Abstract
To investigate combined effect of the anticancer drug cisplatin (CP) and the opiate analgesic morphine (MOR) on liver, rats were administered MOR (10 mg/kg/day i.p. for 10 days), with or without CP (7.5 mg/kg i.p. once at day 5 of the study). MOR or CP alone caused deterioration of liver function tests and induced damage to histological architecture of liver. In addition, each drug alone caused hepatic oxidative stress, as evident by significant increase of malondialdehyde and nitric oxide, as well as the significant decrease in GSH, catalase and SOD compared to control. Administration of either MOR or CP also caused liver inflammation, evident by the increase in the pro-inflammatory cytokines; TNF-α and IL-6. In addition, either MOR or CP induced liver apoptosis, as shown by significant increase in expression of the pro-apoptotic marker; caspase 3 compared to control. Either MOR or CP also caused up-regulation of the efflux transporter P-glycoprotein (P-gp). Combining MOR with CP caused deterioration in all parameters tested compared to CP alone. Thus, treatment with MOR worsened CP-induced hepatotoxicity through oxidative stress, inflammation and apoptosis mechanisms. In addition, both drugs contributed to the up-regulation of P-gp, which might be a new mechanism for their hepatotoxic effects.
1. Introduction
Hepatotoxicity is one of the major side effects of cancer chemotherapy, especially when using one of the platinum-based drugs as cisplatin (CP) [1]. To date, the mechanisms involved in CP-induced hepatotoxicity are not fully elucidated. Oxidative stress has been one of the factors suggested [2], mainly due to the direct effect of CP toxic metabolites on the tissues, causing the generation of reactive oxygen species that consume the antioxidant defenses of the liver. In addition, several pro-inflammatory cytokines have been shown to increase after CP treatment [3,4], suggesting a strong role of inflammation as a mechanistic process in CP-induced hepatotoxicity. Administration of CP was also reported to induce hepatic programmed cell death as evident by up-regulating a number of pro-apoptotic markers [5,6].
In addition to hepatotoxicity, CP might cause chemotherapy-induced neuropathic pain as a side effect [7], which may be a limiting factor for continuation of CP use as an anticancer drug. In many cases, the pain might be too severe to endure without administration of strong analgesics, as opioids. Thus, morphine (MOR), the prototype of opiates, might be used concomitantly with CP for treatment of its chemotherapeutic-induced pain [8]. However, to date, the effect of combining MOR with CP on the liver has not yet been explored.
P-glycoprotein (P-gp), also called multidrug resistance 1 (MDR1) transporter protein, is a member of ATP-binding cassette (ABC) superfamily, encoded by ABCB1 gene, and acts as an active efflux transporter that actively pumps its various substrates across sanctuary membranes at the expense of ATP [9]. In the liver, P-gp is expressed in the canalicular membrane of the liver, and acts as an efflux transporter for its various xenobiotic substrates from the intracellular compartment of hepatocytes to be excreted in bile [10]. MOR has been reported as one of the substrates of P-gp [11]. The aim of the current study was to investigate the effect of administration of MOR concomitantly with CP on the liver, and to explore the mechanisms involved. In addition, the current study aimed at examining the effect of MOR and/or CP on hepatic P-gp level.
2. Methods
2.1. Drugs and Chemicals
Morphine sulphate was purchased from Misr Co. Pharma (Cairo, Egypt) and cisplatin was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Commercial kits were acquired from Biodiagnostic Co. (Giza, Egypt) to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin and albumin, as well as the antioxidant markers reduced glutathione (GSH), superoxide dismutase (SOD) and catalase. For immunohistochemistry, caspase 3 rabbit polyclonal ready-to-use antibody was brought from Thermo Fisher Scientific Inc./Lab Vision (Fremont, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) to measure tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). ELISA kit to measure P-gp was obtained from USBiological life Sciences (Salem, MA, USA). Other chemicals were acquired at analytical grade from commercial sources.
2.2. Experimental Animal Protocol
Male Wistar rats of 165–200 g were left to acclimatize for one week in a standard laboratory animal house under controlled conditions, with free access to chow and water. Ethical approval of the animal protocol was issued by the Institutional Review Board of Princess Nourah bint Abdulrahman University, Saudi Arabia (IRB number 17-0194). Animals were allocated into four groups (n = 10); control group, MOR-treated group receiving 10 mg/kg/day of MOR i.p. for 10 days [12], CP-treated group receiving a single i.p. of 7.5 mg/kg CP at day 5 of the experiment [13], and combined MOR/CP-treated group receiving both drugs as described.
2.3. Blood and Tissue Sample Preparation
At the end of the experiment, animals were fasted over-night and weighed, then blood was sampled, centrifuged at 3000× g for 10 min, and the sera were collected for measurement of biochemical parameters. After animal sacrifice, liver was excised, washed with normal saline and weighed. Liver index was calculated according to the following equation: (Liver index = liver weight/total body weight × 1000). A small slice from each liver was cut and fixed in 10% neutral buffered formalin. These sections were dehydrated using a range of ethanol from 70% to 100%, and then cleared in xylene. The tissues were embedded in paraffin blocks and cut by a microtome into 5 μm thick sections to be mounted on glass slides for examination under light microscope and for immunohistochemical staining. The rest of the liver tissues were homogenized in 20% w/v ice-cold phosphate buffer (0.01M, pH 7.4) by Glas-Col homogenizer. After homogenization, centrifugation was performed for 15 min at 3000× g at 4 °C to obtain the supernatant, which was aliquoted and kept frozen at −20 °C until used.
2.4. Determination of Serum Liver Function Tests and Liver Tissue Oxidant/Antioxidant Markers
Using commercial diagnostic kits for colorimetric analysis, liver function was assessed by evaluating serum ALT, AST, ALP, total bilirubin and albumin, in accordance to the kits manufacturer’s instructions. In liver homogenate, several oxidant/antioxidant stress markers were evaluated. To determine tissue content of lipid peroxidation products, malondialdehyde (MDA) was assessed in liver homogenate through biochemical evaluation of its equivalent thiobarbituric acid reacting substance by spectrophotometric measurement, employing 1,1,3,3-tetramethoxypropane as standard [14]. For evaluation of the level of nitric oxide (NO), total nitrate/nitrate was used because they are the stable oxidation products of NO, as NO itself cannot be measured due to its short half-life. Total nitrate/nitrite was determined using Griess reaction, where nitrate is converted to nitrite by copperized cadmium granules [15]. For evaluation of GSH, a kit was used, whose principal depended on that the -SH component of GSH interacted with Ellman’s reagent (5,5-dithio-bis-2-nitrobenzoic acid) generating 5-thio-2-nitrobenzoic acid with yellow color that could be detected colorimetrically (Beckman DU-64 UV/VIS spectrophotometer). Kits were also used to determine the enzymatic activity of catalase and SOD (shown in terms of U/mg protein) in liver homogenate in accordance to the manufacturer’s instructions.
2.5. Liver Histopathological Examination
Liver specimens were stained using hematoxylin and eosin (H&E) dyes and were examined under light microscopy (Olympus CX41) for detection of any degeneration, necrosis, sinusoidal dilatation, biliary hyperplasia, liver cell vacuolation or cellular infiltration. Liver pathological features were scored as previously described [16], where 3 fields from each rat were analysed and considered normal (score 0) if the pathological findings were seen in less than 25% of the total fields, and considered mildly (+), moderately (++) or severely (+++) affected if they showed pathological changes of 25 to less than 50%, 50% to less than 75%, or 75% or more, respectively.
2.6. Caspase 3 Immunohistochemical Staining of Liver Sections
Liver tissue sections were pre-treated with Triology reagents for de-paraffinization, re-hydration, and antigen revealing. The ready-to-use rabbit polyclonal antibody against caspase 3 was applied to the slides as it is in accordance with the manufacturer’s specification. Slides were incubated with the antibody for 18 h at 4 °C, and then, after washing, incubated for 30 min with the secondary antibody (1:2000 biotinylated goat anti-rabbit). Additional 30 min incubation was performed in vectastain ABC reagent, followed by 6 min incubation in diaminobenzidine to give brownish discoloration of immunopositive sites. Mayer hematoxylin was used as a counter-stain before microscopic examination. For semi-quantitative analysis of the immunohistochemical results, ImageJ 1.41 (freeware; rsbweb.nih.gov/ij) was used to calculate total number of cells in a field by counting hematoxylin-positive and immunopositive cells. Data were presented as the mean of counting three fields from each rat and were expressed as percentage of immunopositive cells in comparison with the total number of cells.
2.7. Determination of TNF-α, IL-6, and P-gp in Liver Tissue Homogenate
The pro-inflammatory cytokines TNF-α and IL-6 levels, as well as the level of the efflux transporter P-gp were determined in rat liver homogenate according to the manufacturer’s instructions of their respective commercial ELISA kits. The color changes were detected using an ELISA 96-wells plate reader spectrophotometrically at a wavelength 450 ± 10 nm. Concentrations of TNF-α, IL-6 or P-gp in the liver homogenate samples were calculated by comparing their optic densities to the standard curve.
2.8. Statistical Analysis
Results were represented in terms of means ± S.E.M. and their statistical analysis was performed using GraphPad Prisms 5.00 for Windows (San Diego, CA, USA), by one-way analysis of variance (ANOVA), followed by Tukey-Kramer post analysis. The results were considered of significance if the p value was less than 0.05.
3. Results
3.1. Effect of MOR on Liver Index and Hepatic Function in CP-Induced Toxicity
At the end of the study, total body weight of each rat was correlated with its liver weight, and the results showed that the liver index was not affected in MOR-treated group, while treatment with CP significantly increased the liver index by nearly 30% compared to control (Table 1). Combined MOR/CP-treated group, however, showed significantly higher liver index by about another extra 10% compared to CP group. The levels of the enzymes ALT, AST, and ALP, as well as the total bilirubin were significantly increased after treatment with either MOR or CP compared to control, and was even more significantly increased in their combined treatment compared with CP-treated group. On the other hand, the level of albumin was significantly decreased by nearly one third or half of the control, respectively, in both MOR- and CP-treated groups compared to control, and further decreased in their combined treatment group compared to the group treated by CP alone.

Table 1.
Effect of morphine (MOR) on liver index and serum liver function tests in cisplatin (CP)-induced toxicity in rats.
3.2. Effect of MOR on Hepatic Oxidant/Antioxidant Markers in CP-Induced Toxicity
The levels of the lipid product of peroxidation MDA and total nitrate/nitrite as equivalent of NO were evaluated. The results showed that treatment with either MOR or CP alone caused a significant increase in both MDA and NO compared to control (Table 2). In addition, their combined treatment caused significantly higher MDA and NO compared to the group treated with CP alone. On the other hand, a significant decrease in GSH, catalase and SOD levels was observed after treatment with either MOR or CP compared to control, while treatment with both drugs combined caused significantly more reduced levels of GSH, catalase and SOD compared to their levels seen in the group treated with CP alone.

Table 2.
Effect of morphine (MOR) on liver oxidative stress markers in cisplatin (CP)-induced toxicity in rats.
3.3. Effect of MOR on Liver Microscopic Features and Caspase 3 Regulation in CP-Induced Toxicity
Microscopically, the liver in the control showed normal hepatic architecture (Figure 1A). On the other hand, the liver microscopic picture of MOR-treated group had large areas of enlarged vacuolated hepatocytes (Figure 1B), while that of the CP-treated group mostly showed dilated liver sinusoids with sporadic necrotic areas (Figure 1C). The histopathologic picture of the liver in the combined MOR/CP-treated group showed even more profound injuries than either treatment alone (Figure 1D). The results were semi-quantitatively scored to confirm these findings (Table 3). For caspase 3, the control group showed minimal background expression of the apoptotic marker (Figure 2A). On the other hand, either MOR or CP treatments alone caused up-regulation of caspase 3 expression by 9- or 17-folds higher levels (Figure 2B,C), respectively, compared to control. The combined treatment of MOR/CP caused synergistic up-regulation of about 34-folds of the control (Figure 2D) which was significantly different compared to CP alone, as evident by the semi-quantitative analysis performed (Figure 2E).
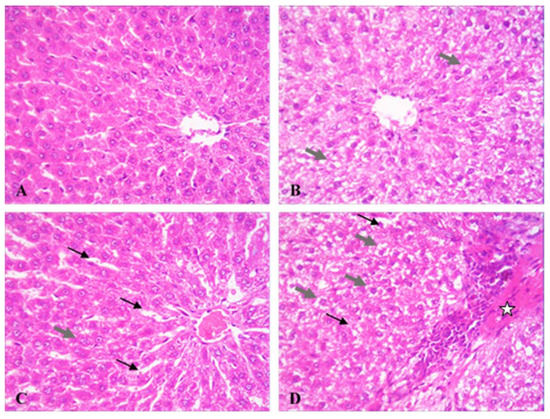
Figure 1.
Effect of morphine (MOR) on liver microscopic picture in cisplatin (CP)-challenged rats. Rat liver photomicrographs (400×) showing microscopic specimens from control (A), MOR-treated group (B), CP-treated group MOR (C), and MOR/CP-treated group (D). Grey thick arrow, vacuolated hepatocyte; black thin arrow, dilated liver sinusoids; star, necrotic hepatic tissue. Semi-quantitative scoring of liver damage is presented in Table 3.

Table 3.
Effect of morphine (MOR) on semi-quantitative scoring of liver microscopic injuries in cisplatin (CP)-induced toxicity in rats.
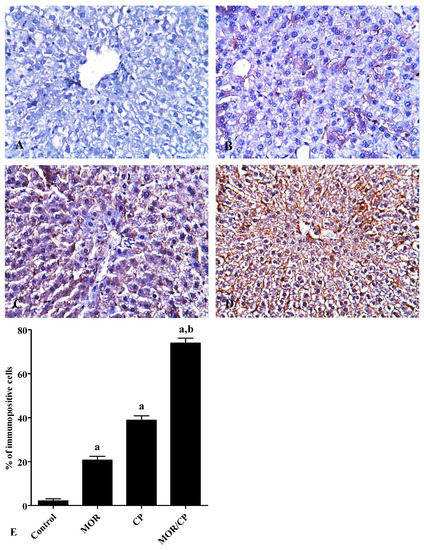
Figure 2.
Effect of morphine (MOR) on liver caspase 3 expression in cisplatin (CP)-challenged rats. Caspase 3 was visualized in rat liver using immunohistochemical staining (400×) of control (A) MOR-treated group (B), CP-treated group (C), and MOR/CP-treated group (D). The results were analyzed semi-quantitatively (E). Data are presented as means ± S.E.M. of % of immune-positive cells per field in each rat (10 rats/group, 3 fields/rat). Significance was reported when p was less than 0.05. a Significantly different compared to control, b significantly different compared to CP group.
3.4. Effect of MOR on Liver TNF-α, IL-6 and P-gp Levels in CP-Induced Toxicity
To investigate the effect of MOR on hepatic inflammation, the level of the pro-inflammatory cytokines TNF-α and IL-6 were tested in liver homogenate (Figure 3A,B, respectively), and it was found that the treatment with either MOR or CP caused significant increase in both markers compared to control. The effect of the combined treatment was even more pronounced and caused significant increase of both cytokines compared to the treatment with CP alone. Similarly, the level of the efflux transporter P-gp in the liver showed a significant increase after treatment with either MOR or CP to reach about 46% or 67% higher levels compared to control (Figure 4). The combined effect of MOR and CP caused almost additive increase in liver P-gp of about 110% compared to control, which was significant from the group treated with CP alone.
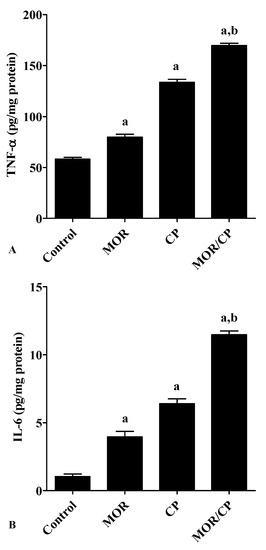
Figure 3.
Effect of morphine (MOR) on liver tissue (A) TNF-α and (B) IL-6 in cisplatin (CP)-challenged rats. Data from 10 rats (n = 3) presented as means ± S.E.M. Results were reported having significant difference if p value was less than 0.05. a Significantly different compared to control, b significantly different compared to CP group.
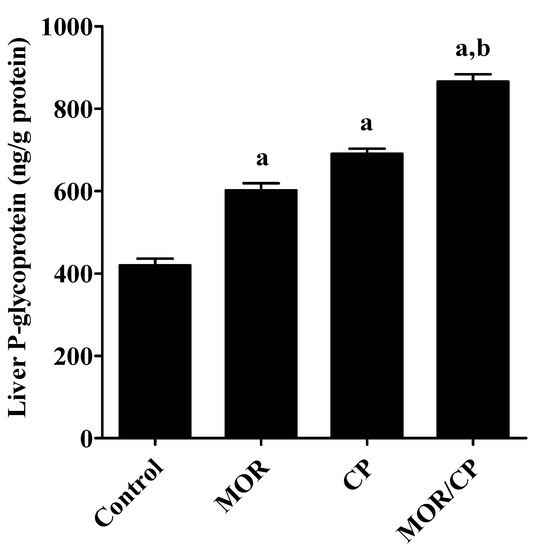
Figure 4.
Effect of morphine (MOR) on liver tissue P-glycoprotein in cisplatin (CP)-challenged rats. Data from each rat (10 rats/group, 3 fields/rat) presented as means ± S.E.M. Results were reported having significant difference if p value was less than 0.05. a Significantly different compared to control, b significantly different compared to CP group.
4. Discussion
Opioids, as MOR, are still considered the mainstay for treatment of severe pain, in particular that associated with cancer treatment and induced by chemotherapeutics as CP. However, the effect of MOR use in conjunction with CP on the liver has not yet been clarified. The current study showed that CP caused deterioration in the function and the structure of the liver, as well as induced hepatic oxidative stress, which was expected from previous studies [3,6]. Similar findings have been shown with MOR alone, which was also in line with previous results [17,18,19]. The combined effect of both drugs in the current study caused more or less additive effect on all liver function parameters, as well as the oxidative stress markers, with the exception of MDA and NO, where the effect seemed synergistic. This synergistic effect can be calculated for MDA, where MOR or CP alone caused an increase by 2.5 or 3.8 μmol/g protein from control, respectively (sum of only 6.3), while their combined effect increased MDA by 8.7 μmol/g protein. Similarly, in NO level, MOR or CP alone caused an increase of 11 or 17 μmol/g protein from control, respectively (sum of only 28), while their combined effect increased NO by 50 μmol/g protein. Interestingly, the effect of MOR/CP combination was previously examined on the kidney and showed that MOR alone had no effect on kidney function or structure, whereas, only when combined with CP, MOR deteriorated both parameters compared to the group treated with CP alone [20].
In the current study, CP caused an increase in liver pro-inflammatory cytokines; TNF-α and IL-6, which was comparable to previous studies [3,4]. Similarly, liver inflammatory cytokines were increased by MOR alone. In literature, the effect of MOR on inflammatory markers like TNF-α is still controversial. While some studies reported that MOR might reduce lipopolysaccharide-induced TNF-α from mast cells [21], others reported that MOR might exacerbate liver inflammation and increase TNF-α during HIV1 infection [22]. Here, the present study showed for the first time that MOR/CP combination enhanced of hepatic inflammation by inducing TNF-α/IL-6 pathway. The present study also showed that CP induced apoptosis by up-regulating caspase 3, which was expected from previous studies [3,6,23]. MOR alone also up-regulated caspase 3 in the liver. Previous studies showed contradicting results concerning the effect of MOR on caspase 3. While some studies reported that MOR had anti-apoptotic effect on rat neonatal cardiomyocytes [24], other studies stated that MOR promoted apoptosis in kidney and liver [25]. The current study showed for the first time that the combined administration of MOR and CP increase apoptosis by significantly elevating liver caspase 3.
The efflux transporter P-gp, in the present study, was increased in liver after CP administration, which is in line with previous studies [26]. Whether CP is a substrate of P-gp or not is still not clear. Several indirect studies suggested CP as a substrate of P-gp. One example was a study showing that the resistance of human osteosarcoma cells to CP-induced cytotoxicity was associated with over-expression of P-gp [27]. Other studies reported that down-regulation of P-gp caused increased sensitivity of human lung adenocarcinoma to CP [28,29]. To the contrary, several studies reported that CP is not a substrate of P-gp [30,31]. Interestingly, these latter studies suggested that up-regulation of P-gp caused resistance against CP, not by effluxing the anticancer drug out of the tumor cells, but rather via decreasing its apoptotic effect through down-regulating caspase 3. Indeed, P-gp, through its antiapoptotic mechanisms, was reported to promote cellular resistance against compounds that were not P-gp substrates [32]. Alternatively, some studies suggested the other way around, that CP could induce up-regulation of P-gp through caspase 3 [33]. Whether P-gp up-regulation is the cause or the effect of hepatic apoptosis, in the current study, CP administration was accompanied by an increase in both P-gp and caspase 3 in liver.
In the current study, MOR increases hepatic level of P-gp. MOR is a known substrate of P-gp [11]. However, its effect on P-gp expression is still controversial. Several studies suggested that MOR up-regulated P-gp in the small intestine [34,35]. Other study suggested that MOR reduced liver P-gp expression [36], which contradicted the current results. Interestingly, it was suggested that the mechanism by which MOR up-regulated P-gp might involve induction of inflammatory pathways [11]. Here, the results showed that MOR increase P-gp as well as the inflammatory cytokines TNF-α and IL-6. The combined effect of MOR and CP was even further increase in hepatic P-gp level in the current study. Previous studies investigated the effect of MOR and/or CP on P-gp expression in the blood-brain barrier [37,38,39], and reported that both CP and MOR might up-regulate P-gp in this sanctuary barrier, causing efflux of the latter and decrease in its central penetration, and consequently, decrease in its analgesic efficacy. Up-regulation of P-gp by CP was not reported to be limited to normal body cells, but extended to include tumor cells [33], where P-gp was related to MDR of cancer cells against chemotherapeutic [40], especially when using P-gp substrates as anticancer drugs concomitantly with CP. Further studies should be performed to test whether addition of MOR might further up-regulate P-gp in cancer cells and jeopardize the therapeutic efficacy of chemotherapy.
In general, up-regulation of hepatic P-gp was reported to be linked to the toxic effects of several compounds, including diethylnitrosamine, zinc pyrithione and uremic toxin indoxyl sulfate [41,42,43]. The effect of the latter compound was tested on 14 different transporter genes, and only the gene encoding P-gp; ABCB1, was up-regulated, indicating its crucial role in hepatotoxicity. The up-regulation of P-gp in these studies was linked to the accumulation and toxicity of the substances causing this genetic induction. It is, thus, possible that, in the current study, the up-regulation of hepatic P-gp is one of the mechanisms involved in MOR/CP hepatotoxicity. It is logic, since P-gp acts as an efflux transporter in the canalicular membrane of the liver, participating in translocating xenobiotics into the bile, where they might undergo entero-hepatic circulation. MOR itself is known to be subjected to entero-hepatic circulation, whatever its route of administration was, and its concentration in bile might reach 100-folds higher than that in blood [44]. The increase of P-gp in the liver seen in the current study might contribute to increasing this level to even higher concentrations in bile, so that, instead of being metabolized and excreted in urine, MOR would preferentially be effluxed in bile, and reabsorbed back for further hepatic processing, subjecting the liver to even more toxicity.
In conclusion, this study discovered that MOR/CP combination can be hazardous on the liver, mainly via oxidant, inflammatory and apoptotic mechanisms, directing the attention of researchers to the necessity of performing clinical studies to explore the impact of such indispensable drug combination during actual chemotherapy. In addition, this study showed that both MOR and CP could increase hepatic P-gp level, which might be a novel mechanism for mediating their hepatotoxic effects, directing the attention to the role of P-gp in hepatotoxicity and its possible linkage to oxidative, inflammatory and apoptotic processes in the liver.
Author Contributions
Conceptualization; Methodology; Analysis; Writing; Project Administration; Funding Acquisition, A.A.K.E.-S. The author has read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Scientific Research at Princess Nourah bint Abdulrahman University (Grant No# 240/S/39).
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CP | Cisplatin |
| ELISA | enzyme-linked immunosorbent assay |
| GSH | reduced glutathione |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| MOR | Morphine |
| NO | nitric oxide |
| P-gp | P-glycoprotein |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor-α |
References
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.B.; Won, D.H.; Shin, Y.S.; Kim, S.Y.; Kang, B.C.; Lim, K.M.; Che, J.H.; Nam, K.T.; Yun, J.W. Ccrn4l as a pre-dose marker for prediction of cisplatin-induced hepatotoxicity susceptibility. Free Radic. Biol. Med. 2020, 148, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hagar, H.; Husain, S.; Fadda, L.M.; Attia, N.M.; Attia, M.M.A.; Ali, H.M. Inhibition of NF-kappaB and the oxidative stress-dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats. Pharmacol. Rep. 2019, 71, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Yay, A.; Onses, M.S.; Sahmetlioglu, E.; Ceyhan, A.; Pekdemir, S.; Onder, G.O.; Sezer, G.; Sarica, Z.S.; Aydin, F. Raman spectroscopy: A novel experimental approach to evaluating cisplatin induced tissue damage. Talanta 2020, 207, 120343. [Google Scholar] [CrossRef]
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-kappaB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018, 9, 3743–3754. [Google Scholar] [CrossRef]
- Pınar, N.; Çakırca, G.; Hakverdi, S.; Kaplan, M. Protective effect of alpha lipoic acid on cisplatin induced hepatotoxicity in rats. Biotech. Histochem. 2019, 1–6. [Google Scholar] [CrossRef]
- Calls, A.; Carozzi, V.; Navarro, X.; Monza, L.; Bruna, J. Pathogenesis of platinum-induced peripheral neurotoxicity: Insights from preclinical studies. Exp. Neurol. 2019, 325, 113141. [Google Scholar] [CrossRef]
- Donertas, B.; Unel, C.C.; Erol, K. Cannabinoids and agmatine as potential therapeutic alternatives for cisplatin-induced peripheral neuropathy. J. Exp. Pharmacol. 2018, 10, 19–28. [Google Scholar] [CrossRef]
- Li, W.; Sparidans, R.; El-Lari, M.; Wang, Y.; Lebre, M.C.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (ABCB1/MDR1) limits brain accumulation and Cytochrome P450-3A (CYP3A) restricts oral availability of the novel FGFR4 inhibitor fisogatinib (BLU-554). Int. J. Pharm. 2020, 573, 118842. [Google Scholar] [CrossRef]
- Hernández, L.I.; Bauer, M.; Wulkersdorfer, B.; Traxl, A.; Philippe, C.; Weber, M.; Häusler, S.; Stieger, B.; Jäger, W.; Mairinger, S.; et al. Measurement of Hepatic ABCB1 and ABCG2 Transport Activity with [(11)C]Tariquidar and PET in Humans and Mice. Mol. Pharm. 2020, 17, 316–326. [Google Scholar] [CrossRef]
- Chaves, C.; Remiao, F.; Cisternino, S.; Decleves, X. Opioids and the Blood-Brain Barrier: A Dynamic Interaction with Consequences on Drug Disposition in Brain. Curr. Neuropharmacol. 2017, 15, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Hole, L.D.; Larsen, T.H.; Fossan, K.O.; Limé, F.; Schjøtt, J. Morphine enhances doxorubicin-induced cardiotoxicity in the rat. Cardiovasc. Toxicol. 2014, 14, 251–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afsar, T.; Razak, S.; Almajwal, A.; Shabbir, M.; Khan, M.R. Evaluating the protective potency of Acacia hydaspica R. Parker on histological and biochemical changes induced by Cisplatin in the cardiac tissue of rats. BMC Complement. Altern. Med. 2019, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Rifaai, R.A. Peroxisome Proliferator Activator Receptor (PPAR)-gamma Ligand, but Not PPAR-alpha, Ameliorates Cyclophosphamide-Induced Oxidative Stress and Inflammation in Rat Liver. PPAR Res. 2014, 2014, 626319. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, R.; Farkhondeh, T. Effect of long-term treatment of morphine on enzymes, oxidative stress indices and antioxidant status in male rat liver. Int. J. Clin. Exp. Med. 2014, 7, 1449–1453. [Google Scholar]
- Shahid, M.; Subhan, F.; Ullah, I.; Ali, G.; Alam, J.; Shah, R. Beneficial effects of Bacopa monnieri extract on opioid induced toxicity. Heliyon 2016, 2, e00068. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Khaleghian, A. Influence of Silybum Marianum on Morphine Addicted Rats, Biochemical Parameters and Molecular Simulation Studies on micro-Opioid Receptor. Drug Res. 2019, 69, 630–638. [Google Scholar]
- Aminian, A.; Javadi, S.; Rahimian, R.; Dehpour, A.R.; Asadi, A.F.; Moghaddas, P.; Ejtemaei, M.S. Enhancement of Cisplatin Nephrotoxicity by Morphine and Its Attenuation by the Opioid Antagonist Naltrexone. Acta Med. Iran. 2016, 54, 422–429. [Google Scholar]
- Madera-Salcedo, I.K.; Cruz, S.L.; Gonzalez-Espinosa, C. Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IkappaB kinase activation and SNAP-23 phosphorylation: Correlation with the formation of a beta-arrestin/TRAF6 complex. J. Immunol. 2013, 191, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, N.; Dever, S.M.; Fitting, S.; Ahmed, T.; Hauser, K.F. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: Increased pathogenesis coincides with uncoordinated host defenses. J. Virol. 2011, 85, 11601–11614. [Google Scholar] [CrossRef] [PubMed]
- Bishr, A.; Sallam, N.; Nour El-Din, M.; Awad, A.S.; Kenawy, S.A. Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/p-ERK. Can. J. Physiol. Pharmacol. 2019, 97, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Barrère-Lemaire, S.; Combes, N.; Sportouch-Dukhan, C.; Richard, S.; Nargeot, J.; Piot, C. Morphine mimics the antiapoptotic effect of preconditioning via an Ins(1,4,5)P3 signaling pathway in rat ventricular myocytes. Am. J. Physiol Heart Circ. Physiol. 2005, 288, H83–H88. [Google Scholar] [CrossRef]
- Luo, F.C.; Zhao, L.; Deng, J.; Liang, M.; Zeng, X.S.; Liu, H.; Bai, J. Geranylgeranylacetone protects against morphine-induced hepatic and renal damage in mice. Mol. Med. Rep. 2013, 7, 694–700. [Google Scholar] [CrossRef]
- Demeule, M.; Brossard, M.; Béliveau, R. Cisplatin induces renal expression of P-glycoprotein and canalicular multispecific organic anion transporter. Am. J. Physiol. 1999, 277, F832–F840. [Google Scholar] [CrossRef]
- He, C.; Sun, Z.; Hoffman, R.; Yang, Z.; Jiang, Y.; Wang, L.; Hao, Y. P-Glycoprotein Overexpression Is Associated With Cisplatin Resistance in Human Osteosarcoma. Anticancer Res. 2019, 39, 1711–1718. [Google Scholar] [CrossRef]
- Cheng, Q.; Liao, M.; Hu, H.; Li, H.; Wu, L. Asiatic Acid (AA) Sensitizes Multidrug-Resistant Human Lung Adenocarcinoma A549/DDP Cells to Cisplatin (DDP) via Downregulation of P-Glycoprotein (MDR1) and Its Targets. Cell Physiol. Biochem. 2018, 47, 279–292. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, L.; Fan, S.; Yang, Z.; Gao, F.; Chen, L.; Xiao, G.G.; Molnar, J.; Wang, Q. Down-regulation of P-glycoprotein is associated with resistance to cisplatin and VP-16 in human lung cancer cell lines. Anticancer Res. 2010, 30, 3593–3598. [Google Scholar]
- Gibalova, L.; Seres, M.; Rusnak, A.; Ditte, P.; Labudova, M.; Uhrik, B.; Pastorek, J.; Sedlak, J.; Breier, A.; Sulova, Z. P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. Toxicol. In Vitro 2012, 26, 435–444. [Google Scholar] [CrossRef]
- Breier, A.; Gibalova, L.; Seres, M.; Barancik, M.; Sulova, Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med. Chem. 2013, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Hano, M.; Tomášová, L.; Šereš, M.; Pavlíková, L.; Breier, A.; Sulová, Z. Interplay between P-Glycoprotein Expression and Resistance to Endoplasmic Reticulum Stressors. Molecules 2018, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shan, Z.; Li, C.; Yang, L. MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed. Pharmacother. 2017, 86, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, W.; Kaneta, M.; Nagae, M.; Yuzuhara, A.; Li, X.; Suzuki, H.; Hanagata, M.; Kitaoka, S.; Suto, W.; Kusunoki, Y.; et al. Mice with neuropathic pain exhibit morphine tolerance due to a decrease in the morphine concentration in the brain. Eur. J. Pharm. Sci. 2016, 92, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, M.; Ochiai, W.; Nagae, M.; Suto, W.; Hanagata, M.; Suzuki, H.; Kitaoka, S.; Hatogai, J.; Ikarashi, N.; Sugiyama, K. Mechanism for Increased Expression of UGT2B in the Liver of Mice with Neuropathic Pain. Biol. Pharm. Bull. 2016, 39, 1809–1814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimura, Y.; Shibata, M.; Tamada, M.; Ozaki, N.; Arai, K. Pharmacokinetics of Morphine in Rats with Adjuvant-induced Arthritis. In Vivo 2017, 31, 811–817. [Google Scholar]
- Balayssac, D.; Cayre, A.; Ling, B.; Maublant, J.; Penault-Llorca, F.; Eschalier, A.; Coudoré, F.; Authier, N. Increase in morphine antinociceptive activity by a P-glycoprotein inhibitor in cisplatin-induced neuropathy. Neurosci. Lett. 2009, 465, 108–112. [Google Scholar] [CrossRef]
- Schaefer, C.P.; Arkwright, N.B.; Jacobs, L.M.; Jarvis, C.K.; Hunn, K.C.; Largent-Milnes, T.M.; Tome, M.E.; Davis, T.P. Chronic morphine exposure potentiates p-glycoprotein trafficking from nuclear reservoirs in cortical rat brain microvessels. PLoS ONE 2018, 13, e0192340. [Google Scholar] [CrossRef]
- Leibrand, C.R.; Paris, J.J.; Jones, A.M.; Masuda, Q.N.; Halquist, M.S.; Kim, W.K.; Knapp, P.E.; Kashuba, A.D.M.; Hauser, K.F.; McRae, M. HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. J. Neurovirol. 2019, 25, 560–577. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar]
- Machado, S.P.; Cunha, V.; Reis-Henriques, M.A.; Ferreira, M. Histopathological lesions, P-glycoprotein and PCNA expression in zebrafish (Danio rerio) liver after a single exposure to diethylnitrosamine. Environ. Toxicol. Pharmacol. 2014, 38, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Fu, G.H.; Liu, T.F.; Hu, K.; Li, H.R.; Fang, W.H.; Yang, X.L. Toxicity and accumulation of zinc pyrithione in the liver and kidneys of Carassius auratus gibelio: Association with P-glycoprotein expression. Fish. Physiol Biochem. 2017, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.T.; Poitevin, S.; Paul, P.; McKay, N.; Jourde-Chiche, N.; Legris, T.; Mouly-Bandini, A.; Dignat-George, F.; Brunet, P.; Masereeuw, R.; et al. Indoxyl Sulfate Upregulates Liver P-Glycoprotein Expression and Activity through Aryl Hydrocarbon Receptor Signaling. J. Am. Soc. Nephrol. 2018, 29, 906–918. [Google Scholar]
- Duflou, J.; Darke, S.; Easson, J. Morphine concentrations in stomach contents of intravenous opioid overdose deaths. J. Forensic Sci. 2009, 54, 1181–1184. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).