Green and Roasted Coffee. Flavour Frag. J. 2006, 21, 637–641. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations I: Structure and Physicochemical Properties, Formation of Complexes, and Types of Complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Frag. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in Aqueous Solution: A Review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef]
- Gingter, S.; Bezdushna, E.; Ritter, H. Chiral Recognition of Macromolecules with Cyclodextrins: pH-and Thermosensitive Copolymers from N-isopropylacrylamide and N-acryloyl-D/L-phenylalanine and Their Inclusion Complexes with Cyclodextrins. Beilstein J. Org. Chem. 2011, 7, 204–209. [Google Scholar] [CrossRef]
- Lee, J.-u.; Lee, S.-S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef] [PubMed]
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; de Mello, J.M.M.; Fiori, M.A. β-Cyclodextrins as Encapsulating Agents of Essential Oils. Cyclodext.-A Versatile Ingred. IntechOpen 2018, 169–200. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Abril-Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids. 2019, 219, 72–78. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Steiner, T.; Koellner, G. Crystalline Beta-cyclodextrin Hydrate at Various Humidities: Fast, Continuous, and Reversible Dehydration Studied by X-ray Diffraction. J. Am. Chem. Soc. 1994, 116, 5122–5128. [Google Scholar] [CrossRef]
- Aree, T.; Hoier, H.; Schulz, B.; Reck, G.; Saenger, W. Novel Type of Thermostable Channel Clathrate Hydrate Formed by Heptakis (2, 6-di-O-methyl)-β-cyclodextrin⋅ 15 H2O—A Paradigm of the Hydrophobic Effect. Angew. Chem. Int. Ed. 2000, 39, 897–899. [Google Scholar] [CrossRef]
- Harata, K.; Rao, C.T.; Pitha, J.; Fukunaga, K.; Uekama, K. Crystal Structure of 2-O-[(S)-2-hydroxypropyl] Cyclomaltoheptaose. Carbohydr. Res. 1991, 222, 37–45. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian16, RevisionB. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Ceborska, M. Structural Investigation of the β-cyclodextrin Complexes with Linalool and Isopinocampheol–Influence of Monoterpenes Cyclicity on the Host–guest Stoichiometry. Chem. Phys. Lett. 2016, 651, 192–197. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, H.M.A.K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Duke, G.; Giambasu, M.K.; Gilson, H.; et al. Amber 2021; University of California: San Francisco, CA, USA, 2021. [Google Scholar]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A Generalizable Biomolecular Force Field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinlikhitkul, N.; Toochinda, P.; Lawtrakul, L.; Kuropakornpong, P.; Itharat, A. Encapsulation of Plumbagin Using Cyclodextrins to Enhance Plumbagin Stability: Computational Simulation, Preparation, Characterization, and Application. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 229–243. [Google Scholar] [CrossRef]
- Connors, K.; Higuchi, T. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- Chatjigakis, A.K.; Donze, C.; Coleman, A.W.; Cardot, P. Solubility Behavior of Beta-cyclodextrin in Water/cosolvent Mixtures. Anal. Chem. 1992, 64, 1632–1634. [Google Scholar] [CrossRef]

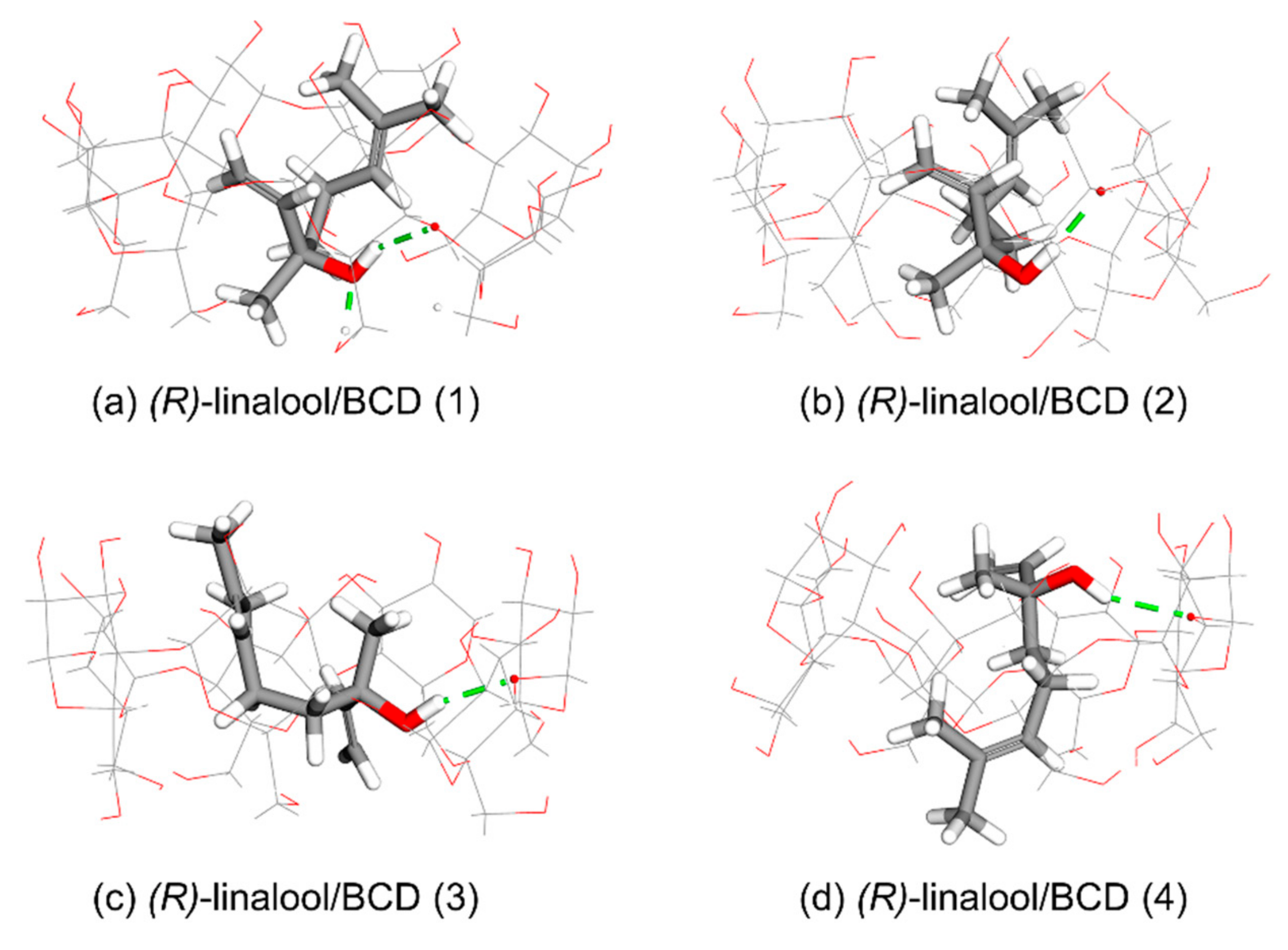
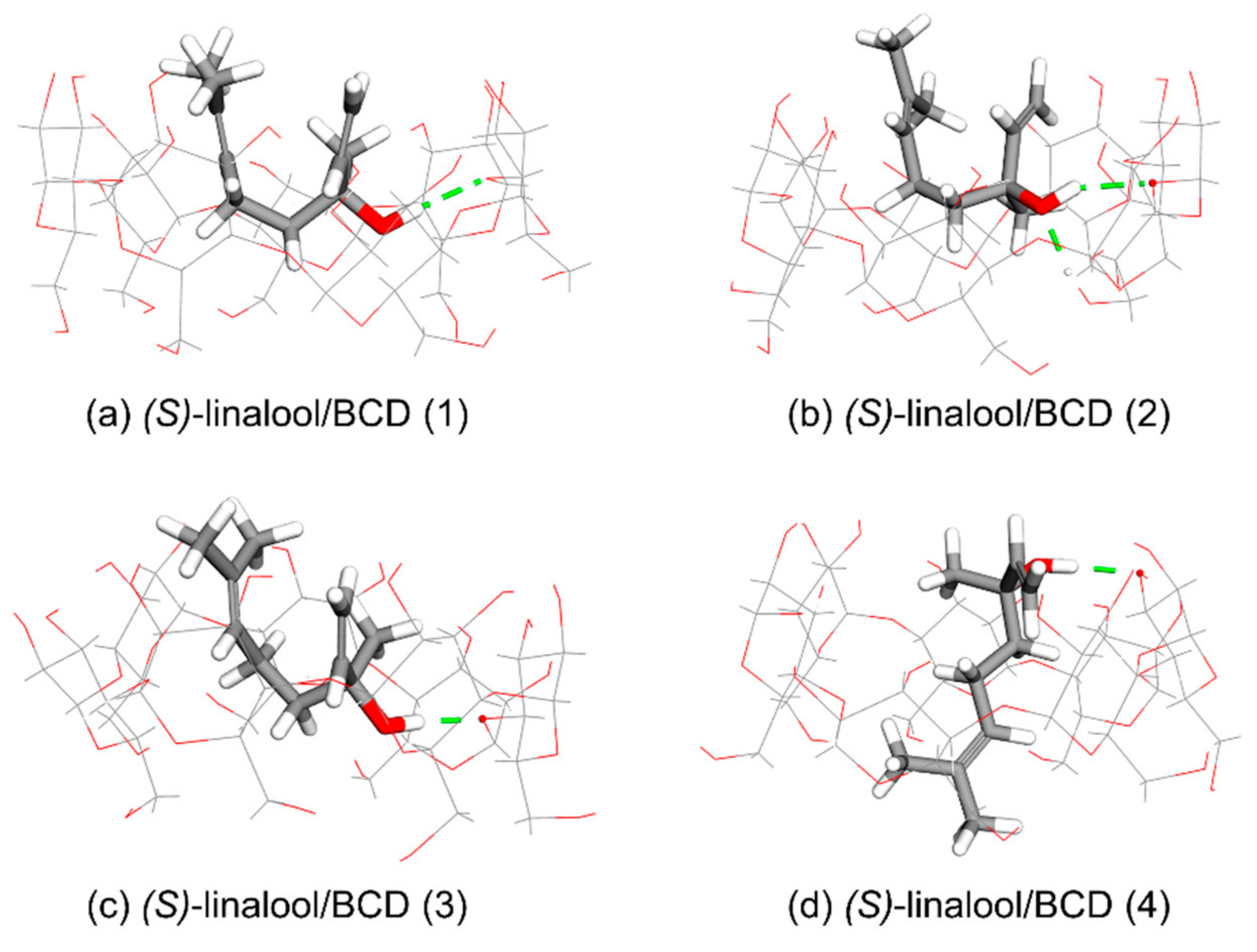

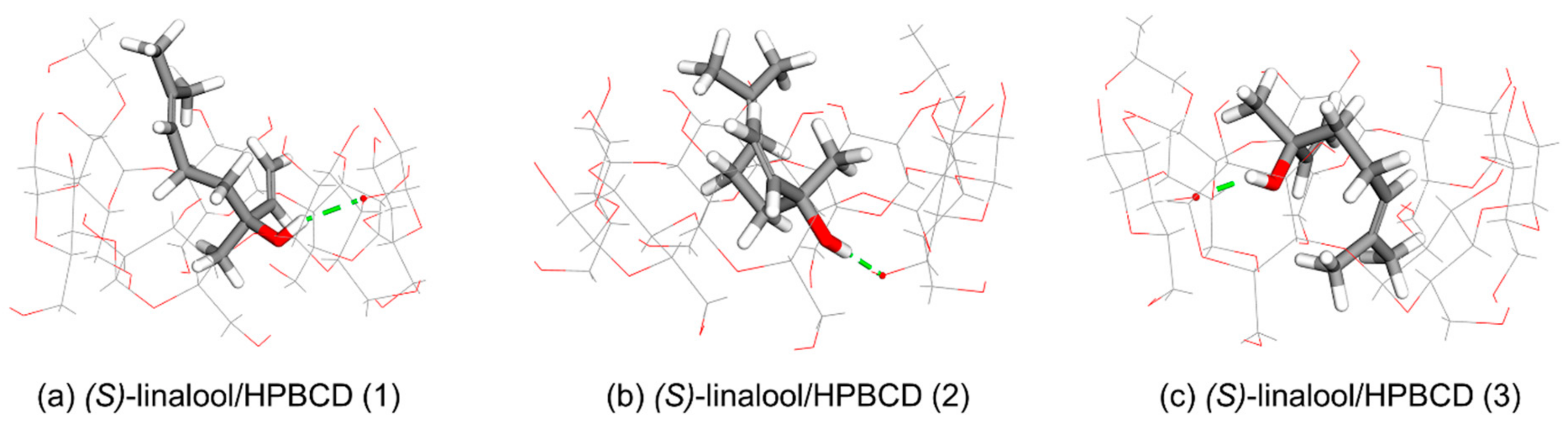

| Cluster | Orientation | Frequency (%) | ΔG (kcal/mol) | ||
|---|---|---|---|---|---|
| Lowest | Average | ||||
| (R)-linalool/BCD | (1) | I | 64 | −4.13 | −4.03 |
| (2) | I | 31 | −4.10 | −4.01 | |
| (3) | I | 1 | −3.95 | −3.95 | |
| (4) | II | 4 | −3.93 | −3.90 | |
| (S)-linalool/BCD | (1) | I | 41 | −4.14 | −4.01 |
| (2) | I | 46 | −4.06 | −3.98 | |
| (3) | I | 12 | −4.04 | −3.96 | |
| (4) | II | 1 | −3.90 | −3.90 | |
| (R)-linalool/MBCD | (1) | I | 90 | −5.02 | −4.86 |
| (2) | I | 6 | −4.83 | −4.75 | |
| (3) | I | 4 | −4.60 | −4.55 | |
| (S)-linalool/MBCD | (1) | I | 87 | −4.98 | −4.79 |
| (2) | I | 13 | −4.78 | −4.71 | |
| (R)-linalool/HPBCD | (1) | I | 69 | −4.36 | −4.23 |
| (2) | I | 22 | −4.30 | −4.23 | |
| (3) | I | 9 | −4.18 | −4.06 | |
| (S)-linalool/HPBCD | (1) | I | 86 | −4.37 | −4.21 |
| (2) | I | 9 | −4.28 | −4.21 | |
| (3) | II | 5 | −4.04 | −4.00 | |
| Orientation | E (kcal/mol) | ΔE (kcal/mol) | µ (Debye) | |
|---|---|---|---|---|
| Isolated molecule | ||||
| (R)-linalool | −57.56 | 1.79 | ||
| (S)-linalool | −57.49 | 2.16 | ||
| BCD | −1608.18 | 7.25 | ||
| MBCD | −1551.01 | 1.55 | ||
| HPBCD | −1664.45 | 5.82 | ||
| Inclusion complex (cluster) | ||||
| (R)-linalool/BCD (1) | I | −1709.68 | −43.94 | 8.48 |
| (R)-linalool/BCD (2) | I | −1706.45 | −40.71 | 7.89 |
| (R)-linalool/BCD (3) | I | −1707.58 | −41.84 | 8.57 |
| (R)-linalool/BCD (4) | II | −1703.56 | −37.82 | 5.19 |
| (S)-linalool/BCD (1) | I | −1705.13 | −39.46 | 7.27 |
| (S)-linalool/BCD (2) | I | −1710.54 | −44.86 | 7.74 |
| (S)-linalool/BCD (3) | I | −1707.42 | −41.75 | 7.08 |
| (S)-linalool/BCD (4) | II | −1709.34 | −43.67 | 3.60 |
| (R)-linalool/MBCD (1) | I | −1651.99 | −43.42 | 3.23 |
| (R)-linalool/MBCD (2) | I | −1652.27 | −43.70 | 4.51 |
| (R)-linalool/MBCD (3) | I | −1646.51 | −37.94 | 3.56 |
| (S)-linalool/MBCD (1) | I | −1650.41 | −41.91 | 1.79 |
| (S)-linalool/MBCD (2) | I | −1649.73 | −41.23 | 4.72 |
| (R)-linalool/HPBCD (1) | I | −1765.59 | −43.59 | 2.85 |
| (R)-linalool/HPBCD (2) | I | −1771.94 | −49.93 | 8.47 |
| (R)-linalool/HPBCD (3) | I | −1759.36 | −37.35 | 6.07 |
| (S)-linalool/HPBCD (1) | I | −1763.53 | −41.59 | 7.70 |
| (S)-linalool/HPBCD (2) | I | −1765.72 | −43.78 | 5.16 |
| (S)-linalool/HPBCD (3) | II | −1764.89 | −42.95 | 4.62 |
| Component | Energy (kcal/mol) |
|---|---|
| Average binding energy (ΔH) | −33.58 ± 2.55 |
| Entropy (TΔS at 298.15 K) | −19.12 |
| Gibbs free energy (ΔGbind) | −14.46 |
| Inclusion Complex | Cluster | ΔE | Distance (Å) | |
|---|---|---|---|---|
| (R)-linalool/BCD | (1) | −43.94 | 2.12 | |
| 1.94 | ||||
| (2) | −40.71 | 1.97 | ||
| (3) | −41.84 | 2.05 | ||
| (4) | −37.82 | 2.25 | ||
| (S)-linalool/BCD | (1) | −39.46 | 2.21 | |
| (2) | −44.86 | 2.09 | ||
| 1.84 | ||||
| (3) | −41.75 | 1.98 | ||
| (4) | −43.67 | 1.96 | ||
| (R)-linalool/MBCD | (1) | −43.42 | 1.98 | |
| (2) | −43.70 | 1.98 | ||
| (3) | −37.94 | 2.08 | ||
| (S)-linalool/MBCD | (1) | −41.91 | 2.03 | |
| (2) | −41.23 | 2.07 | ||
| (R)-linalool/HPBCD | (1) | −43.59 | 2.10 | |
| (2) | −49.93 | 2.12 | ||
| (3) | −37.35 | 1.01 | ||
| (S)-linalool/HPBCD | (1) | −41.59 | 2.16 | |
| (2) | −43.78 | 2.01 | ||
| (3) | −42.95 | 1.99 |
| Inclusion Complex | Cluster | Frequency (%) | ΔE (kcal/mol) | ||
|---|---|---|---|---|---|
| ΔE | Average | Δ Enantiomers | |||
| (R)-linalool/BCD | (1) | 64 | −43.94 | −42.67 | 0.41 |
| (2) | 31 | −40.71 | |||
| (3) | 1 | −41.84 | |||
| (4) | 4 | −37.82 | |||
| (S)-linalool/BCD | (1) | 41 | −39.46 | −42.26 | |
| (2) | 46 | −44.86 | |||
| (3) | 12 | −41.75 | |||
| (4) | 1 | −43.67 | |||
| (R)-linalool/MBCD | (1) | 90 | −43.42 | −43.22 | 1.40 |
| (2) | 6 | −43.70 | |||
| (3) | 4 | −37.94 | |||
| (S)-linalool/MBCD | (1) | 87 | −41.91 | −41.82 | |
| (2) | 13 | −41.23 | |||
| (R)-linalool/HPBCD | (1) | 69 | −43.59 | −44.42 | 2.56 |
| (2) | 22 | −49.93 | |||
| (3) | 9 | −37.35 | |||
| (S)-linalool/HPBCD | (1) | 86 | −41.59 | −41.86 | |
| (2) | 9 | −43.78 | |||
| (3) | 5 | −42.95 | |||
| Inclusion Complex | %Enantiomer in the Inclusion Complexes | |
|---|---|---|
| (R)-Linalool | (S)-Linalool | |
| Racemic linalool (standard) | 49.50 ± 0.16 | 50.50 ± 0.16 |
| Linalool/BCD | 54.53 ± 0.34 | 44.62 ± 0.34 |
| Linalool/MBCD | 52.49 ± 0.20 | 47.51 ± 0.20 |
| Linalool/HPBCD | 51.51 ± 0.10 | 48.49 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poonphatanapricha, T.; Katanyutanon, S.; Jitapunkul, K.; Lawtrakul, L.; Toochinda, P. The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins. Sci. Pharm. 2021, 89, 42. https://doi.org/10.3390/scipharm89030042
Poonphatanapricha T, Katanyutanon S, Jitapunkul K, Lawtrakul L, Toochinda P. The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins. Scientia Pharmaceutica. 2021; 89(3):42. https://doi.org/10.3390/scipharm89030042
Chicago/Turabian StylePoonphatanapricha, Tanaporn, Sasimas Katanyutanon, Kulpavee Jitapunkul, Luckhana Lawtrakul, and Pisanu Toochinda. 2021. "The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins" Scientia Pharmaceutica 89, no. 3: 42. https://doi.org/10.3390/scipharm89030042
APA StylePoonphatanapricha, T., Katanyutanon, S., Jitapunkul, K., Lawtrakul, L., & Toochinda, P. (2021). The Preservation and Enantiomeric Selection of Linalool by Nanoencapsulation Using Cyclodextrins. Scientia Pharmaceutica, 89(3), 42. https://doi.org/10.3390/scipharm89030042






