Water-Soluble Fiber from Bengkoang (Pachyrhizus erosus (L.) Urban) Tuber Modulates Immune System Activity in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material Collection
2.3. Animals
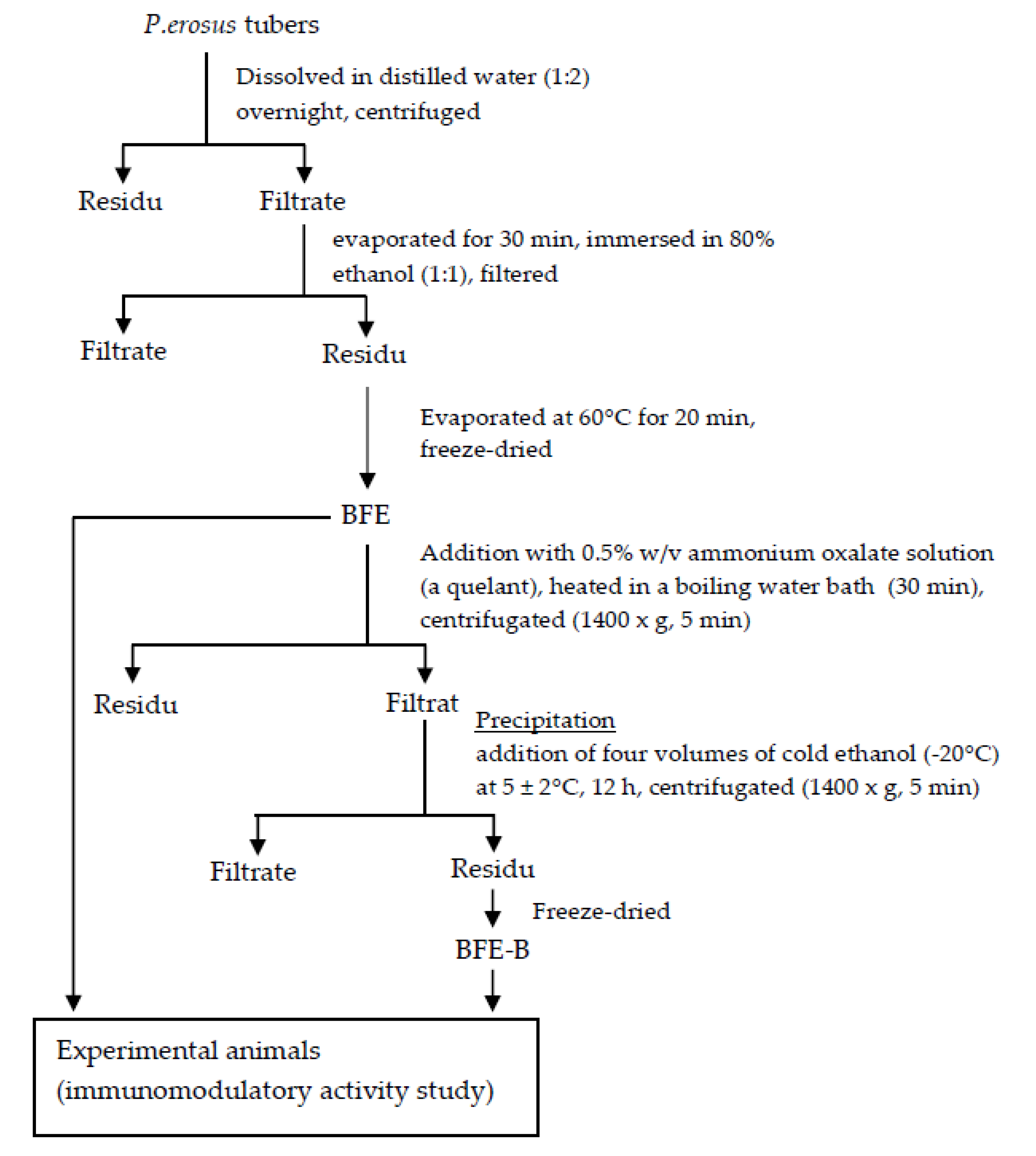
2.4. Preparation of the Soluble Fibers of P. erosus Tubers
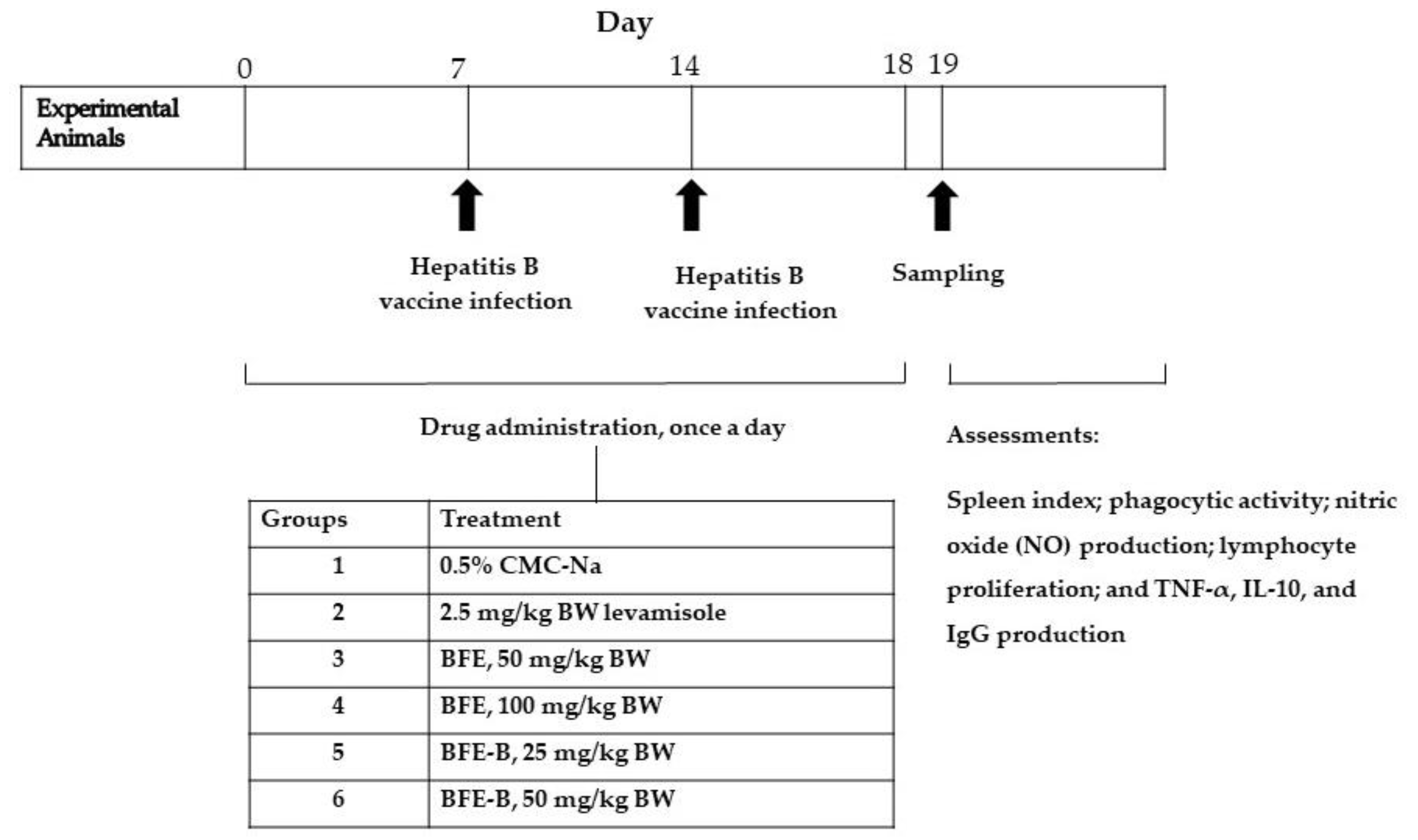
2.5. Experimental Animals
2.6. Phagocytic Assay of Macrophages
2.7. Nitric Oxide (NO) Production Assay
2.8. Spleen Index Analysis
2.9. Lymphocyte Proliferation Assay
2.10. Production of TNF-α, IL-10, and IgG
2.11. Statistical Analysis
3. Results
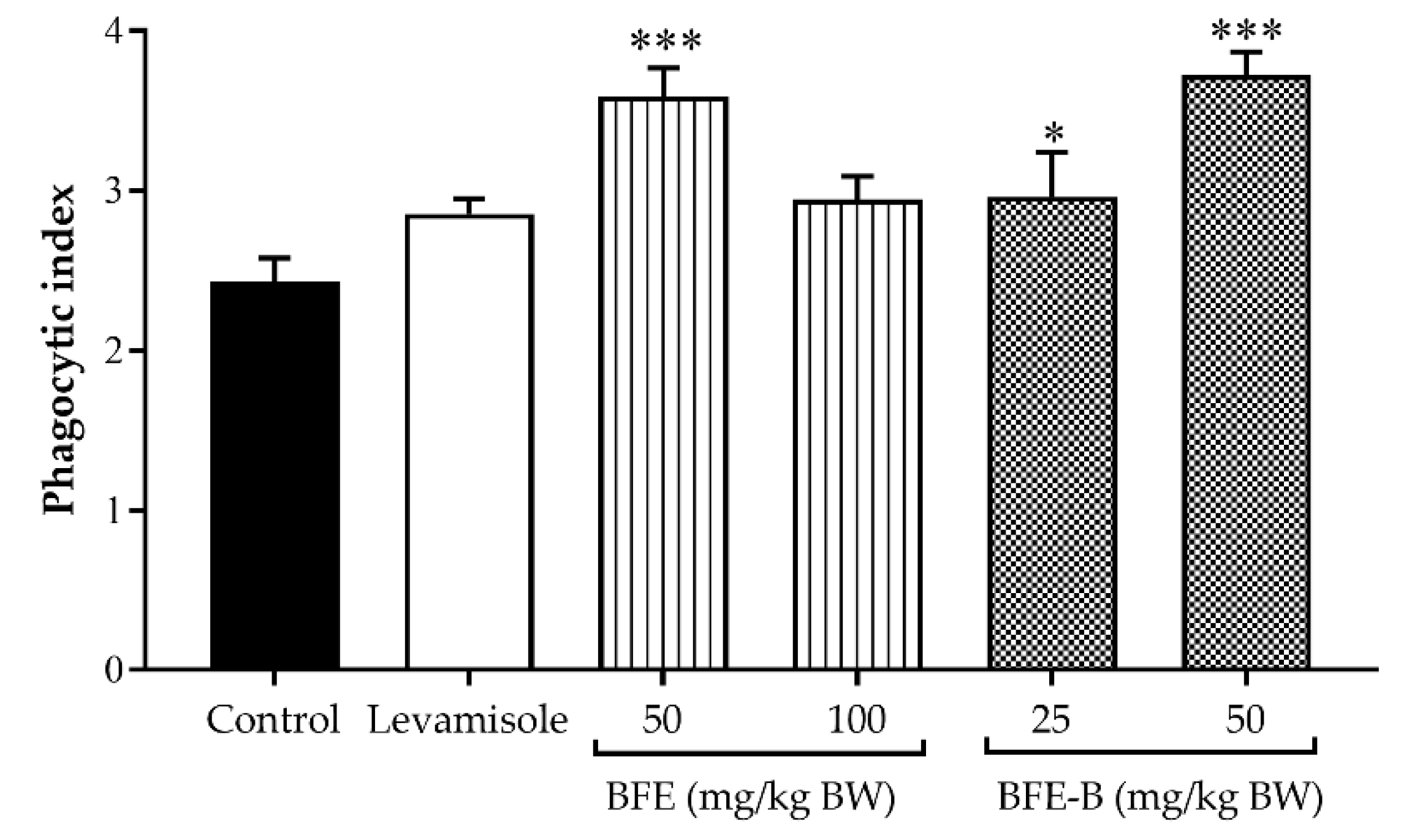
3.1. Phagocytic Activity of Macrophages
3.2. Nitric Oxide Production in Mouse Peritoneal Macrophage
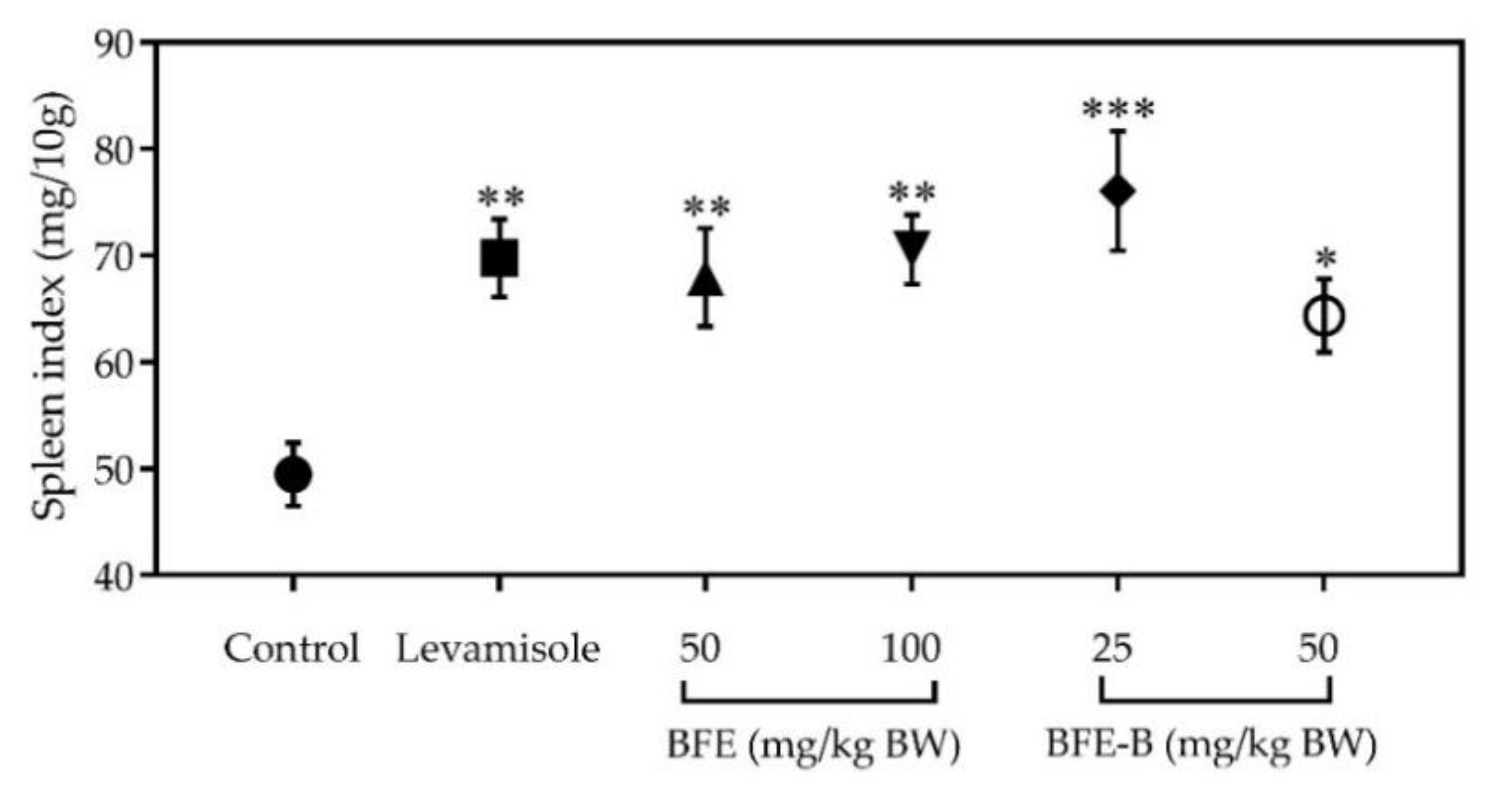
3.3. Spleen Index
3.4. Lymphocyte Cell Proliferation
3.5. Cytokines Production
3.6. Immunoglobulin G Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology E-Book. 2018. Available online: https://usmlebooksdownload.com/cellular-and-molecular-immunology-9th-edition-2018-pdf-free-download/ (accessed on 10 May 2020).
- Lee, S.J.; Chinen, J.; Kavanaugh, A. Immunomodulator therapy: Monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J. Allergy Clin. Immun. 2010, 125, S314–S323. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohyd. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.S.M.; Hoque, M.A.; Haque, M.M.; Pervin, F.; Karim, M.R. Nutritional and anti-nutritional components in Pachyrhizus erosus L. tuber. Food Chem. 2007, 102, 1112–1118. [Google Scholar] [CrossRef]
- Lukitaningsih, E. Bioactive Compound in Bengkoang (Pachyrhizus erosus) as Antioxidant and tyrosinase inhibiting agents. Indonesian J. Pharm. 2014, 25, 68. [Google Scholar] [CrossRef]
- Ramirez-Santiago, C.; Ramos-Solis, L.; Lobato-Calleros, C.; Peña-Valdivia, C.; Vernon-Carter, E.J.; Alvarez-Ramírez, J. Enrichment of stirred yogurt with soluble dietary fiber from Pachyrhizus erosus L. Urban: Effect on syneresis, microstructure and rheological properties. J. Food Eng. 2010, 101, 229–235. [Google Scholar] [CrossRef]
- Kumalasari, I.D.; Nishi, K.; Putra, A.B.N.; Sugahara, T. Activation of macrophages stimulated by the bengkoang fiber extract through toll-like receptor 4. Food Funct. 2014, 5, 1403–1408. [Google Scholar] [CrossRef]
- Kumalasari, I.D.; Nishi, K.; Harmayani, E.; Raharjo, S.; Sugahara, T. Effect of bengkoang (Pachyrhizus erosus) fiber extract on murine macrophage-like J774. 1 cells and mouse peritoneal macrophages. J. Funct. Foods 2013, 5, 582–589. [Google Scholar] [CrossRef]
- Kumalasari, I.D.; Nishi, K.; Harmayani, E.; Raharjo, S.; Sugahara, T. Immunomodulatory activity of Bengkoang (Pachyrhizus erosus) fiber extract in vitro and in vivo. Cytotechnology 2014, 66, 75–85. [Google Scholar] [CrossRef]
- Baroroh, H.N.; Nugroho, A.E.; Lukitaningsih, E.; Nurochmad, A. Immune-enhancing effect of bengkoang (Pachyrhizus erosus (L.) Urban) fiber fractions on mouse peritoneal macrophages, lymphocytes, and cytokines. J. Nat. Sci. Biol. Med. accepted.
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Huang, H. Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity. J. Funct. Foods 2017, 37, 574–585. [Google Scholar] [CrossRef]
- Winanta, A.; Hertiani, T. In vivo Immunomodulatory Activity of Faloak Bark Extract (Sterculia quadrifida R.Br). Pak. J. Biol Sci. 2019, 22, 590–596. [Google Scholar] [PubMed]
- Sumardi, T.H.; Sasmito, E. Ant plant (Myrmecodia tuberosa) hypocotyl extract modulates TCD4+ and TCD8+ cell profile of doxorubicin-induced immune-suppressed sprague dawley rats in vivo. Sci. Pharm. 2013, 81, 1057. [Google Scholar] [CrossRef] [PubMed]
- Nurrochmad, A.; Lukitaningsih, E.; Monikawati, A.; Septhea, D.B.; Meiyanto, E. Combination of low-concentration of novel phytoestrogen (8,9)-furanyl-pterocarpan-3-ol from Pachyrhizus erosus attenuated tamoxifen-associated growth inhibition on breast cancer T47D cells. Asian Pac. J. Trop. Biomed. 2013, 3, 847–852. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nie, S.; Cai, H.; Zhang, G.; Cui, S.W.; Xie, M.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan): Part IV. Immunomodulatory activity in vivo. J. Funct. Foods 2015, 15, 525–532. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, W.; Shen, W.; Wang, K. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell. Immunol. 2010, 262, 69–74. [Google Scholar] [CrossRef]
- Anudeep, S.; Prasanna, V.K.; Adya, S.M.; Radha, C. Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int. J. Biol. Macromol. 2016, 91, 656–662. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.; Martinez, G.R.; Noleto, G.R. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef]
- Chandy, M.L.; Soman, C.; Kumar, S.P.; Kurup, S.; Jose, R. Understanding molecular mechanisms in multivariant actions of levamisole as an anti-helminthic, anti-inflammatory, antioxidant, anti-neoplastic and immunomodulatory drug. J. Oral Maxillofac. Surg. Med. Pathol. 2016, 28, 354–357. [Google Scholar] [CrossRef]
- Mohamed, E.H.; Baiomy, A.A.-A.; Ibrahim, Z.S.; Soliman, M.M. Modulatory effects of levamisole and garlic oil on the immune response of Wistar rats: Biochemical, immunohistochemical, molecular and immunological study. Mol. Med. Rep. 2016, 14, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.H.; Mahfouz, M.E. Immune status of Oreochromis niloticus experimentally infected with Aeromonas hydrophila following feeding with 1, 3 β-glucan and levamisole immunostimulants. Aquaculture 2019, 509, 40–46. [Google Scholar] [CrossRef]
- Oft, M. Immune regulation and cytotoxic T cell activation of IL-10 agonists—Preclinical and clinical experience. Semin. Immunol. 2019, 44, 101325. [Google Scholar] [CrossRef]
- Nakamae, S.; Kimura, D.; Miyakoda, M.; Sukhbaatar, O.; Inoue, S.-I.; Yui, K. Role of IL-10 in inhibiting protective immune responses against infection with heterologous Plasmodium parasites. Parasitol. Int. 2019, 70, 5–15. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37. [Google Scholar] [CrossRef]
- Parham, P. The Immune system, 3rd ed.; Garland Science: New York, NY, USA, 2009. [Google Scholar]
- Coico, R.; Sunshine, G. Immunology: A Short Course, 7th ed.; Wiley Blackwell: Chichester, West Sussex, UK, 2015. [Google Scholar]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Farahmand, H.; Mirvaghefi, A.; Hoseinifar, S.H. Dietary fermentable fiber upregulated immune related genes expression, increased innate immune response and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas hydrophila. Fish Shellfish Immunol. 2014, 41, 326–331. [Google Scholar] [CrossRef]
- Schley, P.D.; Field, C.J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2002, 87, S221–S230. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Andrade, M.E.R.; Araújo, R.S.; de Barros, P.A.V.; Soares, A.D.N.; Abrantes, F.A.; Generoso, S.D.V.; Fernandes, S.O.A.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
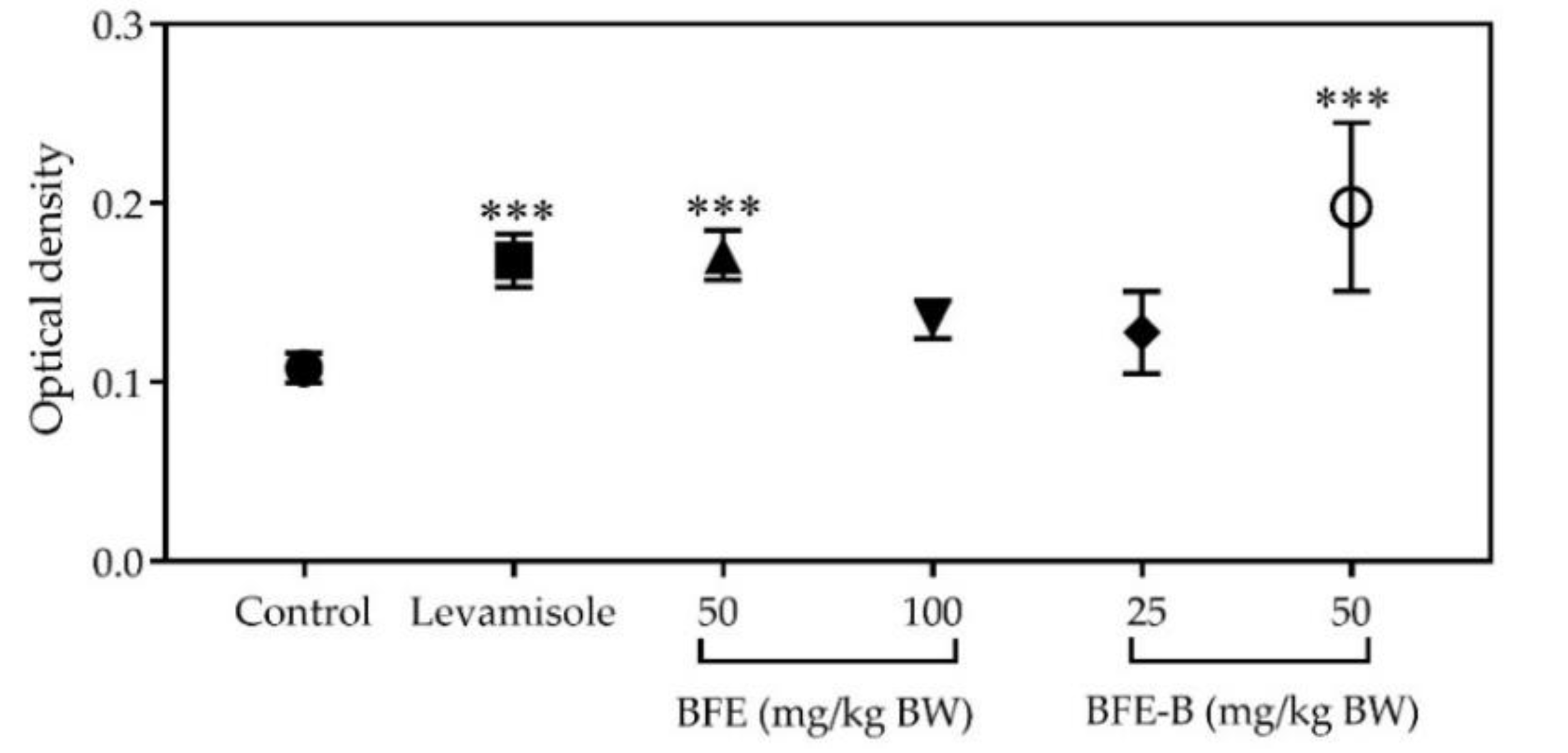
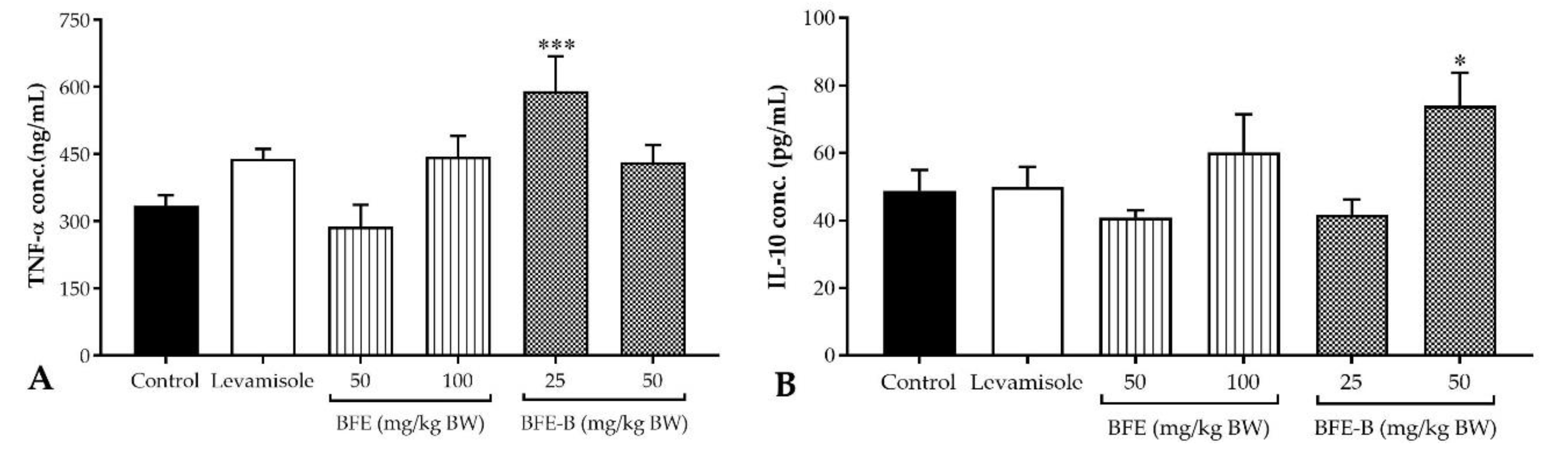
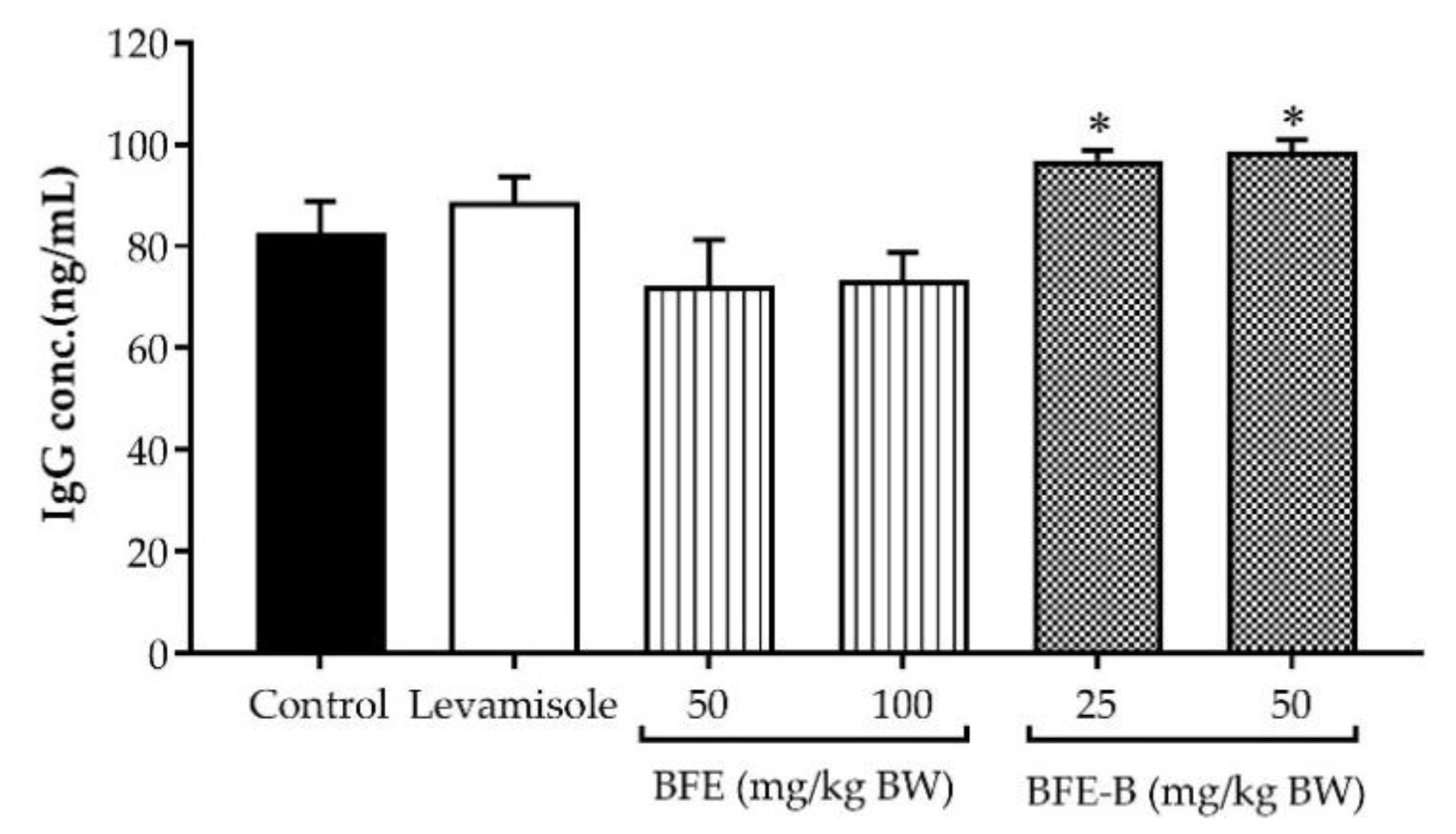
| Group | Phagocytic Capacity (%) ± SEM |
|---|---|
| BFE, 50 mg/kg BW | 49.60 ± 7.12 *** |
| BFE, 100 mg/kg BW | 54.60 ± 6.21 *** |
| BFE-B, 25 mg/kg BW | 48.40 ± 2.13 *** |
| BFE-B 50 mg/kg BW | 50.50 ± 3.26 *** |
| Levamisole, 2.5 mg/kg BW | 53.75 ± 2.93 *** |
| Control (CMC-Na) | 22.20 ± 5.80 |
| Group | Nitric Oxide Concentration (µM) ± SEM |
|---|---|
| BFE, 50 mg/kg BW | 1.77 ± 0.26 |
| BFE, 100 mg/kg BW | 0.821 ± 0.04 |
| BFE-B, 25 mg/kg BW | 4.199 ± 0.36 *** |
| BFE-B, 50 mg/kg BW | 3.47 ± 0.98 ** |
| Levamisole, 2.5 mg/kg BW | 2.59 ± 0.06 * |
| Control (CMC-Na) | 0.57 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroroh, H.N.; Nugroho, A.E.; Lukitaningsih, E.; Nurrochmad, A. Water-Soluble Fiber from Bengkoang (Pachyrhizus erosus (L.) Urban) Tuber Modulates Immune System Activity in Male Mice. Sci. Pharm. 2020, 88, 34. https://doi.org/10.3390/scipharm88030034
Baroroh HN, Nugroho AE, Lukitaningsih E, Nurrochmad A. Water-Soluble Fiber from Bengkoang (Pachyrhizus erosus (L.) Urban) Tuber Modulates Immune System Activity in Male Mice. Scientia Pharmaceutica. 2020; 88(3):34. https://doi.org/10.3390/scipharm88030034
Chicago/Turabian StyleBaroroh, Hanif Nasiatul, Agung Endro Nugroho, Endang Lukitaningsih, and Arief Nurrochmad. 2020. "Water-Soluble Fiber from Bengkoang (Pachyrhizus erosus (L.) Urban) Tuber Modulates Immune System Activity in Male Mice" Scientia Pharmaceutica 88, no. 3: 34. https://doi.org/10.3390/scipharm88030034
APA StyleBaroroh, H. N., Nugroho, A. E., Lukitaningsih, E., & Nurrochmad, A. (2020). Water-Soluble Fiber from Bengkoang (Pachyrhizus erosus (L.) Urban) Tuber Modulates Immune System Activity in Male Mice. Scientia Pharmaceutica, 88(3), 34. https://doi.org/10.3390/scipharm88030034






