Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review
Abstract
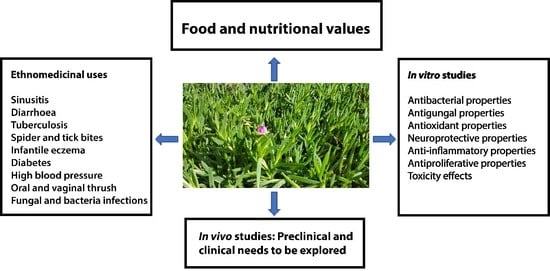
1. Introduction
2. Research Methodology
2.1. Distribution and Survival of Carpobrotus edulis
2.2. Taxonomic Classification of Carpobrotus edulis
2.3. Traditional Use of Carpobrotus edulis
2.4. Phytochemistry of Carpobrotus edulis
2.5. Toxicity Studies on Carpobrotus edulis
3. Biological Activity of Carpobrotus edulis
3.1. Antibacterial Properties
3.2. Antifungal Properties
3.3. Antioxidant Properties
3.4. Neuroprotective Properties
3.5. Antidiabetic Properties
3.6. Anti-Inflammatory Properties
3.7. Antiproliferative Properties
4. Dietary Uses of Carpobrotus edulis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Omoruyi, B.E.; Bradley, G.; Afolayan, A.J. Antioxidant and phytochemical properties of carpobrotus edulis (l.) bolus leaf used for the management of common infections in HIV/AIDS patients in eastern cape province. Bmc Complement. Altern. Med. 2012, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.E.; D’Antonio, C.M.; Schierenbeck, K.A. Hybridization and introgression in carpobrotus spp.(Aizoaceae) in california. I. Morphological evidence. Am. J. Bot. 1997, 84, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Wisura, W.; Glen, H. The South African species of carpobrotus (mesembryanthema–Aizoaceae). Contrib. Bolus Herb. 1993, 15, 76–107. [Google Scholar]
- D’antonio, C. Invasion of coastal plant communities by the introduced succulent, carpobrotus edulis (Aizoaceae). Roles Fire Herbiv. 1992, 95, 14–21. [Google Scholar]
- Roiloa, S.R.; Rodríguez-Echeverría, S.; Freitas, H.; Retuerto, R. Developmentally-programmed division of labour in the clonal invader carpobrotus edulis. Biol. Invasions 2013, 15, 1895–1905. [Google Scholar] [CrossRef]
- Vilà, M.; Siamantziouras, A.S.D.; Brundu, G.; Camarda, I.; Lambdon, P.; Médail, F.; Moragues, E.; Suehs, C.M.; Traveset, A.; Troumbis, A.Y. Widespread resistance of Mediterranean island ecosystems to the establishment of three alien species. Divers. Distrib. 2008, 14, 839–851. [Google Scholar] [CrossRef]
- Traveset, A.; Moragues, E.; Valladares, F. Spreading of the invasive Carpobrotus aff. Acinaciformis in Mediterranean ecosystems: The advantage of performing in different light environments. Appl. Veg. Sci. 2008, 11, 45–54. [Google Scholar] [CrossRef]
- Campoy, J.G.; Acosta, A.T.; Affre, L.; Barreiro, R.; Brundu, G.; Buisson, E.; González, L.; Lema, M.; Novoa, A.; Retuerto, R. Monographs of invasive plants in europe: Carpobrotus. Bot. Lett. 2018, 165, 440–475. [Google Scholar] [CrossRef]
- D’Antonio, C.M. Mechanisms controlling invasion of coastal plant communities by the alien succulent carpobrotus edulis. Ecology 1993, 74, 83–95. [Google Scholar] [CrossRef]
- Kubitzki, K.; Rohwer, J.G.; Bittrich, V. Flowering Plants·Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Springer Science & Business Media: Berlin, Germany, 2013; Volume 2. [Google Scholar]
- Gonçalves, M. Carpobrotus. In Flora Ibérica: Plantas Vasculares de la Península Ibérica e Islas Baleares; Spanish National Research Council: Madrid, Spain, 1990. [Google Scholar]
- Baker, J.T.; Borris, R.P.; Carté, B.; Cordell, G.A.; Soejarto, D.D.; Cragg, G.M.; Gupta, M.P.; Iwu, M.M.; Madulid, D.R.; Tyler, V.E. Natural product drug discovery and development: New perspectives on international collaboration. J. Nat. Prod. 1995, 58, 1325–1357. [Google Scholar] [CrossRef]
- Almeida, J.; Marchante, E.; Marchante, H.; Freitas, H. A brief report on the invasive flora of portugal. Aliens 2003, 18, 16–18. [Google Scholar]
- De Almeida, J.D.; Freitas, H. The exotic and invasive flora of portugal. Bot. Complut. 2001, 25, 317–327. [Google Scholar]
- Weber, E.; D’antonio, C.M. Phenotypic plasticity in hybridizing carpobrotus spp.(Aizoaceae) from coastal california and its role in plant invasion. Can. J. Bot. 2000, 77, 1411–1418. [Google Scholar] [CrossRef]
- Howell, C. Consolidated List of Environmental Weeds in New Zealand; Science & Technical Pub., Department of Conservation Wellington: Wellington, New Zealand, 2008. [Google Scholar]
- Brandes, D. Urban Flora of Sousse (Tunisia); Institut für Botanik: Hannover, Germany, 2001. [Google Scholar]
- Schmalzer, P.A.; Hinkle, C.R. Species Biology and Potential for Controlling Four Exotic Plants (Ammophila Arenaria, Carpobrotus Edulis, Cortaderia Jubata and Gasoul Crystallinum) on Vandenberg Air Force Base, California; NASA Kennedy Space Center: Cocoa Beach, FL, USA, 1987.
- Dufour-Dror, J.; Fragman-Sapir, O.; Avishai, M.; Valczak, M.; Yaacoby, T.; Kagan, S.; Vered-Leshner, H.; Galon, I.; Heller, A.; Gotlieb, A. Israel’s Least Wanted Alien Ornamental Plant Species; The Ministry of Environmental Protection, Israel Nature & Parks Authority and the Ministry of Agriculture: Jerusalem, Israel, 2013.
- Verlaque, R.; Affre, L.; Diadema, K.; Suehs, C.M.; Médail, F. Unexpected morphological and karyological changes in invasive carpobrotus (Aizoaceae) in Provence (se france) compared to native South African species. Comptes Rendus Biol. 2011, 334, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Suehs, C.; Affre, L.; Médail, F. Invasion dynamics of two alien carpobrotus (Aizoaceae) taxa on a Mediterranean island: I. Genetic diversity and introgression. Heredity 2004, 92, 31. [Google Scholar] [CrossRef]
- Washburn, J.; Frankie, G. Dispersal of a scale insect, pulvinariella mesembryanthemum (Homoptera: Coccoidea) on ice plant in California. Environ. Entomol. 1981, 10, 724–727. [Google Scholar] [CrossRef]
- Novoa, A.; González, L. Impacts of carpobrotus edulis (l.) ne br. On the germination, establishment and survival of native plants: A clue for assessing its competitive strength. PLoS ONE 2014, 9, e107557. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Silva, S.E.; Marabuto, E.; Silva, D.N.; Wilson, M.R.; Thompson, V.; Yurtsever, S.; Halkka, A.; Borges, P.A.; Quartau, J.A. New mitochondrial and nuclear evidence support recent demographic expansion and an atypical phylogeographic pattern in the spittlebug Philaenus spumarius (Hemiptera, aphrophoridae). PLoS ONE 2014, 9, e98375. [Google Scholar] [CrossRef][Green Version]
- Vilà, M.; D’antonio, C.M. Fitness of invasive carpobrotus (Aizoaceae) hybrids in coastal california. Ecoscience 1998, 5, 191–199. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Mahall, B.E. Root profiles and competition between the invasive, exotic perennial, carpobrotus edulis, and two native shrub species in california coastal scrub. Am. J. Bot. 1991, 78, 885–894. [Google Scholar] [CrossRef]
- Thring, T.; Weitz, F. Medicinal plant use in the Bredasdorp/Elim region of the southern Overberg in the western cape province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa; Briza publications: Pretoria, South Africa, 1997. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E. and S. Livingstone Ltd.: Edinburgh, UK, 1962. [Google Scholar]
- Van Wyk, B.-E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa; Briza: Pretoria, South Africa, 2009. [Google Scholar]
- Cock, I.; Van Vuuren, S. Anti-proteus activity of some South African medicinal plants: Their potential for the prevention of rheumatoid arthritis. Inflammopharmacology 2014, 22, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E. A review of Khoi-san and cape dutch medical ethnobotany. J. Ethnopharmacol. 2008, 119, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Henley-Smith, C.; Botha, F.; Lall, N. The use of plants against oral pathogens. Formatex 2013, 30, 1375–1384. [Google Scholar]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef]
- Cock, I.; Van Vuuren, S. South African food and medicinal plant extracts as potential antimicrobial food agents. J. Food Sci. Technol. 2015, 52, 6879–6899. [Google Scholar] [CrossRef]
- Eman, A. Phytochemical screening on different plants parts of some succulent plants of Egypt. N. Y. Sci. J. 2011, 4, 15–18. [Google Scholar]
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.; Zengin, G.; Uysal, A.; Jeko, J.; Cziáky, Z.; Reis, C.P.; Gaspar, M.M. If you cannot beat them, join them: Exploring the fruits of the invasive species Carpobrotus edulis (l.) ne br as a source of bioactive products. Ind. Crop. Prod. 2020, 144, 112005. [Google Scholar] [CrossRef]
- Chokoe, P.K.; Masoko, P.; Mokgotho, M.P.; Howard, R.L.; Mampuru, L.J. Does seasonal variation influence the phytochemical and antibacterial properties of Carpobrotus edulis? Afr. J. Biotechnol. 2008, 7, 4164–4171. [Google Scholar]
- Van der Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Ibtissem, B.; Abdelly, C.; Sfar, S. Antioxidant and antibacterial properties of mesembryanthemum crystallinum and carpobrotus edulis extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte mesembryanthemum edule L. Ind. Crop. Prod. 2011, 34, 1066–1071. [Google Scholar] [CrossRef]
- Martins, A.; Vasas, A.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Antibacterial properties of compounds isolated from carpobrotus edulis. Int. J. Antimicrob. Agents 2011, 37, 438–444. [Google Scholar] [CrossRef]
- Martins, A.; Vasas, A.; Schelz, Z.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Constituents of carpobrotus edulis inhibit p-glycoprotein of mdr1-transfected mouse lymphoma cells. Anticancer Res. 2010, 30, 829–835. [Google Scholar]
- Omoruyi, B.E.; Afolayan, A.J.; Bradley, G. Chemical composition profiling and antifungal activity of the essential oil and plant extracts of mesembryanthemum edule (l.) bolus leaves. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 19–30. [Google Scholar] [CrossRef]
- Huang, D.-J.; Chun-Der, L.; Hsien-Jung, C.; Yaw-Huei, L. Antioxidant and antiproliferative activities of sweet potato (ipomoea batatas [l.] lamtainong 57′) constituents. Bot. Bull. Acad. Sin. 2004, 45, 179–186. [Google Scholar]
- El Hilaly, J.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicological studies of ajuga iva in experimental animals. J. Ethnopharmacol. 2004, 91, 43–50. [Google Scholar] [CrossRef]
- Gayathri, R.; Venkataraman, A.; Vishnupriya, V.; Jainu, M. Acute toxicity studies of acetone mace extract of myristica fragrans houtt on rats. Drug Invent. Today 2018, 10, 1508–1510. [Google Scholar]
- Jooste, C.S. Brine Shrimp Lethality Test and Acetylcholine Esterase Inhibition Studies on Selected South African Medicinal Plants; University of the Western Cape: Cape Town, South Africa, 2012. [Google Scholar]
- Meddeb, E.; Charni, M.; Ghazouani, T.; Cozzolino, A.; Fratianni, F.; Raboudi, F.; Nazzaro, F.; Fattouch, S. Biochemical and molecular study of Carpobrotus edulis bioactive properties and their effects on dugesia sicula (turbellaria, tricladida) regeneration. Appl. Biochem. Biotechnol. 2017, 182, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Ferreira, A.C.; Pereira, H.; Silvestre, L.; Vizetto-Duarte, C.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. The marine halophytes Carpobrotus edulis l. And arthrocnemum macrostachyum l. Are potential sources of nutritionally important PUFAS and metabolites with antioxidant, metal chelating and anticholinesterase inhibitory activities. Bot. Mar. 2012, 55, 281–288. [Google Scholar] [CrossRef]
- Rocha, M.; Rodrigues, M.; Pereira, C.; Pereira, H.; da Silva, M.; da Rosa Neng, N.; Nogueira, J.; Varela, J.; Barreira, L.; Custódio, L. Biochemical profile and in vitro neuroprotective properties of carpobrotus edulis l., a medicinal and edible halophyte native to the coast of south africa. S. Afr. J. Bot. 2017, 111, 222–231. [Google Scholar] [CrossRef]
- Ordway, D.; Hohmann, J.; Viveiros, M.; Viveiros, A.; Molnar, J.; Leandro, C.; Arroz, M.J.; Gracio, M.A.; Amaral, L. Carpobrotus edulis methanol extract inhibits the MDR efflux pumps, enhances killing of phagocytosed S. Aureus and promotes immune modulation. Phytother. Res. 2003, 17, 512–519. [Google Scholar] [CrossRef]
- Martins, M.; Ordway, D.; Kristiansen, M.; Viveiros, M.; Leandro, C.; Molnar, J.; Amaral, L. Inhibition of the Carpobrotus edulis methanol extract on the growth of phagocytosed multidrug-resistant mycobacterium tuberculosis and methicillin-resistant staphylococcus aureus. Fitoterapia 2005, 76, 96–99. [Google Scholar] [CrossRef]
- Steenkamp, V.; Fernandes, A.; Van Rensburg, C. Screening of Venda medicinal plants for antifungal activity against candida albicans. S. Afr. J. Bot. 2007, 73, 256–258. [Google Scholar] [CrossRef]
- Falleh, H.; Trabelsi, N.; Bonenfant-Magné, M.; Le Floch, G.; Abdelly, C.; Magné, C.; Ksouri, R. Polyphenol content and biological activities of mesembryanthemum edule organs after fractionation. Ind. Crop. Prod. 2013, 42, 145–152. [Google Scholar] [CrossRef]
- Fan, L.-Y.; Chiu, M.-J. Pharmacological treatment for Alzheimer’s disease: Current approaches and future strategies. Acta Neurol. Taiwan 2010, 19, 228–245. [Google Scholar]
- Mulaudzi, R.B.; Aremu, A.O.; Rengasamy, K.R.; Adebayo, S.A.; McGaw, L.J.; Amoo, S.O.; Van Staden, J.; Du Plooy, C.P. Antidiabetic, anti-inflammatory, anticholinesterase and cytotoxicity determination of two Carpobrotus species. S. Afr. J. Bot. 2019, 125, 142–148. [Google Scholar] [CrossRef]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Dietary polyunsaturated fatty acids improve cholinergic transmission in the aged brain. Genes Nutr. 2009, 4, 309. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Omoruyi, S.I.; Ekpo, O.E. Antioxidant and apoptosis-inhibition potential of carpobrotus edulis in a model of Parkinson’s disease. J. Afr. Assoc. Physiol. Sci. 2018, 6, 126–135. [Google Scholar]
- Zarrouk, A.; Smach, M.A.; Hafsa, J.; Sghaier, R.; Hammami, M.; Charfeddine, B. Effects of carpobrotus edulis extract on oxidative stress and 158n oligodendrocyte death. Biomed. Environ. Sci. 2019, 32, 291–299. [Google Scholar] [PubMed]
- Da Silva Morrone, M.; de Assis, A.M.; da Rocha, R.F.; Gasparotto, J.; Gazola, A.C.; Costa, G.M.; Zucolotto, S.M.; Castellanos, L.H.; Ramos, F.A.; Schenkel, E.P. Passiflora manicata (Juss.) aqueous leaf extract protects against reactive oxygen species and protein glycation in vitro and ex vivo models. Food Chem. Toxicol. 2013, 60, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Laoufi, H.; Benariba, N.; Adjdir, S.; Djaziri, R. In vitro α-amylase and α-glucosidase inhibitory activity of Ononis angustissima extracts. J. Appl. Pharm. Sci. 2017, 7, 191–198. [Google Scholar]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) and murta (ugni molinae turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Neuser, D.; Benson, A.; Brückner, A.; Goldberg, R.B.; Hoogwerf, B.J.; Petzinna, D. Safety and tolerability of acarbose in the treatment of type 1 and type 2 diabetes mellitus. Clin. Drug Investig. 2005, 25, 579–587. [Google Scholar] [CrossRef]
- Kazeem, M.; Adamson, J.; Ogunwande, I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of morinda lucida benth leaf. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Fawole, O.; Amoo, S.; Ndhlala, A.; Light, M.; Finnie, J.; Van Staden, J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. 2010, 127, 235–241. [Google Scholar] [CrossRef]
- Iwalewa, E.; McGaw, L.; Naidoo, V.; Eloff, J. Inflammation: The foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr. J. Biotechnol. 2007, 6. [Google Scholar] [CrossRef]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014, 13, 465. [Google Scholar] [CrossRef]
- White, H.L.; Glassman, A.T. A simple radiochemical assay for prostaglandin synthetase. Prostaglandins 1974, 7, 123–129. [Google Scholar] [CrossRef]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010, 78, 33–46. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ta, Q.T.H.; Pham, Q.T.; Luong, T.N.H.; Phung, V.T.; Duong, T.-H.; Vo, V.G. Anticancer activity of novel plant extracts and compounds from adenosma bracteosum (bonati) in human lung and liver cancer cells. Molecules 2020, 25, 2912. [Google Scholar] [CrossRef]
- Baldivia, D.D.S.; Leite, D.F.; Castro, D.T.H.D.; Campos, J.F.; Santos, U.P.D.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; de Picoli Souza, K.; Dos Santos, E.L. Evaluation of in vitro antioxidant and anticancer properties of the aqueous extract from the stem bark of stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Chanda, S.; Nagani, K. In vitro and in vivo methods for anticancer activity evaluation and some Indian medicinal plants possessing anticancer properties: An overview. J. Pharmacogn. Phytochem. 2013, 2, 140–152. [Google Scholar]
- Omoruyi, S.I.; Enogieru, A.B.; Ekpo, O.E. Preliminary cytotoxic activity of sutherladia frutescens and carpobrotus edulis on malignant glioblastoma cells. Trop. J. Nat. Prod. Res. 2019, 3, 175–179. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868. [Google Scholar] [CrossRef]
- Broomhead, N.K.; Moodley, R.; Jonnalagadda, S.B. Chemical and elemental analysis of the edible fruit of five Carpobrotus species from South Africa: Assessment of nutritional value and potential metal toxicity. Int. J. Environ. Health Res. 2019, 1–15. [Google Scholar] [CrossRef]


| Part of Plant Used or Administered | Traditional Use | Reference |
|---|---|---|
| Leaves—Leaf juice or leaf pulp | Used to treat inflammation, dysentery, digestive troubles, tuberculosis, toothache, earache. The juice is used as an antiseptic for wounds and burns and vaginal thrush. | [29,31,32] |
| Leaves—The juice from the powdered leaf | Treatment of oral thrush. | [27,29,33,34,35] |
| Leaves—Leaf juice or leaf pulp | Treatment of throat infections, burn stomach problems chilblains, mouth ulcers, sinusitis and diabetes. | [1,36,37] |
| Fruit | Food and antimicrobial Food agent. The dried fruits are eaten directly or used to make jams, preserves and act as a flavor. | [38] |
| Phytochemicals | Aqueous | Ethanol | Acetone | Hexane |
| Phenols mg TE/g | 517 ± 0.40 | 330.8 ± 0.04 | 557 ± 0.23 | 64.14 ± 0.15 |
| Flavonoids mg QE/g | 0.29 ± 0.01 | 0.28 ± 0.01 | 0.65 ± 0.04 | 1.19 ± 0.04 |
| Flavonols mg QE/g | 0.05 ± 0.001 | 0.05 ± 0.001 | 0.023 ± 0.05 | 0.19 ± 0.03 |
| Proanthocyanidins mg CE/g | 896 ± 0.05 | 115.28 ± 0.007 | 753.87 ± 0.02 | 134.91 ± 0.01 |
| Tannins (mg/g) | 461 ± 0.07 | 489 ± 0.38 | 384 ± 0.14 | 64 ± 0.14 |
| Saponins (mg/g) | 34 ± 0.21 | 45 ± 0.26 | 11 ± 0.071 | 2 ± 0.035 |
| Alkaloids (mg/g) | 45 ± 0.06 | 38 ± 0.02 | 31 ± 0.021 | 3 ± 0.015 |
| Compounds | Chemical Formula |
|---|---|
| Monoterpenes | |
| Isoterpinolene | C10 H16 |
| Nephthalene, 1,2-dihydro-2,5,8-tri | C12H10 |
| Nephthalene, 1,2-dihydro-2,5,8-tri | C12H10 |
| Bistrimethylsilyl N-acetyl EICOSAS | C15H33NO5Si3 |
| Oxygenated monoterpenes | |
| Mercaptoacetic acid, bis (trismethylsilyl) | C8H20O2SSi2 |
| Eicosamethylcyclodecasiloxane | C8H24O4Si4 |
| N-Octanol | C8H18O |
| Nonylaldehyde | C9H18O |
| Trans-β-demascenone | C13H18 O |
| Trans-2-tridecenal | C13H24O |
| Tetradecamethylcycloheptasiloxane | C14H42O7Si7 |
| Tetradecamethylcycloheptasiloxane | C14H42O7Si7 |
| Tetradecamethylcycloheptasiloxane | C14H42O7Si7 |
| Sesquiterpenes | |
| Octadecane | C18H38 |
| Octadecane | C18H38 |
| 1-octadecene | C18H36 |
| Nonadecane | C18H40 |
| Oxygenated sesquiterpene | |
| 2-pentadecanone,6,10,14-trimethyl | C18H36O |
| Diterpenes | |
| Eicosane | C20H42 |
| Eicosane | C20H42 |
| Oxygenated diterpenes | |
| Phytol (2-Hexadecen-1-o1, 3,7,11,15-tetramethyl) | C20H40O |
| Trisiloxane,1,1,1,5,5,5-hexamethyl-3-[(trimethylsilyl)oxy] | C24H72O12Si12 |
| Tetrasiloxane,1,1,1,5,7,7,7-heptamethyl-3,bis[(trimethylsilyl)oxy] | 2C24H72O12Si12 |
| 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tri(trismethylsiloxy) tetrasiloxane | 2C24H72O12Si12 |
| Tetrasiloxane-1,1,1,5,7,7,7-heptamethyl-3,3 bis[(trismethylsilyl)oxy)] | C24H72O12Si12 |
| Fatty acids | |
| Benzoic acid, 2,5-bis (trimethylsiloxy-,trimethylsilyl ester) | C16H30O4Si3 |
| Hexadecanoic acid, ethyl ester | C18H36O2 |
| Hexadecanoic acid, 1-methyl ethyl ester | C19H38O2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyede, K.A.; Ekpo, O.E.; Oguntibeju, O.O. Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Sci. Pharm. 2020, 88, 39. https://doi.org/10.3390/scipharm88030039
Akinyede KA, Ekpo OE, Oguntibeju OO. Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Scientia Pharmaceutica. 2020; 88(3):39. https://doi.org/10.3390/scipharm88030039
Chicago/Turabian StyleAkinyede, Kolajo Adedamola, Okobi Eko Ekpo, and Oluwafemi Omoniyi Oguntibeju. 2020. "Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review" Scientia Pharmaceutica 88, no. 3: 39. https://doi.org/10.3390/scipharm88030039
APA StyleAkinyede, K. A., Ekpo, O. E., & Oguntibeju, O. O. (2020). Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Scientia Pharmaceutica, 88(3), 39. https://doi.org/10.3390/scipharm88030039