1. Introduction
The structure of adamantane influences its atypic physicochemical properties, good thermal and oxidative stability, extreme lipophilicity, and low energy [
1,
2]. Memantine (MEM) is an uncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist with low- to moderate-affinity [
3]. It was initially synthesized by Eli Lilly company and patented in 1968 as a derivative of amantadine [
4]. In various studies, the memantine was reported to be a neuroprotective agent that positively impacts both neurodegenerative and vascular processes [
5]. While excessive levels of glutamate result in neurotoxicity, in part through the over-activation of N-methyl-D-aspartate receptors, memantine—as a partial NMDARs antagonist, blocks these receptors to normalize the glutamatergic system and ameliorate cognitive and memory deficits [
6]. Memantine contains three rings with a bridgehead amine (-NH
2) function, which under physiological conditions carries a positive charge (-NH
3+) capable to bind on or near the Mg
2+ site in the NMDARs-associated channel [
7]. The NMDARs participates in regulation of synaptic plasticity and plays a role in learning and memory. Memantine is able to modulate the pathologic activation of NMDARs, presumably occurring in the process of AD, whilst retaining physiological activity important for learning and memory [
8]. Memantine can replace Mg
2+ during activation of NMDARs by interaction with NMDARs with a higher affinity than Mg
2+ ions. It is also able to inhibit the prolonged influx of Ca
2+ ions, which are responsible for excitotoxicity of neurons. The low affinity and rapid off-rate kinetics of memantine preserve the physiological function of the receptor, as it can still be activated by the relatively high concentration of glutamate release following depolarization of the presynaptic neuron [
9,
10]. Like other adamantane analogues, the most common side effects with memantine are dizziness, headache, constipation, drowsiness, and high blood pressure [
11].
In modern medicine, the main problem for drug delivery is passing through cell membrane as well as transfer to the target cell. Prodrugs are bioreversible derivatives of drug molecules used to overcome some barriers to the utility of the parent drug molecule [
12]. Classical prodrug design often represents a nonspecific chemical approach to mask undesired drug properties such as limited bioavailability, lack of site specificity, and hydrolytic as well as chemical instability [
13]. One possible approach to facilitate crossing of biologically active compounds through the cell membrane is to bond them to specific transport molecules such as amino acids or peptides [
14]. There are many examples in the literature for modification of known medical drugs with amino acids, which lead to increasing biological activity and often decreasing parent molecule toxicity [
15]. Amino acids are involved in many cellular metabolic and signaling pathways, so the effects of altered amino acid metabolism in AD brain are far-reaching [
16]. Taking into account all mentioned above, new memantine analogues with various amino acids-alanine, β-alanine, glycine, phenylalanine, and valine were synthesized as potential agents for treatment of illness related to cognitive and memory deficits.
There have been relations between Alzheimer’s disease (AD) and infections that cause a long-term activation of the immune system, a process known as chronic inflammation. In our previous work, the investigated amino acid memantine analogs showed inhibitory effects against model Gram-positive (Staphylococcus aureus NBIMCC (6538) and Bacillus megaterium) and Gram-negative bacteria (Escherichia coli (NBIMCC 3397) and Salmonella enterica (NBIMCC 869)) as well as yeasts (Rhodotorula sp. (BF 16-25) and Candida lusitaniae (BF 74-4)) [
17]. Comparing analysis of antimicrobial activity shows that compounds Gly-MEM and Val-MEM show inhibition activity against Gram (−) bacteria. Compound Val-MEM also inhibits proliferation of model strain Gram (+) bacteria
Bacillus megaterium. Any of tested compounds do not show antifungal activity against used model strains yeasts.
Thus, the newly synthesized molecules have double effect and could be attractive as a possible alternative for treatment of diseases like AD and infections. The obtained analogues were tested in conditions corresponding to stomach pH 2.0 and human plasma pH 7.4 at 37 °C.
2. Materials and Methods
2.1. Reagents and Methods
The buffer components HCl, KCl, NaCl, Na2HPO4 and H3PO4 were purchased from Sigma Aldrich (St. Louis, MO, USA). The kinetic study were performed on Agilent 8453 UV/Vis Spectrophotometer Agilent Technologies, 5301 Stevens Creek Blvd, Santa Clara, CA 95051, United States. Software: UV-Visible ChemStation. Light sources: low pressure deuterium lamp (wavelength range from 190 nm to approximately 800 nm); low-noise tungsten lamp (wavelength range from 370 nm to 1100 nm). Detector: Diode Array: 1024 individual photodiodes and control circuits etched onto a semiconductor chip. Cuvette: 10 mm cuvette
2.2. Solutions
The buffer solutions used in determination of hydrolytic stability of aim compounds were prepared according to the European Pharmacopoeia, 6th Edition as follows:
- (i)
buffer pH 2.0—6.57 g KCl were dissolved in water (CO2 free) and 119.0 mL 0.1mol/L HCl were added. The obtained solution was diluted to 1000.0 mL with dH2O;
- (ii)
buffer pH 7.4—2.38 g Na2HPO4, 0.19 g KH2PO4 and 8.0 g NaCl were dissolved in dH2O. The obtained solution was diluted to 1000.0 mL with dH2O;
- (iii)
standard solutions for UV-VIS-NIR spectrometry.
2.3. Kinetic Study
The kinetic study was performed by measuring the concentration of the memantine compounds and their decomposition products every 30 min for a period of 300 min under defined conditions. UV-VIS Spectrophotometric method was used for analyte quantification at relevant λ(max) for the respective compound (range 205–220 nm).
For this purpose, memantine amides containing amino acids (Ala, β-Ala, Gly, Phe and Val) were incubated at pH 2.0 and pH 7.4. The stock solutions of the respective compounds were prepared immediately before the stability studies. Aliquots (9.8 mL) of the buffer were placed in a screw-capped vial and tempered at 37 °C. A studied compound stock solution (0.2 mL) was added to the buffer. The vial was placed in a magnetic stirrer with a bath at the same temperature and agitated at 60 rpm for 300 min. Each sample was directly analyzed by UV-VIS-NIR spectrometry.
2.4. General Methodology for Peptide Synthesis in Solution Using 2-(1H-Benzotriazole-1-yl)-1,1,3,3-Tetramethylaminium Tetrafluoroborate (TBTU) as a Coupling Reagent
One eq of memantine was dissolved in in a minimal amount of dichloromethane. The corresponding amino acids Boc-AA-OH (1.3 eq), TBTU (1.3 eq) and TEA (1.3 eq), were added to the solution. The reaction mixture was stirred for 24 h at a room temperature. After 24 h, the solvent was evaporated. The obtained product was extracted by 3 × 25 mL dichloromethane. Further, combined organic layers were washed with 10% citric acid in water (3 × 25 mL), 5% NaHCO3 (3 × 25 mL) and water (3 × 25 mL). Organic layers were finally dried on anhydrous Na2SO4, and solvent was removed under vacuum.
2.5. General Methodology for Boc-Group Deprotection
One eq of corresponding Boc-AA-memantine was dissolved in 10-fold excess of trifuoroacetic acid (TFA) at 0 °C. The reaction mixture was stirred until Boc group running out (the chromatographic control was carried out in systems chloroform-methanol 95:5 ratio). The solvent was evaporated and the residue was dissolved in 10 mL methanol. Twenty-five percent ammonia solution was added until the pH reached approximately 9. The solvent was evaporated under vacuum. Obtained crystals were dissolved in ethyl acetate and washed with water (3 × 25 mL). The organic layer dried on anhydrous Na2SO4 and solvent was removed. The yield on each compound is various, but it is within 65–82%.
3. Results
This investigation aimed to study hydrolytic stability of memantine analogues with amino acids at pH 2.0 and pH 7.4 at 37 °C. UV-VIS Spectrophotometric method was specially developed for quantification of the amide concentrations. The hydrolytic stability of memantine derivatives: alanyl-memantine (1), β-alanyl-memantine (2), glycyl-memantine (3), phenylalanyl-memantine (4), and valyl-memantine (5) was studied under experimental conditions of biological relevance, i.e., at pH 2.0 and pH 7.4, at a temperature of 37 °C [
15,
16]. All studied compounds (
Figure 1) were synthesized using TBTU as a coupling reagent.
In most of the cases, the calibration function in UV-VIS spectrometry is linear and rather stable. Exceptions are relatively rare, but existing. In the region of high concentrations, negative deviations from the Beer’s law are always expected. Six to seven calibration standard solutions, covering the working range, were prepared for all substances studied in exactly the same way as for the kinetic study described above. All calibration points in the higher concentration region, exceeding 15% negative deviation from the straight line were rejected. The nature of the experiment, the possibility to prepare blank solution precisely matching the matrix in all standards and samples measured, and automatic blank correction used allows including the point at concentration 0 in calculation of the regression line. It is generally not needed if the calibration function is linear. However, in the case of nonlinearity, it is important and recommended for correct calculation of the regression coefficients.
Correct selection of calibration model and estimation of the concentration of the studied compounds is a problem of crucial importance for the reliability of the kinetic studies. Preliminary kinetic experiment was performed to ensure that all data points are situated within the working range of the corresponding calibration function. If some of the data points appear to be outside the concentration range between the lowest and the highest calibration standard, either calibration range or concentration of the solution used in kinetic study were respectively adjusted.
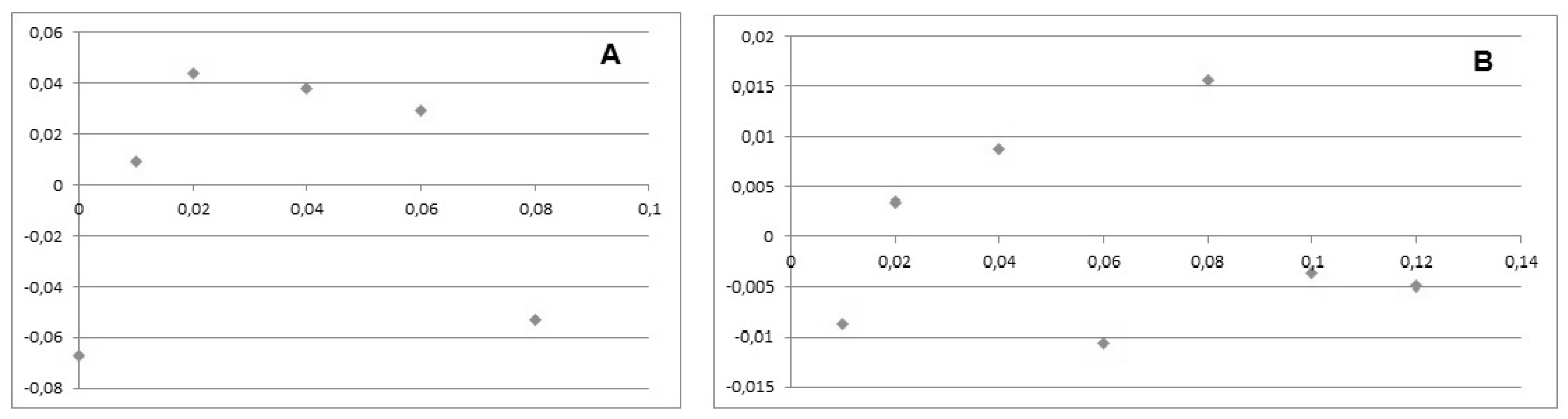
The linearity of the calibration functions was examined visually by the residual deviation plot graphical method [
18]. It is recommended by IUPAC for quick evaluation, as a part of the calibration validation procedure. This is due to the fact that frequently used statistical tests are generally not sensitive to the distribution of the deviations along the regression line. When the statistic, related to some of the other statistical tests, is close to the critical value, the residual deviation plot is helpful for making the final decision. Additionally, it is a simple qualitative homoscedasticity test. When the lowest and highest points of the calibration graphic are on the same side of the most probable straight line, while the middle part is on the other side, this is a clear indication that the real function is non-linear. In such cases, second order polynomials were tested as a calibration model. The final decision for use of linear or non-linear calibration functions was based on the Fisher’s F-test [
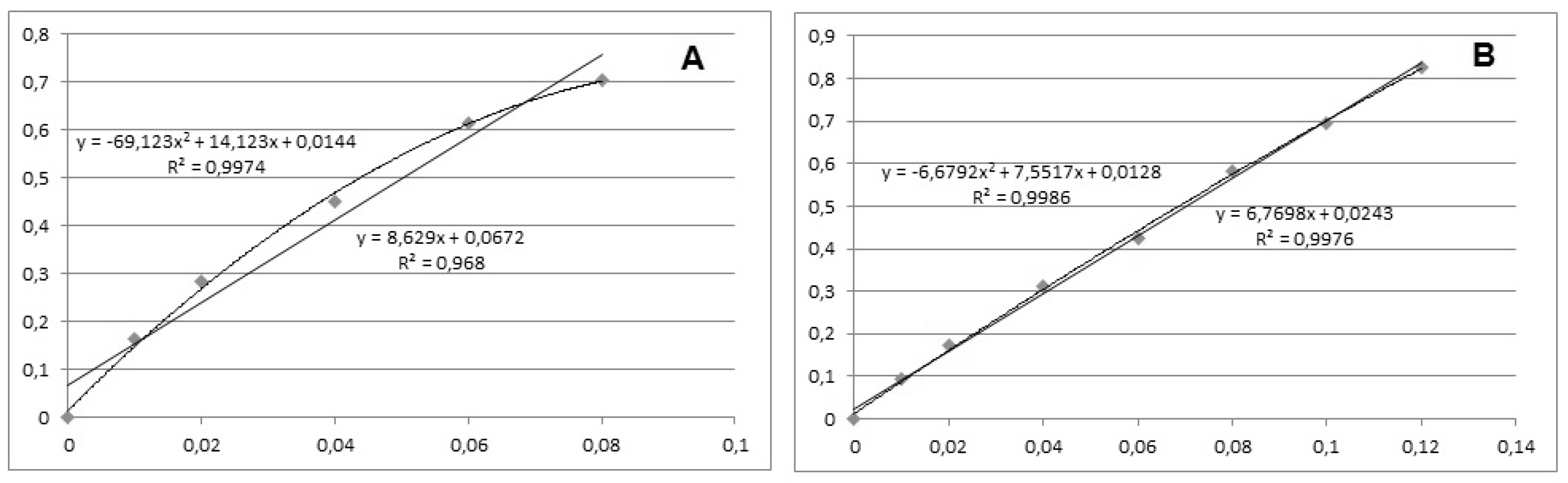
19]. In most of the cases, the calibration function appeared to be non-linear with only one exception—the case of Ala-MEM, where the function was suggested as linear. Since the calibration model based on second order polynomial function could be successfully validated, more complicated mathematical functions were not tested. The finally selected calibration equations for all compounds studied are listed in
Table 1. All kinetic experiments were performed under repeatability conditions, immediately after the respective calibration, in order to avoid the effect of any potential variability between days.
The residual deviation plots for representative cases of Val-MEM for non-linear and Ala-MEM for linear are shown in
Figure 2.
Respective comparisons between linear and second order polynomial calibration graphics for the same examples are presented in
Figure 3.
4. Discussion
Correct selection of calibration model and estimation of the concentration of the studied compounds is a problem of crucial importance for the reliability of the kinetic studies. All calibration standards were prepared using the same compounds as in kinetic as described above. Preliminary kinetic experiment was performed to ensure that all data points are situated within the working range of the corresponding calibration function. If some of the data points appear to be outside the concentration range between the lowest and the highest calibration standard, either calibration range or concentration of the solution used in kinetic study were respectively adjusted.
The linearity of the calibration functions was examined visually by the residual deviation plot graphical method [
18]. When the lowest and highest points of the calibration graphic are on the same side of the most probable straight line, while the middle part is on the other side, this is a clear indication that the real function is non-linear. In such cases, second order polynomials were tested as a calibration model. The final decision for use of non-linear calibration functions was confirmed by the Fisher’s F-test [
19]. In most of the cases, the calibration function appeared to be non-linear with only one exception—the case of Ala-MEM where the function suggested as linear. According to the literature data and previous studies, in most of the cases, the hydrolysis of drugs is following zero or first order kinetics. Zero order implies concentration-independent rate and linear function:
. In case of first or pseudo-first order kinetics, the reaction rate depends on the concentration of one reactant and linearity can be achieved in coordinates:
. The linearity of the kinetic curves in the present study was evaluated by Pearson’s correlation coefficient and Student’s t-test. It should be noted that practically always when
, the application of other linearity tests also provides positive results. These tests were applied both for examination of zero order (first function) first or pseudo first order (second function). In both cases, the slope of the regression line is equal to the rate constant of the process.
It should be noted that the conclusion based on formal kinetic evaluation of the reaction order might be significantly complicated if the concentration of the studied compound varies in a narrow concentration range. In such a case, the function trends to linear. Thus, both regression functions might show similar linearity estimated by values of R2 and the statistic for Students t-test. To distinguish between zeroth and first order kinetics, additional information is needed.
One of the possible solutions is to compare kinetic curves build for different initial concentrations of the studied compounds. If the process is of zeroth order, the slope of the curve should be independent from C0 value. To the contrary, in the case of first order reaction, the slope of the curve in the region close to should be different for different initial concentrations.
Both models were tested for all compounds at pH = 1 and in buffer solution (pH = 7).
In the present study, the R2 values for linear and logarithmic kinetic curves were not significantly different, as could be expected considering the narrow concentration range. Comparing data from the preliminary experiments and the final investigations, differences were found between the slopes of the linear kinetic curves. Since the rate constant in all kinetic models should be independent from the concentration changes, first or pseudo-first order kinetics was assumed.
Values of the rate constants were obtained as a slope of the linear regression line in coordinates
. Standard deviation of the slope (
) was calculated according to Equation (1) [
20]:
where:
xi is the time value for the
ith data point from each kinetic curve, and
SR is the regression standard deviation, calculated using Equation (2).
where:
yi and
are respectively the measured and evaluated, according to regression equation, absorbance values for the
ith data point from the each kinetic curve,
n is the number of points and
c is the number of coefficients in the regression equation (in the present case:
c = 2).
Since the measured value is the slope of the linear regression line, its standard deviation may be assumed as an estimate of the combined standard measurement uncertainty [
21] of the rate constant (
).
Reaction half-time (
) can be estimated according to Equation (3):
where
is the rate constant of the reaction. Applying the uncertainty propagation law [
21] to Equation (3), an expression can be derived for evaluation of the combined standard measurement uncertainty of the reaction half-time (
).
Expanded uncertainty (half-width of the confidence interval) [
20,
21] for the rate constant (
) and the reaction half-time (
) can be calculated according to Equation (5):
where
k is coverage factor and
uc is the corresponding combined standard measurement uncertainty. Typically, for confidence probability 0.95 (level of significance 0.05):
.
The results from the kinetic investigations are summarized in
Table 2.
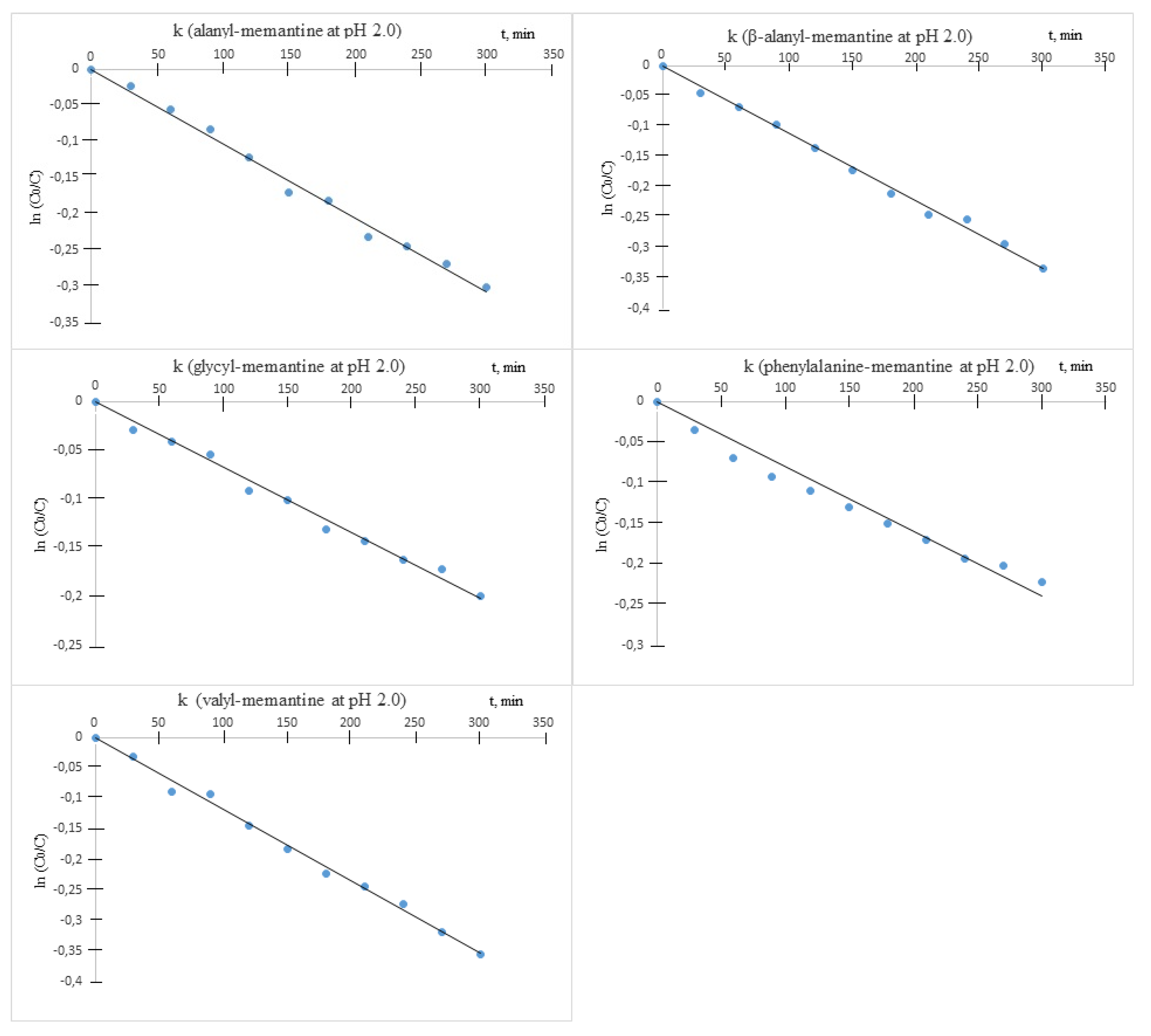
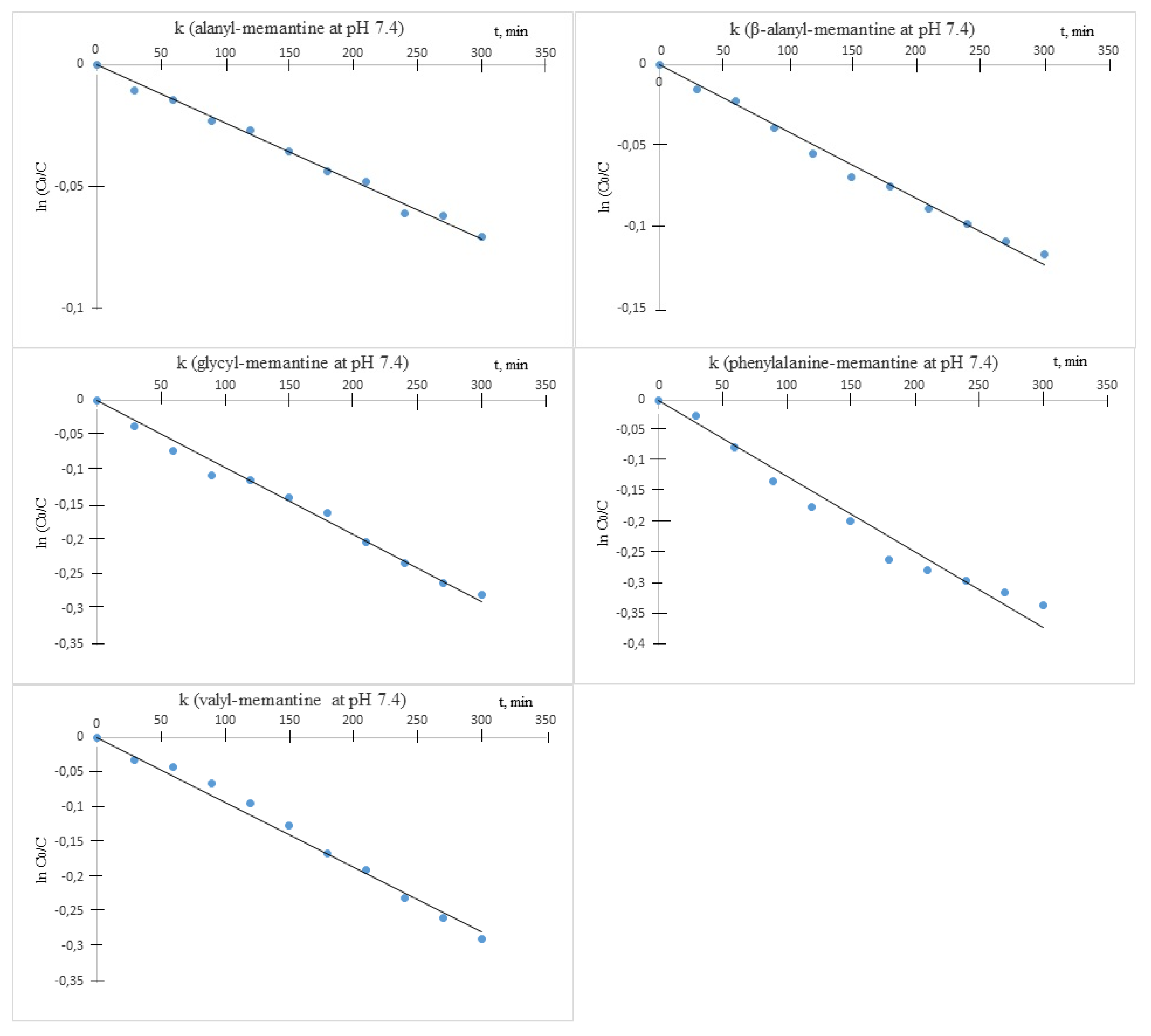
It was established that under the described experimental conditions, all amides underwent decomposition by hydrolysis. The hydrolysis followed apparent first order kinetics, and the rate constants (k) were obtained as slopes from the semi-logarithmic plots of the unchanged ester concentration versus time. The chemical stability was assessed by means of the decomposition half-times of live
t1/2 = ln2/k (
Table 2).
Hydrolytic stability of measurements revealed that the compounds were relatively stable at acid pH (
Figure 4).
Under these conditions, all of the observed half-lives were more than 10 h. All tested compounds were also stable at pH 7.4 (
Figure 5).