Abstract
Possessing the quinone moiety, ilimaquinone (1), a sponge–derived sesquiterpene quinone, has been hypothesised to express its cytotoxicity through a redox cycling process, yielding active product(s) that can cause DNA damage. To determine the DNA damaging effects of 1 and examine whether a redox transformation may participate in its functions, the DNA damaging properties of 1, the corresponding hydroquinone (2) and hydroquinone triacetates (3) and their 5-epimeric counterparts (4–6) were tested and compared. When incubated directly with plasmid DNA, the hydroquinones were the only active species capable of cleaving the DNA. In cell-based assays, however, the quinones and hydroquinone triacetates were active in the same range as that of the corresponding hydroquinones, and all damaged the cellular DNA in a similar manner. The in situ reduction of 1 and 4 were supported by the decreases in the cytotoxicity when cells were pre-exposed to dicoumarol, an NAD(P)H:quinone oxidoreductase 1 (NQO1) inhibitor. The results confirmed the DNA damaging activities of the ilimaquinones 1 and 4, and indicated the necessity to undergo an in-situ transformation into the active hydroquinones, thereby exerting the DNA damaging properties as parts of the cytotoxic mechanisms.
1. Introduction
Quinone and hydroquinone moieties have long been appreciated as ones of the biologically active functionalities, especially in the anticancer-antitumor chemotherapy. Centering on the cytotoxicity of quinone/hydroquinone functionalities, two mechanisms—both of which involve the interconversion between the quinone and hydroquinone species through a redox cycling process—have been proposed. On one hand, quinone and quinonoid moieties can alkylate onto biological nucleophiles after being reduced into the hydroquinone and/or semiquinone radical. This is the primary mechanism associated with the anticancer activity of mitomycin C [1]. On the other hand, upon being reduced to the corresponding hydroquinone, an autoxidation back to the parent structure of the quinone resulted in the reactive oxygen species that can cause the oxidative stress and cell death. Diaziquone exerts the anticancer activity, in part through this autoxidation pathway [2].
Recently, we reported the isolation of sesquiterpene quinones and hydroquinones from the sponges Dactylospongia elegans [3] and Verongula rigida [4], among which ilimaquinone (1) constituted the major component. The isolated compounds showed a good to moderate cytotoxicity against cancer cell lines. Compound 1, for instance, was active against PC3 prostate cancer cells with an IC50 of 10.1 μM, which was in good agreement with the previous report [5]. The compound reportedly expresses its cytotoxicity through a wide range of oncologic pathways and cell proliferation processes, including the induction of cell cycle arrests, activation of apoptotic and autophagic processes and interferences with gene regulation in oncologic pathways [5,6,7,8,9,10].
The quinone functionality of 1 and other related sesquiterpene quinones is predicted to play a crucial role similar to those of other cytotoxic quinones. For example, having the quinone moieties parallel to 1, avarone and avarol were reported to cause the single strand DNA break via the generation of reactive oxygen species [11]. It is therefore of our interest to examine the effects of the quinone functionality of 1 and its related derivatives on DNA, particularly within a regard of the interconversion between the quinone and hydroquinone species. Using the ilimaquinones to represent the quinone-containing cytotoxic agents, this investigation is taking a close look into the direct effects of the quinone functionality on DNA as a part of the cytotoxic mechanisms. Here, we report the DNA damaging effects of compound 1, its hydroquinone (2) and hydroquinone triacetate (3) congeners, and their 5-epimeric counterparts (compounds 4–6) on a cell-free and cell-based assays. In addition, the transformation of the quinone into the active hydroquinone moieties via an in-situ reduction are explored and discussed.
2. Materials and Methods
2.1. General Experimental Procedures
Unless stated otherwise, all the chemicals and solvents were used as purchased without further purification. All the reactions were carried out in oven-dried vessels under a N2 atmosphere. UV spectra were performed on a Thermo Scientific Genesys 6, and IR were on a Bruker Vertex 70 spectrophotometers. NMR experiments were performed on an NMR Varian Unity Inova 500 spectrometer (Office of Scientific Equipment and Testing, PSU), referencing the according solvent signals as internal standards. ESI mass spectra were obtained from a Waters Alliance 2690 Micromass LCT spectrometer (Office of Scientific Equipment and Testing, PSU).
2.2. Dithioite Reduction and Reductive Peracetylation of 1 and 4
Ilimaquinone (1) and 5-epi-ilimaquinone (4) used as the starting materials in this investigation were readily available from our previous project [4], and were used with no additional purification. The NMR spectra were obtained and re-examined to ensure the integrity and purity of both starting materials prior to the further experiments; no significant signals of impurity were observed in the spectra of both compounds (Figures S1, S2, S7 and S8).
2.2.1. Dithionite Reduction of 1
To an ice-cold solution of 1 (30 mg, 0.084 mmol) in THF (3 mL) was added dropwise an aqueous solution (1.5 mL) of Na2S2O4 (146.3 mg, 0.84 mmol) [12]. The mixture was stirred at 0 °C for 30 min, at which time brine (10 mL) was added. The mixture was extracts with Et2O (3 × 10 mL). The Et2O solution was brought to dryness, yielding compound 2 (22 mg, 72%), which can be used without the chromatographic separation.
Ilimaquinol (2). Brown solid. UV (MeOH) λmax (log ε) 286 (4.00) nm; IR (neat) νmax 3535, 2928, 2858, 1609, 1480, 1352, 1248 cm−1; 1H NMR (DMSO-d6, 500 MHz, Figure S3) δ 8.36 (s, 18-OH), 7.45 (s, 17-OH), 7.32 (s, 21-OH), 6.32 (s, H-19), 4.41 (dd, J = 1.7, 1.7 Hz, H-11a), 4.38 (brs, H-11b), 3.63 (3H, s, 20-OCH3), 2.55 (d, J = 13.2 Hz, H-15a), 2.54 (d, J = 13.2 Hz, H-15b), 2.28 (ddd, J = 13.7, 13.7, 5.5 Hz, H-3ax), 2.03 (m, H-1eq), 2.00 (m, H-3eq), 1.73 (m, H-2eq), 1.53 (m, H-6eq), 1.42 (overlapped, H-1ax), 1.34 (overlapped, H-2ax), 1.28 (overlapped, H-6ax), 1.24 (3H, overlapped, H2-7 & H-8), 1.06 (brd, J = 11.3 Hz, H-10), 0.99 (3H, s, H3-12), 0.82 (3H, s, H3-14), 0.81 (3H, d, J = 6.4 Hz, H3-13); 13C NMR (DMSO-d6, 125 MHz, Figure S4) δ 159.7 (C, C-4), 139.0 (C, C-18), 138.9 (C, C-17), 138.7 (C, C-21), 135.9 (C, C-20), 115.6 (C, C-16), 102.6 (CH2, C-11), 99.8 (CH, C-19), 56.5 (CH3, 20-OCH3), 50.8 (CH, C-10), 42.3 (C, C-9), 39.8 (C, C-5), 38.0 (CH, C-8), 36.4 (CH2, C-6), 32.5 (CH2, C-3), 30.4 (CH2, C-15), 28.2 (CH2, C-2), 28.1 (CH2, C-7), 23.1 (CH2, C-1), 20.1 (CH3, C-12), 18.7 (CH3, C-13), 16.7 (CH3, C-14); ESIMS m/z 359.2218 [M−H]− (calcd for C22H31O4, 359.2214).
2.2.2. Reductive Peracetylation of 1
To a solution of 1 (20 mg, 0.056 mmol) in Ac2O (3 mL) and Et3N (3 mL) was added Zn dust (20 mg) in one portion [13]. The mixture was refluxed for 70 min. Once cooled, brine was added, and the mixture was extracted with Et2O (3 × 10 mL). The organic phase was brough to dryness. Compound 3 (22 mg, 80%) was cleanly obtained with no further purification.
Ilimaquinol triacetate (3). Brown solid. UV (MeOH) λmax (log ε) 281 (3.61) nm; IR (neat) νmax 2929, 1773, 1479, 1369, 1180, 1016 cm−1; 1H NMR (benzene-d6, 500 MHz, Figure S5) δ 6.52 (s, H-19), 4.63 (dd, J = 1.7, 1.7 Hz, H-11a), 4.60 (brs, H-11b), 3.12 (3H, s, 20-OCH3), 2.60 (d, J = 14.0 Hz, H-15a), 2.52 (d, J = 14.0 Hz, H-15b), 2.33 (ddd, J = 13.6, 13.6, 5.4 Hz, H-3ax), 2.15 (dd, J = 13.6, 4.4 Hz, H-3eq), 1.99 (3H, s, 21-OCOCH3), 1.93 (3H, s, 18-OCOCH3), 1.79 (3H, s, 17-OCOCH3), 1.77 (2H, m, H-7), 1.73 (m, H-1eq), 1.65 (dd, J = 3.5, 1.6 Hz, H-8), 1.54 (m, H-6eq), 1.39 (m, H-1ax), 1.38 (m, H-2eq), 1.35 (m, H-6ax), 1.31 (m, H-2ax), 1.25 (dd, J = 11.8, 1.9 Hz, H-10), 1.02 (3H, s, H3-12), 0.93 (3H, s, H3-14), 0.88 (3H, d, J = 6.5 Hz, H3-13); 13C NMR (benzene-d6, 125 MHz, Figure S6) δ 167.7 (C, 18-OCOCH3), 167.6 (2C, 17-OCOCH3 & 21-OCOCH3), 160.2 (C, C-4), 149.9 (C, C-20), 141.3 (C, C-18), 138.1 (C, C-21), 136.4 (C, C-17), 129.4 (C, C-16), 106.2 (CH, C-19), 103.9 (CH2, C-11), 55.9 (OCH3, 20-OCH3), 54.1 (CH, C-10), 43.8 (C, C-9), 41.4 (C, C-5), 40.5 (CH, C-8), 39.4 (CH2, C-15), 37.5 (CH2, C-6), 33.7 (CH2, C-3), 29.7 (CH2, C-7), 29.2 (CH2, C-2), 24.5 (CH2, C-1), 20.8 (2CH3, C-12 & 21-OCOCH3), 20.5 (2CH3, 17-OCOCH3 & 18-OCOCH3), 19.3 (CH3, C-13), 16.7 (CH3, C-14); ESIMS m/z 509.2516 [M+Na]+ (calcd for C28H38O7Na, 509.2505).
2.2.3. Dithionite Reduction of 4
The reduction of 4 (30 mg, 0.084 mmol) was carried out in a similar manner to that for the reduction of 1 toward the hydroquinone 2. The resulting compound 5 (20 mg, 66%) was obtained also without any necessity of further purification.
5-Epi-ilimaquinol (5). Brown solid. UV (MeOH) λmax (log ε) 286 (3.95) nm; IR (neat) νmax 3338, 2928, 1644, 1608, 1232 cm−1; 1H NMR (DMSO-d6, 500 MHz, Figure S9) δ 8.37 (s, 18-OH), 7.44 (s, 17-OH), 7.35 (s, 21-OH), 6.32 (s, H-19), 4.66 (brs, H-11a), 4.62 (brs, H-11b), 3.63 (3H, s, 20-OCH3), 2.62 (d, J = 13.2 Hz, H-15a), 2.59 (d, J = 13.2 Hz, H-15b), 2.36 (ddd, J = 13.8, 6.1, 6.1 Hz, H-3ax), 2.06 (m, H-1eq), 2.04 (m, H-3eq), 1.94 (ddd, J = 13.8, 3.3, 3.3 Hz, H-1ax), 1.80 (overlapped, H-6eq), 1.68 (overlapped, H-2eq), 1.59 (overlapped, H-2ax), 1.41 (overlapped, H-7eq), 1.30 (overlapped, H-8), 1.22 (overlapped, H-10), 1.16 (overlapped, H-7ax), 1.08 (overlapped, H-6ax), 1.03 (3H, s, H3-12), 0.84 (3H, s, H3-14), 0.80 (3H, d, J = 6.3 Hz, H3-13); 13C NMR (DMSO-d6, 125 MHz, Figure S10) δ 153.4 (C, C-4), 139.2 (C, C-18), 138.7 (C, C-17), 138.6 (C, C-21), 136.1 (C, C-20), 116.0 (C, C-16), 105.4 (CH2, C-11), 99.7 (CH, C-19), 56.4 (CH3, 20-OCH3), 49.2 (CH, C-10), 43.9 (C, C-9), 39.2 (CH, C-8), 39.0 (C, C-5), 37.5 (CH2, C-6), 32.8 (CH3, C-12), 32.7 (CH2, C-3), 31.6 (CH2, C-15), 27.9 (CH2, C-7), 24.6 (CH2, C-2), 22.3 (CH2, C-1), 18.8 (CH3, C-13), 17.8 (CH3, C-14); ESIMS m/z 359.2221 [M−H]− (calcd for C22H31O4, 359.2214).
2.2.4. Reductive Peracetylation of 4
Compound 4 (20 mg, 0.056 mmol) was subjected to the reductive peracetylation in the same manner as that for 1 toward compound 3. Also similar to that for 3, compound 6 (21 mg, 78%) was obtained cleanly, and no further purification was needed.
5-Epi-ilimaquinol triacetate (6). Brown solid. UV (MeOH) λmax (log ε) 280 (3.66) nm; IR (neat) νmax 2931, 2864, 1773, 1369, 1181 cm−1; 1H NMR (benzene-d6, 500 MHz, Figure S11) δ 6.55 (s, H-19), 4.80 (dd, J = 1.7, 1.7 Hz, H-11a), 4.77 (dd, J = 1.7, 1.7 Hz, H-11b), 3.13 (3H, s, 20-OCH3), 2.64 (d, J = 14.0 Hz, H-15a) 2.60 (d, J = 14.0 Hz, H-15b), 2.41 (ddd, J = 13.6, 6.6, 6.6 Hz, H-3ax), 2.12 (m, H-1eq), 2.06 (m, H-3eq), 2.02 (m, H-6eq), 2.00 (3H, s, 21-OCOCH3), 1.97 (m, H-1ax), 1.94 (3H, s, 18-OCOCH3), 1.83 (m, H-2eq), 1.80 (3H, s, 17-OCOCH3), 1.73 (m, H-1ax), 1.66 (brd, J = 6.6 Hz, H-2ax), 1.59 (2H, overlapped, H-7eq & H-8), 1.53 (m, H-10), 1.22 (m, H-7ax), 1.16 (3H, s, H3-12), 1.10 (m, H-6ax), 1.04 (3H, s, H3-14), 0.93 (3H, d, J = 5.9 Hz, H3-13); 13C NMR (benzene-d6, 125 MHz, Figure S12) δ 167.7 (3C, 17-OCOCH3, 18-OCOCH3 & 21-OCOCH3), 153.6 (C, C-4), 149.8 (C, C-20), 141.2 (C, C-17), 138.3 (C, C-21), 136.5 (C, C-18), 127.9 (C, C-16), 106.8 (CH, C-19), 106.1 (CH2, C-11), 55.9 (OCH3, 20-OCH3), 50.3 (CH, C-10), 45.5 (C, C-9), 41.7 (CH, C-8), 40.3 (C, C-5), 38.8 (CH2, C-6), 38.4 (CH2, C-15), 33.6 (CH3, C-12), 32.7 (CH2, C-3), 28.7 (CH2, C-7), 25.7 (CH2, C-2), 24.1 (CH2, C-1), 20.9 (CH3, 21-OCOCH3), 20.6 (2CH3, 17-OCOCH3 & 18-OCOCH3), 19.6 (CH3, C-13), 18.6 (CH3, C-14); ESIMS m/z 509.2513 [M+Na]+ (calcd for C28H38O7Na, 509.2505).
2.3. Cytotoxic Activity Determinaiton
The cytotoxic activities were determined using a standard MTT assay protocol readily described [14]. PC3 prostate cancer cell line (8000 cell/well, ×3, 24-h exposure period) was used in this investigation. The activities are reported as IC50s (±SDs), referencing docetaxel and cisplatin as standard drugs.
2.4. DNA Damaging Experiments and Agarose Gel Electrophoreses
The plasmid DNA of pCMV6-Entry Tagged Cloning Vector (4.9 kb, Promega) was used as a model in the DNA damaging assay. To a 500-ng DNA was added a 1 μL of each tested sample in DMSO (diluted to the according concentrations). The mixtures were incubated at 37 °C for 45 min, at which time 1 μL of loading dye (DNA Gel Loading Dye 6X, Thermo Fischer Scientific) was added. Each incubate was developed on a 1% (w/v) agarose gel in 1× TAE buffer (100 v, 70 min) on Biorad PowerPac Basic (Biorad). Visualised at 254 nm, the resulting bands were captured using EpiChemi3 Darkroom Gel Imaging System (LabworkTM image acquisition and analysis software, UVP Bioimaging Systems). A non-incubated DNA and DMSO-DNA (1 μL) incubate were referred to as negative controls, and cisplatin-DNA incubate was as a positive one.
2.5. Hoechst 33342 Nuclear Staining
To observe the damaging effects on the intact cellular DNA, fluorescence Hoechst 33342 nuclear staining [15] was performed on the PC3 prostate cancer cells, cultured in the same incubating medium and conditions used for the cytotoxic assay [14]. Cells (100,000 cells/well) were seeded on sterile cover slips submerged in six-well plates over a 24-h period. A 5- and 10-μM solutions of each tested sample in DMSO (×3, each) were added, and the cells were incubated for an additional 24 h, at which time the medium was removed. Cells were washed with 1× PBS buffer, and fixed with absolute EtOH. After 10 min, EtOH was removed, and cells were stained with Hoechst 33342 dye (2 mg/mL) for 30 min in a dark room. Each cell-attached coverslip was mounted to a microscope glass slide using a buffered glycerol. Cell and nuclear morphology were observed and photographed under a fluorescence microscope (Olympus BX53). Cisplatin was referred to as a positive standard. Representative micrographs (×3 for each cultured slide) were selected and used to determine the percentages of cells with damaged DNAs. Cells present in 250 × 250-μm2 frames (a total of nine frames per treatment) were counted manually, and percentages of cells with damaged DNAs (condensed and fragmented DNAs combined) over total cell counts were calculated.
2.6. NQO1 Inhbiting Assays
NAD(P)H:quinone oxidoreductase 1 (NQO1) is the primary enzyme participating in the in-situ reduction of several quinone-containing anticancer drugs [16,17,18]. The enzyme activity can be inhibited by dicoumarol [16]. This information opens up an opportunity to explore whether the ilimaquinones may undergo an in situ transformation into the active hydroquinone species, and whether the enzyme may be involved in the conversion.
The inhibition of NQO1 by dicoumarol was performed on PC3 prostate cancer cells using an SRB assay method [19]. PC3 cells, primarily maintained in RPM1 1640 medium (Gibco) (supplemented with 10% FBS and 1% streptomycin/penicillin), were plated on a 96-well plate (8000 cells/well) for 24 h. Four hours prior to exposure to the tested samples, cells were pretreated with 100 μL of dicoumarol (10 μM) [20]. A serial dilution of each tested compound (100 μL, 1.25–120 μM, ×3 each) were added, and the incubation was continued for 24 h. The SRB staining was performed as described [19]. The resulting pink solutions were measured at 560 nm (Versamex microplate reader, Softmax Pro). The activities were reported as IC50s ± SDs.
3. Results and Discussion
3.1. Preparation of Hydroquinones and Hydroquinone Triacetates
The reduction procedures of 1 toward the hydroquinone counterparts have been reported [13], although the chemical properties and biological activities of all the derivatives have not been fully documented. Dithionite reduction of compounds 1 and 4 towards the hydroquinones proceeded fast and smoothly to provide 2 and 5 (72% and 66%, respectively) in clean, good yields (Scheme 1). Predictably, the 2-hydroxy-hydroquinone moiety of 2 and 5 was not stable, and the two compounds converted to their parent quinones promptly, particularly in an alkaline medium. This autoxidation has been reported and is widely acknowledged [21]. The chemical structures of 2 and 5 in fact have been projected [13]; however, the complete spectroscopic data of both compounds have never been available. Here, with a quick and immediate removal of reaction media and all involving solvents, we successfully obtained both compounds in adequately genuine conditions for all the spectroscopic experiments and bioassays. As dry solids, 2 and 5 can be stored (N2 atmosphere, −20 °C) for up to two weeks. In DMSO, both hydroquinones remained intact up to two days before the signals of the parent quinones become gradually visible in the 1H NMR spectra. Other solvents, however, including CHCl3 and benzene, failed to preserve the compounds in the hydroquinone forms for even a period as short as 15 min.
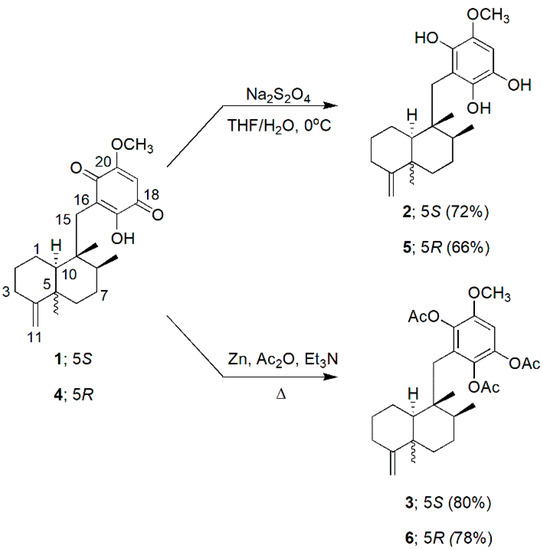
Scheme 1.
Preparation of compounds 2, 3, 5 and 6.
On the other hand, the hydroquinone triacetates 3 and 6 are well stable. Reduction of 1 and 4 with Zn dust, followed by an immediate trapping with Ac2O [22,23] led to 3 and 6 in clean, good yields (80 and 78%, respectively, Scheme 1).
3.2. Cytotoxic Activities and DNA Damaging Effects of Ilimaquinone and Derivatives
3.2.1. Cytotoxic Activities
Compounds 1–6 were first subjected to a cytotoxicity determination against PC3 cancer cells using an MTT assay with a 24-h exposure period. This is to compare the cytotoxicity of the tested samples with the results in our previous reports, and to justify the further investigation. All the quinones and hydroquinones were active in a range of 10–20 μM, comparable to the activity of cisplatin, but approximately ten times less active than the standard docetaxel (Table 1).

Table 1.
Cytotoxic activity of compounds 1–6 against PC3 prostate cancer cells (MTT assay).
3.2.2. DNA Damaging Effects in a Cell-Free System
The direct effects of compounds 1–6 on DNA were determined by ways of a DNA migratory experiment (Figure 1), in which each compound was directly incubated with plasmid DNA before being electrophoresed on an agarose gel. In its quinone form, 1 (lane 4) casted no direct effects, and the DNA retained its supercoiled conformation (form I). A couple of unknown faint bands were observed; however, these were not in an agreement with other identifiable forms of DNA nor were coherent with the band of tested samples. While these were certainly not caused by the DNA-drug adducts, we were yet unable to account for the presence of these minor bands.
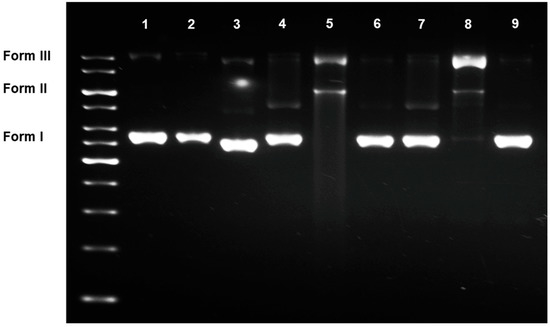
Figure 1.
Effects of compounds 1–6 on plasmid DNA; lanes (1) DNA; (2) DNA + DMSO; (3) DNA + cisplatin (10 mM); (4–9) DNA + compounds 1–6 (10 mM, each), respectively.
When 1 was reduced to the hydroquinone 2 (lane 5), the compound became active. The supercoiled plasmid DNA was cleaved and uncoiled into the nicked, circular (single-strand break, form III) and linear (double-strand break, form II) conformations. A faint smear of DNA fragments was found on the lower half of the agarose gel. Being fully acetylated, the hydroquinone triacetate 3 (lane 6) lost its DNA damaging activities, and the plasmid DNA remained supercoiled similar to that observed with 1.
The effects of the three 5-epimers (4–6, lanes 7–9) were almost parallel to those of 1-3. The quinone 4 caused no significant effects, while the hydroquinone 5 cleaved the DNA into nicked, circular and linear forms. Similar to 3, the hydroquinone triacetate 6 lost its activities once the hydroquinone moiety was fully protected.
The tested samples also migrated on the agarose gel, yielding a faint band at the bottom of the agarose gel (not visible at 254 nm). This, however, was incoherent with any bands of DNAs described above. With the samples clearly separated from the DNAs, this indicated the indirect effects of the compounds on the targeted DNA. That is, the tested compounds might not necessarily bind to DNA directly to cause the damages as observed. An alternative explanation is that the direct interactions, if any, were reversible, and the tested compounds dissociated from the binding sites completely during the electrophoreses.
Having observed the contrasting effects of the quinones 1 and 4 vs. hydroquinones 2 and 5 on the DNA, we now turned our attention towards the concentration-responsiveness of 2 and 5 (Figure 2). The results from both hydroquinones were parallel, and indicated their successive impacts on DNA damages. Upon increasing the concentration of tested compounds, the supercoiled plasmid DNA was cleaved (at 1 and 5 mM), nicked, uncoiled and opened into a linear form (at 10 mM), before being cut completely into small fragments (at 25 mM). Particularly focused were the smears of fragmented DNA, which gradually enlarged from faintly observable patches at 5 mM to large diffusing smudges at 50 mM of the tested samples. Interestingly, at the highest concentration of 50 mM of both compounds, despite the presence of the fragmented DNA smears, the bands of supercoiled DNA remained. The consistent results from both compounds suggested that the incidence was genuine. To our knowledge, however, such an anomaly has not been rationalised either theoretically or experimentally in any documents. At this moment, we are unable to account for the seemingly reverted activities of both compounds.
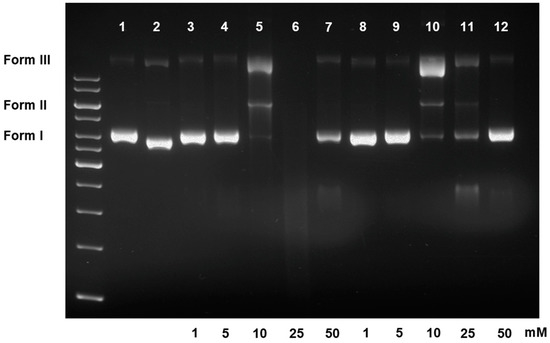
Figure 2.
Concentration-responsive effects of compounds 2 and 5 on plasmid DNA; lanes (1) DNA; (2) DNA + cisplatin (10 mM); (3–7) DNA + 2 (1, 5, 25 and 50 mM, respectively); (8–12) DNA + 5 (1, 5, 25 and 50 mM, respectively).
3.2.3. DNA Damaging Effects in a Cell-Based Assay
To examine the effects of the tested quinones and hydroquinones on the cellular DNA, compounds 1–6 were subjected to the cell-based assay targeting PC3 prostate cancer cells. The cellular DNAs were stained with Hoechst 33324 dye after a 24-h exposure, and the condensed and fragmented DNAs (white solid and pink dashed arrows, respectively) were observed (Figure 3). The use of Hoechst 33324 staining, while not providing an immediate quantification, allowed the simple and direct observation of cells with damaged DNAs, therefore aligning the results from the cell-free system to a cell-based assay. The percentages of cells with damaged DNAs (condensed and fragmented) calculated from direct cell counts were in comparable ranges of 35–50% and 55–65% when cells were treated with 5 and 10 μM of tested samples, respectively (Figure 4). The results from the tested compounds were all parallel; i.e., all caused cellular DNA damages, and the effects were concentration-dependent.
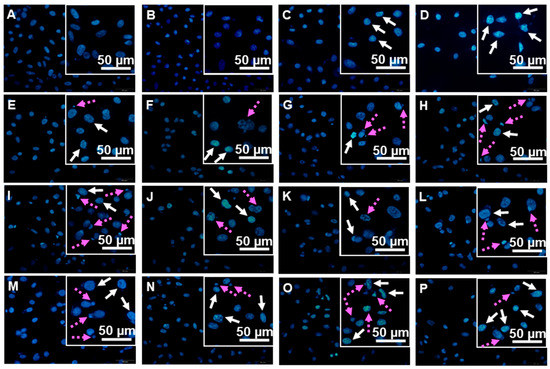
Figure 3.
Hoechst 33342 nuclear staining on PC3 cancer cells after an exposure to compounds 1–6 (condensed DNA, white solid arrows; fragmented DNA, pink dotted arrows); (A) untreated; (B) DMSO; (C,D) cisplatin (10 and 15 μM); (E,F) 1 (5 and 10 μM); (G,H) 2 (5 and 10 μM); (I,J) 3 (5 and 10 μM); (K,L) 4 (5 and 10 μM); (M,N) 5 (5 and 10 μM); and (O,P) 6 (5 and 10 μM), respectively.
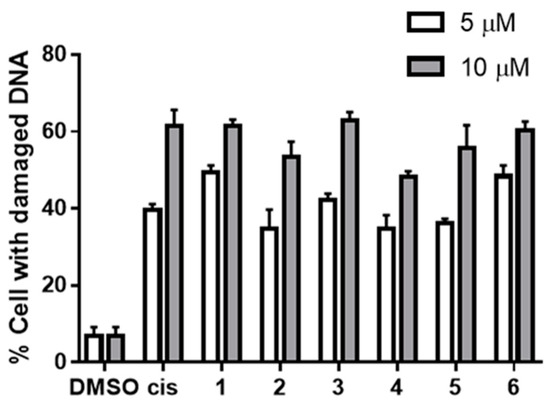
Figure 4.
Percentages of PC3 cells with damaged DNAs upon exposure to compounds 1–6 at 5 and 10 μM, referencing cisplatin (cis) at 10 and 15 μM (white and grey bars, respectively); p ≤ 0.0001 in all treatments.
It is fascinating when comparing the effects of the tested samples on the cellular DNA with the results from the cell-free experiments. When incubated directly with the plasmid DNA, the quinones 1 and 4 caused no observable effects, while the hydroquinones 2 and 5 promptly cleaved the DNA. However, when the cell-based assays were carried out, all four compounds were cytotoxic against PC3 cells. The damaging effects on the cellular DNA of the quinone 1 and hydroquinone 2 were in a comparable manner, as were the effects of the quinone 4 and hydroquinone 5. The results indicated that, as quinones, compounds 1 and 4 were unable to cause DNA damage directly. The compounds presumably need an in-situ transformation into their reactive corresponding hydroquinones to interact with DNA.
The hydroquinone triacetates 3 and 6 lost their direct effects on DNA due to the acetyl protecting groups, but were able to damage the DNA in the cell-based assays. Presumably, the compounds were hydrolysed in situ into the deacetylated hydroquinone, therefore enabling the DNA-damaging activity and exerting their cytotoxicity once exposed to the targeted cancer cells.
Note here that it is arguable whether the DNA condensation and fragmentation observed here could be either a direct consequence of the DNA-damaging effects, or a post-incidence induced by programmed cell death. While our results did not allow a conclusive explanation to be drawn, the coherence with the results from cell-free experiments suggested that the DNA-damaging effects, whether entirely or in part, may as well precede the upcoming incidences that lead to cell death.
The similarity among the DNA-damaging effects of the tested samples in the cell-based experiments were reflected on the cytotoxicity results. The IC50s of all six compounds were in the same magnitude (Table 1, Section 3.2.1). The comparable IC50s indicated that, being hydroquinones, 2 and 5 did not have any superior cytotoxicity to the other four compounds, nor did the hydroquinone moiety exert a preferable structure-activity relationship. The results confirm our hypothesis that the hydroquinones strictly act as the active forms of the quinones and hydroquinone triacetates, all of which, once being transformed into the active hydroquinones, can interact with the DNA in the same manner.
3.3. Effects of NQO1 Inhibition on the Cytotoxicity of Ilimaquinone Derivatives
The DNA-damaging results described above led to the hypothesis that the cytotoxicity of ilimaquinone may involve an in-situ transformation of the quinone functionality to the reactive hydroquinone. As one to the most common in situ transformations of the quinone-containing anticancer drugs towards the hydroquinone species is through a reduction catalysed by the enzyme NQO1 [16], we therefore extended our investigation to examine whether the same route may involve in the activities of the sesquiterpene quinones.
Compounds 1–6 were subjected to a cytotoxic assay on PC3 cells that had been pre-exposed to dicoumarol, an NQO1 inhibitor [16]. Note that, in this experiment, the SRB assay was used instead of the MTT one, as dicoumarol had been reported to interfere with the oxidation of MTT dye [24]. Additionally, although PC3 cells might not be as sensitive to NQO1 as other cancer cells, such as colon and breast cancers [18,25], being an alternative choice, the PC3 cell line was proved adequate for the study. Dicoumarol, in fact, was weakly cytotoxic against PC3 cells (IC50 148.8 μM). At the recommended concentration (10 μM) [26], however, the cytotoxic effects of dicoumarol were negligible. Hence, we opted to carry out this part of the investigation targeting PC3 cells, to thus observe the continuity in the results from the previous sections.
As expected, PC3 cells became less susceptible to the quinones 1 and 4 when pretreated with dicoumarol. IC50s of 1 and 4 increased approximately 1.5- and 1.3-fold in the cells that had been exposed to the inhibitor (Table 2); that is, pre-exposure to dicoumarol decreased the cytotoxicity of 1 and 4 by approximately 50 and 35%, respectively. Cell viability, when treated with the inhibitor, also increased significantly with each concentration of compound 1 (Figure 5a). With compound 4, the effects from the inhibitor were not as consistent as those observed with 1, and did not yield significant increases in cell viability until the highest concentration (120 μM) was reached (Figure 5d). Nonetheless, the difference between the IC50s from cells not treated and pretreated with dicoumarol was parallel to that of compound 1 (Table 2). This indicated the overall impacts from the inhibitors that caused the cancer cells to become less susceptible to quinones 1 and 4.

Table 2.
Cytotoxic activity of compounds 1-6 against PC3 prostate cancer cells (pretreated vs. untreated with 10 μM dicoumarol; SRB assay).
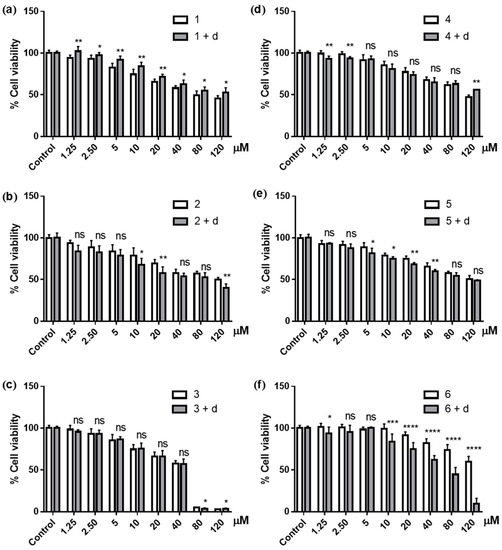
Figure 5.
Cytotoxic effects of compounds 1–6 (a–f, respectively) against PC3 cancer cells untreated (white bars) and 4-h pre-treated with 10 μM dicoumarol (d; gray bars); ns = non-significant; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001.
With hydroquinones 2 and 5, on the other hand, pretreatment with dicoumarol increased the activity of the compounds by approximately 25 and 20%, respectively (Table 2). Coherently, the cell viabilities were also lowered with the treatment of the inhibitor at most of the concentration of both compounds (Figure 5b,e).
Combined with the results from the DNA-damaging experiments, these confirmed our hypothesis that part of the cytotoxic mechanisms of ilimaquinone (1) and 5-epi-ilimaquinone (4) involves DNA-damaging effects, particularly through the in-situ transformation to their reactive hydroquinone counterparts. This is in good agreement with a recent work by van Stuijvenberg et al. [27], in which 5-epi-ilimaquinone (4) and 5-epi-nakijiquinone Q were reported to be genotoxic, as the expression of γH2AX, a marker of DNA damage response, was induced upon an exposure to the compounds. Dicoumarol, as NQO1 inhibitor, dimmed the cytotoxicity of 1 and 4. Nonetheless, the mediocre effects of dicoumarol on 1 and 4 suggested that the DNA damaging effects might not be the major cytotoxic mechanisms for 1 and 4. An alternative explanation is that, in addition to NQO1—well-known to be the primary enzyme that catalyses the redox process of quinone-containing compounds—other oxidoreductases may also participate in the reduction of 1 and 4 towards the hydroquinones.
As described earlier, two mechanisms of quinone-containing anticancer drugs have been proposed. Upon being reduced, the resulting hydroquinones can either react as a reactive electrophile, or induce an oxidative stress that leads to cell damages [17]. For the ilimaquinone analogs, the mechanisms as a reactive electrophile were unlikely, as the bands of the liberated parent quinones, but not the bands of hydroquinone-DNA adducts, were observed on the agarose gel (see Section 3.2.2.). On the other hand, hydroquinones 2 and 5 caused the DNA cleavages that resulted in a combination of single-strand (nicked, circular DNA, form III), double-strand (linear DNA, form II), and fragmented DNAs in a single treatment (Figure 2, Section 3.2.2.). This coincided with the hypothesis that the resulting hydroquinones damage DNA indirectly through the oxidative stress caused either by themselves, or by the reactive semiquinone species generated during the autoxidation back to their parent structures. A recent report by Lin et al. [28] on the ability of ilimaquinone to generate reactive oxygen species strongly confirmed this oxidative stress hypothesis.
Inhibition of NQO1 was expected to have little effect on the cytotoxicity of the hydroquinone triacetates. Pretreatment with dicoumarol barely changed the activity of 3, and even increased the cytotoxicity of 6 by approximately 45% (Table 2, Figure 5c,f). The effects of NQO1 inhibition on the activity of 3 and 6—proposed here to undergo the hydrolysis, not reduction, prior to becoming the reactive hydroquinones—were in a comparable manner to the inhibitory effects of compounds 2 and 5.
4. Conclusions
Several marine-derived sesquiterpene quinones have been hypothesised to express parts of their cytotoxic mechanisms through the in situ redox cycling on the quinone functionalities, which leads to reactive products that could cause DNA damages. Here, the DNA damaging effects of ilimaquinone (1) and 5-epi-ilimaquinone (4) were compared with the corresponding hydroquinones (2 and 5) via the direct incubation with plasmid DNA and the cell-base DNA staining assays. The results indicated that, as parts of the mechanisms, 1 and 4 were unable to interact directly with the DNA and required an in-situ activation towards the corresponding hydroquinones to induce the DNA damages and fragmentation. The diminished cytotoxicity of 1 and 4 upon the inhibition of NQO1 activity supported this in-situ transformation hypothesis. Our results complement the report by van Stuijvenberg et al. [27], in which compound 4 and 5-epi-nakijiquinone Q were found to induce the expression of γH2AX, and confirm the hypothesis described therein that the quinone moieties of the ilimaquinones need to undergo an in-situ redox cycling to exert their activities on the DNA.
Similar to their parent quinones, the hydroquinone triacetates 3 and 6 also required an in-situ transformation into the deacetylated hydroquinones to cause damages on cellular DNA. Quinones (1 and 4) and fully acetylated hydroquinones (3 and 6) can be considered prodrugs, being administered in the inactive forms before being transformed into their active species. The need for 1 and 4 to undergo a reduction catalysed by NQO1 prior to becoming reactive could be inspiring for further studies on whether the cytotoxicity may take place—and if so, how potent the activity would be—in the NQO1-dependent cancer cell lines. The extended investigation may lead to an invention of an alternative combination between cytotoxins and enzyme inhibitors for the anticancer chemotherapy.
Supplementary Materials
The 1H and 13C NMR spectra of compounds 1–6 (Figures S1–S12) are available online at https://www.mdpi.com/article/10.3390/scipharm89020026/s1.
Author Contributions
Conceptualization, A.P.; Data curation, A.J.; Funding acquisition, A.P.; Investigation, A.J., L.Y. and S.J.; Supervision, K.C. and A.P.; Writing–original draft, A.J.; Writing–review & editing, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thailand Research Fund (DBG6080005). A.J. thanks the financial support from the Royal Golden Jubilee PhD Program (PHD/0094/2556).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The reagents used in the SRB assay were kindly provided by Sukanya Dej-Adisai, Department of Pharmacognosy and Pharmaceutical Botany, Prince of Songkla University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, G.S.; Lipman, R.; Cummings, J.; Tomasz, M. Mitomycin C-DNA adducts generated by DT-diaphorase. Revised mechanism of the enzymatic reduction activation of mitomycin C. Biochemistry 1997, 36, 14128–141360. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, P.L. Mechanism(s) of bioreductive activation: The example of diaziquinone (AZQ). Free Radic. Biol. Med. 1989, 6, 405–445. [Google Scholar] [CrossRef]
- Balansa, W.Q.; Mettal, U.; Wusam, Z.G.; Plubrukarn, A.; Ijong, F.G.; Liu, Y.; Schäberle, T.F. A new sesquiterpene aminoquinone from an Indonesian marine sponge. Mar. Drugs 2019, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- Jiso, A.; Kittiwisut, S.; Chantakul, R.; Yuenyongsawad, S.; Putchakarn, S.; Schäberle, T.F.; Temkitthaworn, P.; Ingkaninan, K.; Chaithirayanon, K.; Plubrukarn, A. Quintaquinone, a merosesquiterpene from the yellow sponge Verongula cf. rigida Esper. J. Nat. Prod. 2020, 82, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Chueh, S.C.; Kung, F.L.; Pan, S.L.; Shen, Y.C.; Guh, J.H. Ilimaquinone, a marine sponge metabolite, display anticancer activity via GADD153-mediated pathway. Eur. J. Pharmacol. 2007, 556, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Namikoshi, M.; Kobayashi, H.; Yao, X.; Cai, G. Sesquiterpene quinones from a marine sponge Hippospongia sp. that inhibit maturation of starfish oocytes and induce cell cycle arrest with HepC2 cells. Pharm. Biol. 2006, 76, 522–527. [Google Scholar] [CrossRef]
- Du, L.; Zhoa, Y.D.; Nagle, D.G. Inducers of hypoxic response: Marine sesterterpene quinones activiate HIF-1. J. Nat. Prod. 2013, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yun, E.; Hwang, H.I.; Yoon, S.; Kim, D.E.; Kim, S.J.; Na, M.; Song, G.Y.; Oh, S. Ilimaquinone and ethylmenoquionone, marine sponge metabolites, suppress the proliferation of multiple myeloma cells by down-regulating the level of β catenin. Mar. Drugs 2014, 12, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chung, J.K.; Hwang, H.I.; Gwak, J.; Park, S.; Ju, G.B.; Yun, E.; Kim, D.E.; Chung, Y.H.; Na, M.; et al. Activation of p53 with ilimaquinone and ethylmenoquinone, marine sponge metabolites, induce apoptosis and autophagy in colon cancer cells. Mar. Drugs 2015, 13, 543–557. [Google Scholar] [CrossRef]
- Ratovitski, A.E. Tumor protein (TP)-p53 members as regulators of autophagy in tumor cells upon marine drug exposure. Mar. Drugs 2016, 14, 154. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Sladić, D.; Zahn, R.K.; Bässler, K.J.; Dogović, N.; Gerner, H.; Gašić, M.J.; Schröder, H.C. Avarol-induced DNA strand breakage in vitro and in erythroleukemia cells. Cancer Res. 1987, 24, 6565–6571. [Google Scholar]
- Watson, A.T.; Park, K.; Wiemer, D.F.; Scott, W.J. Application of the nickel mediated neopentyl coupling in the total synthesis of the marine natural products arenarol. J. Org. Chem. 1995, 60, 5102–5106. [Google Scholar] [CrossRef]
- Luibrand, R.T.; Erdman, T.R.; Vollmer, J.J.; Scheuer, P.J.; Finer, J.; Clardy, J. Ilimaquinone, a sesquiterpene quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar] [CrossRef]
- Mosman, T. Rapid colorimetric assay for cellylar growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 9, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L. DT diaphorase. Methods Enzymol. 1967, 10, 309–317. [Google Scholar]
- Ross, D.; Siegel, D.; Beall, H.; Prakash, A.S.; Mulcahy, R.T.; Gibson, N.W. DT-diaphorase in activation and detoxification of quinones. Cancer Metastasis Rev. 1993, 12, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.T.; Park, H.J. Implication of NQO1 in cancer therapy. BMB Rep. 2015, 48, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Cullen, J.J.; Hinkhouse, M.M.; Grady, M.; Gaut, A.W.; Liu, J.; Zhang, Y.P.; Weydert, C.J.D.; Domann, F.E.; Oberley, L.W. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanisms. Cancer Res. 2003, 63, 5513–5520. [Google Scholar] [PubMed]
- Brunmark, A.; Cadenas, E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic. Biol. Med. 1989, 7, 435–477. [Google Scholar] [CrossRef]
- Popov, A.M.; Stekhova, S.I.; Utkina, N.K.; Rebachuk, A.M. Antimicrobial and cytotoxic activity of sesquiterpene quinones and brominated diphenyl esters isolated from marine sponges. Pharm. Chem. J. 1999, 33, 71–73. [Google Scholar] [CrossRef]
- Evanno, L.; Lachkar, D.; Lamali, A.; Boufridi, A.; Séon-Méniel, B.; Tintillier, F.; Saulnier, S.; Denis, S.; Genta-Jouve, G.; Jullian, J.-C.; et al. A ring distortion strategy from marine natural product ilimaquinone leads to quorum sensing modulators. Eur. J. Org. Chem. 2018, 2018, 2486–2497. [Google Scholar] [CrossRef]
- Collier, A.C.; Pritsos, C.A. The mitochondrial uncoupler dicumarol disrupts the MTT assay. Biochem. Pharmacol. 2003, 66, 281–287. [Google Scholar] [CrossRef]
- Siegel, D.; Ross, D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic. Biol. Med. 2000, 29, 246–253. [Google Scholar] [CrossRef]
- Watanabe, J.; Nishiyama, H.; Matsui, Y.; Ito, M.; Kawanishi, H.; Kamoto, T.; Ogawa, O. Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in p53 wild-type urogenital cancer cell lines. Oncogene 2006, 25, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- van Stuijvenberg, J.; Proksch, P.; Fritz, G. Targeting the DNA damage response (DDR) by natural compounds. Bioorganic Med. Chem. 2020, 28, 115279. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Bai, L.Y.; Su, J.H.; Chiu, C.F.; Lin, W.Y.; Huang, W.T.; Shih, M.C.; Huang, Y.T.; Hu, J.L.; Weng, J.R. Ilimaquinone induces apoptosis and autophagy in human oral squamous cell carcinoma cells. Biomedicines 2020, 8, 296. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).