1. Introduction
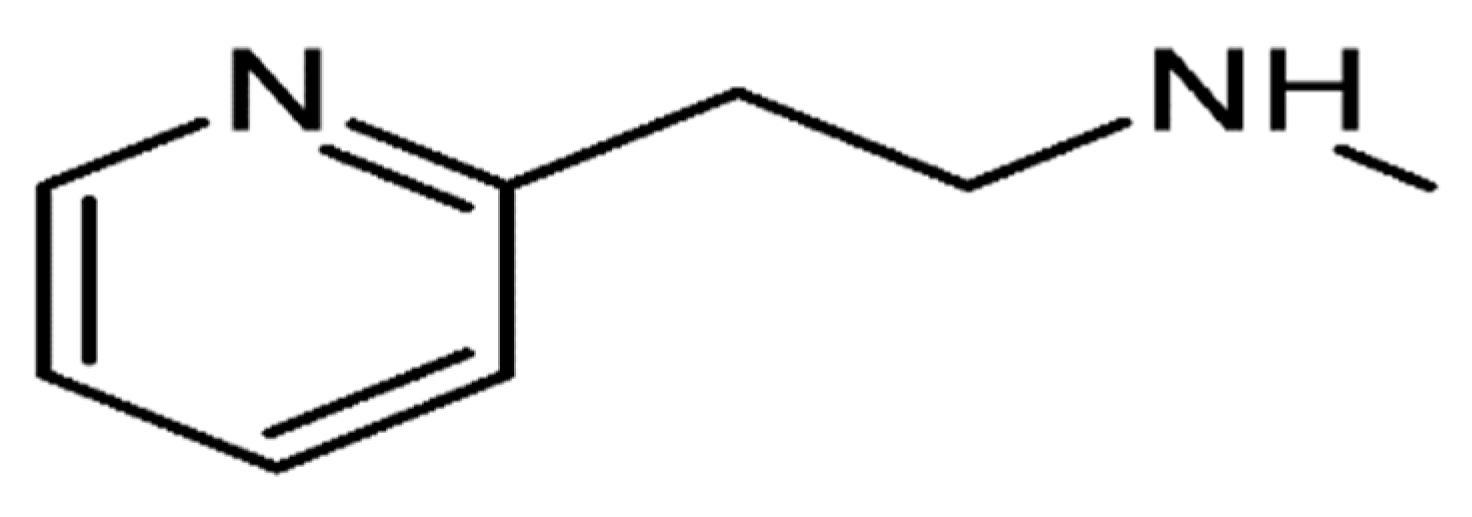
Betahistine is a synthetic, orally active analogue of histamine. Betahistine dihydrochloride is 2-(2-methylamino-ethyl) pyridine dihydrochloride, and the molecular formula is C
8H
12N
2.2HCl. The structure of betahistine is shown in
Figure 1.
Betahistine dihydrochloride is indicated for treatment of Ménière’s syndrome, which is associated with the following symptoms: hardness of hearing or even hearing loss, feeling of dizziness with nausea and vomiting even when standing still (vestibular vertigo), feeling of sound in the ear in the absence of corresponding external sound such as ringing (tinnitus). Betahistine has been found to have a partial histamine H1-receptor agonistic and histamine H3-receptor antagonistic activities in neuronal tissues; however, betahistine has a negligible H2-receptor property. Betahistine dihydrochloride oral tablets are available in 8, 16, and 24 mg doses. The recommended dosage for adults is 24–48 mg administered across several doses during the day. The drug is generally well tolerated [
1]. Recently, betahistine has been studied for treating subjects with attention deficit hyperactivity disorder (ADHD) in once-daily doses of 50 mg, 100 mg, or 200 mg [
2].
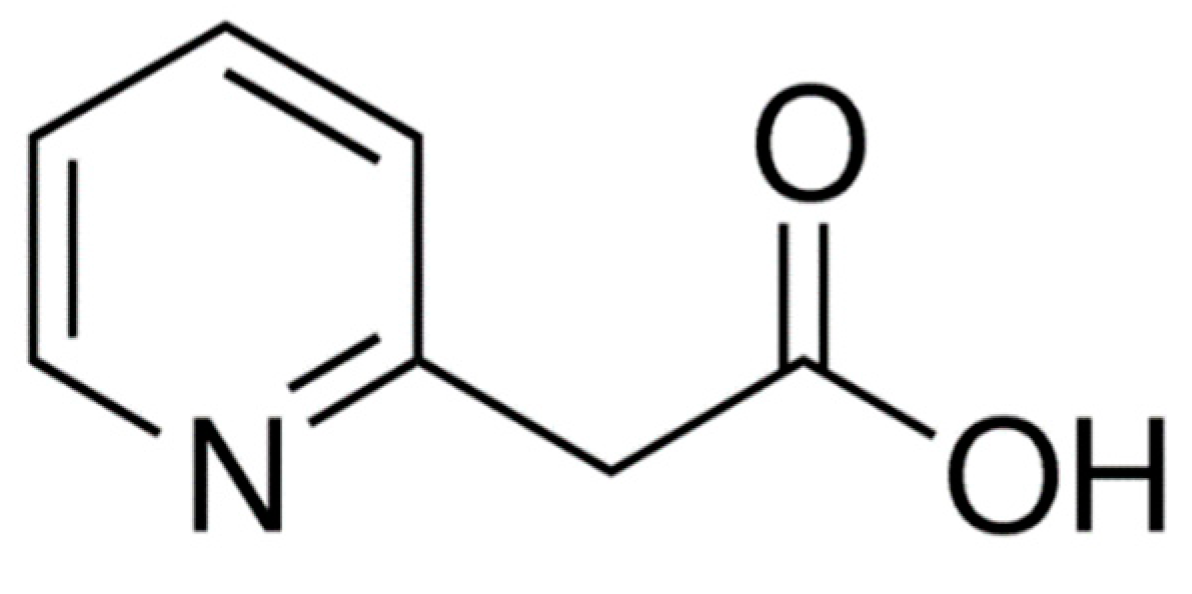
Orally administered betahistine dihydrochloride demonstrates rapid and almost complete absorption from all parts of the gastrointestinal tract. Food intake has no significant impact on the extent of drug absorption (area under plasma concentration–time; AUC), however, the rate of absorption (C
max) is higher under fasting compared to a fed state. The drug is metabolized rapidly and almost completely in the liver to the biologically inactive metabolite 2-pyridylacetic acid (2-PAA). The structure of 2-PAA is shown in
Figure 2. Betahistine shows linear pharmacokinetics over the oral therapeutic dose range of 8–48 mg, indicating that the metabolic pathway is not saturated. Less than 5% of betahistine is bound to plasma proteins. 2-PAA is rapidly excreted in the urine [
1].
Plasma levels of betahistine are extremely low (below 100 pg/mL plasma). Therefore, the pharmacokinetics of betahistine is based on the measurement of 2-PAA in plasma or urine, rather than of the parent drug [
1,
2,
3,
4,
5,
6]. Plasma level (C
max) of 2-PAA reaches its maximum (T
max) about 1 h after betahistine intake [
1,
3,
4,
5,
6]. The terminal elimination half-life of 2-PAA is approximately 3 h [
1,
3,
5,
6]. However, a longer terminal elimination half-life of about 5 h (range 2–11 h) has been reported in Chinese individuals [
4]. Interestingly, total drug exposure (area under plasma concentration–time; AUC) and C
max of 2-PAA have demonstrated great variations among different national populations [
3,
4,
5,
6].
There is limited information concerning the assessment of pharmacokinetics and dose proportionality of betahistine administered in the therapeutic dose range [
1]. Moreover, to date, there are no published data concerning the pharmacokinetics of the drug in an Arabic population. Therefore, the present investigation was conducted to study the pharmacokinetics and dose proportionality of betahistine given in therapeutic ranges of 8–24 mg to healthy Arabic subjects in order to obtain basic pharmacokinetic information about betahistine which can be utilized for further future investigation to find out the proper optimal therapeutic dose of the drug for Arabic patients suffering from Ménière’s disease. The current study was based on the measurement of 2-PPA as the key surrogate for evaluating the pharmacokinetics of the parent drug betahistine.
2. Subjects and Methods
2.1. Study Protocol
A study protocol including an informed consent form was prepared by the principal investigator and approved by the clinical investigator and the institutional review board (IRB) following ICH guidelines for good clinical practice (GCP) and the Declaration of Helsinki [
7,
8]. Each subject willing to participate in the study provided written informed consent prior to the screening procedures. The participants were thoroughly informed about the details and purpose of the study. Consent procedures were carried out by the clinical staff under the supervision of the clinical investigator. Each subject personally signed the consent form with two witnesses and the clinical investigator. Each participant was given an original signed copy of the consent form.
2.2. Subjects
Thirty-eight Arabic healthy adult male subjects with age ranging between 18 and 40 years and body mass index between 18 and 28 were selected to participate in this study. The subjects were considered healthy if they were in good physical and clinical condition, and had normal clinical laboratory tests. The clinical examinations included vital signs (blood pressure, pulse, and temperature) and electrocardiogram (ECG). The clinical laboratory tests included biochemistry, hematology, and routine urine analysis. Additionally, the subjects had to have negative results for illicit drugs, hepatitis (B & C) antigens, and human immunodeficiency virus (HIV). The subjects were considered not illegible for participation in the study if they were heavy smokers (more than 10 cigarettes per day), had a history of hypersensitivity and/or contraindications to betahistine or any related compounds, or had history of any chronic illness including gastrointestinal, hepatic, renal, cardiovascular, pulmonary, hematological, endocrinal, immunological, dermatological, neurological, or psychiatric diseases.
Each subject was given a random identification number upon admission to the clinical site during Period 1 of the study. The subjects retained the same number throughout the entire study. Subjects were free to leave the study at any time they wished without undue delay. Additionally, no subject was allowed to continue the study if there was a potential risk to his health or if there was any violation of the study protocol.
2.3. Study Conduct
The subjects were admitted to the clinical site about 14 h (18:00) before dose administration and they were confined until 24 h after dosing (end of each study period). The subjects were served a standard dinner at about 8 p.m. On the morning of next day (at about 7:00), an indwelling cannula was placed in the forearm antecubital vein of each subject for blood sampling. The cannula was flushed directly with 0.5 mL of heparinized normal saline (20 units/mL) to prevent blood clotting, and the flushing was repeated after each blood sample withdrawal. Moreover, two drops of blood were discarded from the cannula before sampling to ensure the absence of residual drug in the cannula from the previous blood sample.
After overnight fasting of 12 h (at 8:00), the investigational drug was given with 240 mL of tap water. Betahistine 8, 16, and 24 mg (Serc®, Abbott) was administered as a single dose in a randomized, open-label, fasting, three-period, three-sequence, cross-over design separated by a one week washout interval between dosing.
A 5 mL blood sample was collected from each subject before drug administration (zero time) and then at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 h post-dosing. A total of 17 blood samples were obtained from each subject. The actual blood sampling time was recorded. The blood samples were immediately placed in heparinized tubes and centrifuged at 4000× g for 5 min to separate the plasma. Each plasma sample was transferred directly using a polypropylene Pasteur pipette to an Eppendorf tubes. The separated plasma samples were stored in a deep freezer at −30 ± 5 °C until the day of drug assay. The subjects were not allowed to have any meals and fluids other than water, which was permitted as desired after 2 h of drug intake. A standardized lunch was served at 4 h after dosing and a standardized dinner at 12 h after dosing. The meals were identical in all three periods of the study. Xanthine-containing beverages were prohibited from the time of drug administration until 24 h post-dosing, which was the time of last blood sample withdrawal and subjects’ discharge. Moreover, the subjects remained ambulatory or seated upright and were not allowed to sleep or lie down for the first 4 h post-dosing.
2.4. Safety Assessment
Vital signs including blood pressure, pulse, and temperature were recorded for each subject at 1 h before dosing, and then at 2, 4, 6, 12, and eventually at 24 h post-dosing (time of discharge). The clinical investigator and staff were available throughout the entire study period (24 h) to observe and monitor any adverse events (AE), adverse drug reactions (ADR), and serious adverse effects (SAE) if they occurred.
2.5. Assay of 2-Pyridyl Acetic Acid in Plasma
A highly sensitive, rapid, specific, accurate, and precise LC-MS/MS analytical method was used to determine betahistine inactive metabolite 2-pyridyl acetic acid (2-PAA) concentrations in plasma [
4]. Best linearity of the analytical method was obtained for concentration ranges from 1.0 to 800.00 ng, with a correlation coefficient (r) equal to 0.9972 and a lower limit of quantification (LLOQ) of 1.0 ng/mL 2-PAA in plasma. Precision and accuracy were considered within acceptable ranges for intra-day and inter-day according to the method validation criteria, applying FDA bioanalytical method-validation guidance [
9,
10]. Each analytical run used to determine 2-PAA concentrations in plasma included standard calibration curve, quality control (QC) sample (low, mid and high), and the unknown authentic samples. No determination was done by extrapolation below the LLOQ or above the upper limit of quantitation (ULOQ) of the standard calibration curve. Plasma samples with levels of 2-PAA above the ULOQ were diluted to within the linear concentrations range of the standard calibration curve. Moreover, all plasma samples collected for each of the investigated doses, 8, 16, and 24 mg betahistine, were analyzed together after the completion of the three clinical phases of the study (end of Period 3), as recommended by the above guidelines [
9,
10].
2.6. Pharmacokinetic and Statistical Analysis
All pharmacokinetic calculations were carried out using Kinetica software. The statistical analysis and data plotting were performed using Microsoft Excel. Standard methods were used for calculating the pharmacokinetic parameters C
max, AUC
0–t, AUC
0–∞, T
max, T
half, and K
elimination (λ
z) obtained from plasma concentration–time profiles of betahistine’s inactive metabolite (2-PAA), applying non-compartmental analysis [
11,
12]. The maximum or peak concentration of the drug in plasma (C
max) and the time of its occurrence (T
max) were compiled directly from the concentration versus time curve of each subject. The terminal elimination rate constant (K
elimination or λ
z) was estimated by linear regression of at least the three last points at the terminal phase of the log-concentration versus time profile of each subject. Terminal elimination half-life (T
half) was measured as 0.693/λ
z. The area under the plasma concentration–time curve from time zero up to the time of last blood sample withdrawal (t
last) at 24 h post-dosing (AUC
0–t) was calculated using the trapezoidal rule. The area under the plasma concentration–time curve from t
last to infinity (AUC
t–∞) was calculated as C
last/λ
z, where C
last is the last measurable concertation at 24 h post-dosing. The area under the plasma concentration–time curve from time zero to infinity (AUC
0–∞) was estimated from the sum of AUC
0–t and AUC
t–∞. The percent extrapolated area (%AUC
extrapolated), which is also called residual area (AUC
residual) or tail area (AUC
tail), was calculated as (AUC
t–∞/AUC
0–∞) × 100 [
13,
14]. The descriptive statistics including mean, standard deviation (SD), coefficient of variation (CV), median, and correlation coefficient (
r) are presented.
3. Results and Discussion
No deviations or anomalies from the protocol were documented in any of the study phases. The study was designed to end up with a minimum of 34 subjects who should complete the three periods of the study (3 weeks) in order to have the adequate sample size to yield a power of more than 80%. Therefore, 38 subjects were enrolled and screened for participation in the study in order to mitigate any dropout and withdrawal of subjects during the study. Thirty-six subjects completed the entire investigation, due to the withdrawal of two subjects the evening before Period 1 for personal reasons. The drug was well tolerated by all participants and they were discharged at the end of the study (3 weeks) without any significant changes in their clinical baseline characteristics. The demographic data and the clinical baseline (vital signs) of the subjects are summarized in
Table 1.
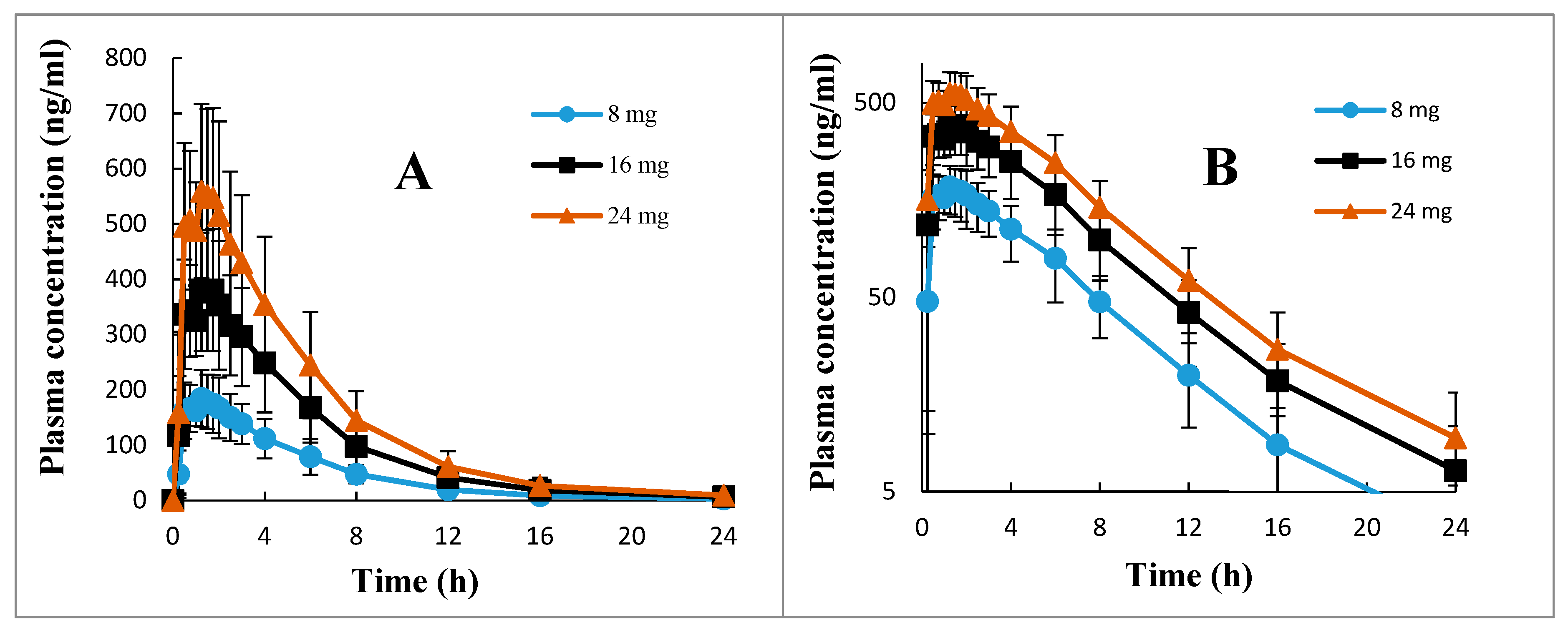
The mean ± SD, the ranges, and the %CV of all pharmacokinetic parameters of 2-PAA calculated after 8, 16, and 24 mg betahistine doses are presented in
Table 2. The mean ± SD plasma concentration versus time profiles of 2-PAA depicted in the normal graph and semi-log graph are shown in
Figure 3. It is apparent from
Table 2 that the drug showed a low inter-individual variation, with %CV of about 25% for the pharmacokinetic parameters C
max, AUC
0–∞, and T
half and for all the investigated doses. However, higher between-subject differences with a %CV of about 40% were observed for T
max values and for all dose ranges of 8–24 mg (
Table 2). The calculated extrapolated AUC had a negligible contribution to the total AUC (AUC
0–∞) for all the investigated doses, with %AUC
extrapolated values of less than 2% (
Table 2). This indicated that blood sampling for 24 h post-dosing of oral betahistine applied in the current research with LLOQ of 1.0 ng.
2-PAA in plasma is quite enough for the reliable estimation of all pharmacokinetic parameters of the drug, particularly the primary parameters, including the total AUC and the terminal elimination half-life (
Table 2).
Statistical evaluation based on ANOVA tests identified no significant differences (
p > 0.05) between the dose-normalized pharmacokinetic parameters, namely C
max, AUC
0–t, and AUC
0–∞, with their corresponding doses of 8, 16, and 24 mg betahistine. These results were supported by ANOVA tests, which revealed no statistically significant differences (
p > 0.05) between the other pharmacokinetic parameters, namely T
max, T
half, and K
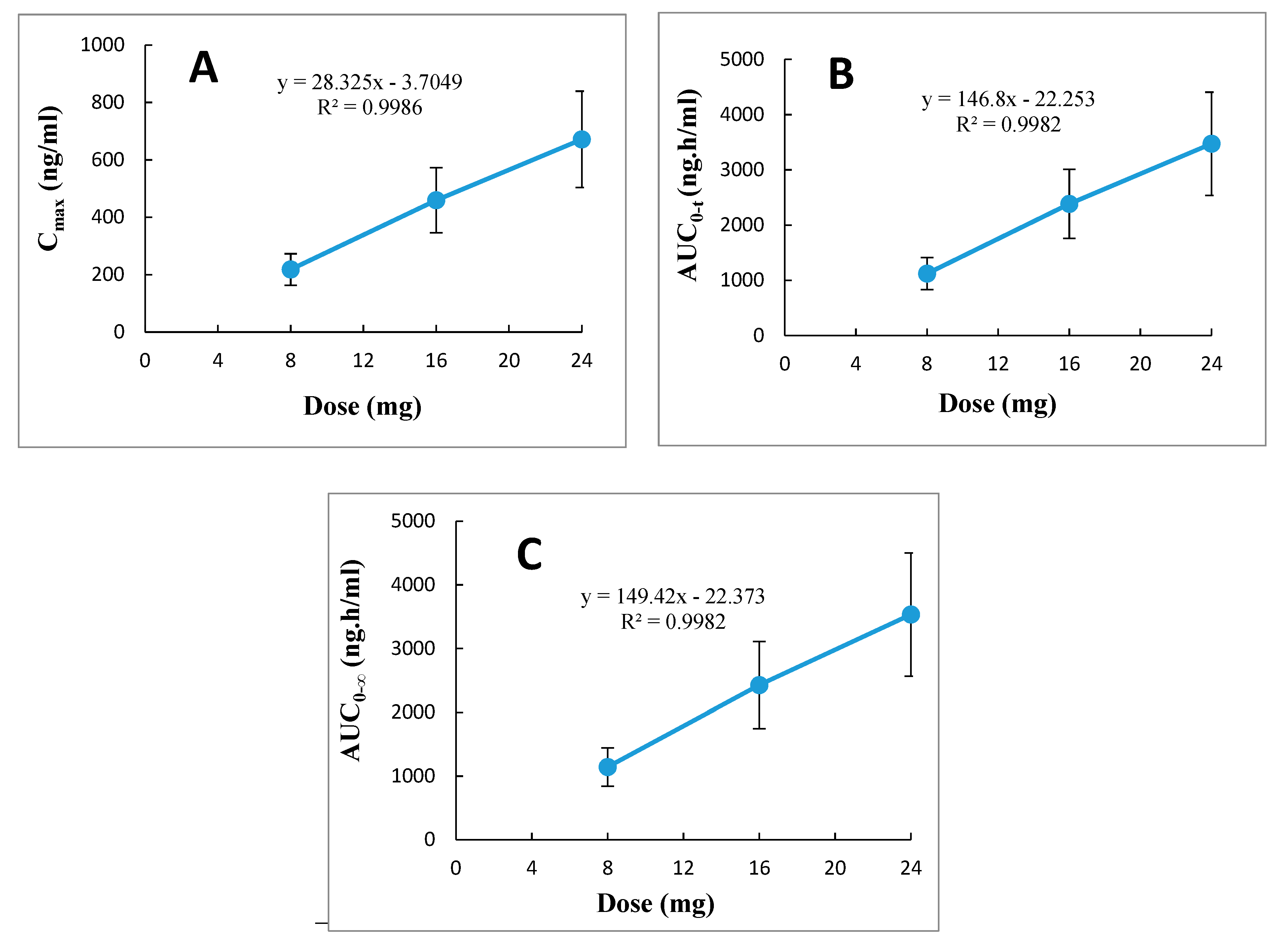
elimination, and their corresponding doses of 8, 16, and 24 mg. Furthermore, evaluation of the relationships between dose ranges of 8 to 24 mg betahistine with the parameters C
max, AUC
0–t, and AUC
0–∞ by linear regression analysis demonstrated a linear positive correlation with correlation coefficient (r
2) approaching unity (range 0.9982–0.9986), as shown in
Figure 4. Thus, it was obvious from these results that betahistine demonstrates linear pharmacokinetics (dose proportionality) within the investigated therapeutic dose ranges of 8 to 24 mg.
The pharmacokinetic parameters of 2-PAA presented in previous investigations conducted in different nations are summarized in
Table 3. After oral administration of 24 mg betahistine tablets, the average C
max value in Thai subjects was 380 ng/mL; for Chinese subjects it was 339 ng/mL and for Mexican subjects it was 1677 ng/mL (
Table 3). In a study conducted in Brazilian subjects, the average C
max value was 527 ng/mL after oral intake of 16 mg betahistine tablets (
Table 3). On the other hand, the average C
max for Arabic subjects in the current investigation was found to be 459 ng/mL and 671 ng/mL after administration of 16 and 24 mg betahistine tablets, respectively (
Table 2). It is obvious from the above-mentioned results that the rate/extent of betahistine absorption (C
max) after oral dosing exhibits high variation among nations. Similar remarkable variability has also been demonstrated for the extent of absorption (AUC) since the average AUC
0–∞ was 1538 ng.h/mL, 1196 ng.h/mL, 6850 ng.h/mL, and 3534 ng.h/mL for Thai, Chinese, Mexican, and Arabic populations, respectively after 24 mg betahistine tablets (
Table 2 and
Table 3). Moreover, the average AUC
0–∞ was 2306 ng.h/mL and 1144 ng.h/mL for Brazilian and Arabic subjects, respectively, after 16 mg betahistine tablets (
Table 2 and
Table 3). Interestingly, the other pharmacokinetic parameters, including T
max, T
half, and K
elimination (λ
z), were almost comparable for different nations except for Chinese individuals, who demonstrated longer terminal elimination half-life, as shown in
Table 2 and
Table 3.
A recent review article focused on the difference in the pharmacokinetics of betahistine based on the usage of the drug as betahistine dihydrochloride or betahistine mesilate after administration in different nations [
15]. This review suggested that the obvious variability in the pharmacokinetics of the drug is related to genetic and environmental factors that can influence the metabolism of betahistine to its principle metabolite 2-pyridyl acetic acid [
15]. Alternatively, another reason for the difference in betahistine pharmacokinetics may be the form of betahistine salt administered (i.e., betahistine dihydrochloride or betahistine mesilate) [
15]. Betahistine is metabolized thoroughly and rapidly to 2-PAA by oxidation via monoamine oxidase, and 2-PAA is the only major metabolite found in humans. Therefore, in pharmacokinetic studies, 2-PAA is the only major metabolite that can be identified, since the plasma concentrations of betahistine are very low or undetectable, suggesting a complete first-pass metabolism [
2]. Consequently, any difference in the pharmacokinetics of betahistine is related to a difference in the hepatic clearance of the drug. Accordingly, the results presented in previous studies and the current investigations (
Table 2 and
Table 3) support the former suggestion (i.e., differences in drug metabolism), since in spite of using the same salt (betahistine dihydrochloride), the variability in drug pharmacokinetics, namely C
max and AUC
0–∞, between nations is obvious (
Table 2 and
Table 3). The usage of betahistine mesilate in Chinese subjects resulted in significant difference in betahistine pharmacokinetics in comparison to other nations; even the terminal elimination half-life was longer for Chinese subjects, as shown in
Table 3 (mean 5, range 2–11 h), relative to other nations, whose populations showed similar terminal elimination half-life values of about 3 h with a range of 2–7 h (
Table 2 and
Table 3).
The considerable differences in the primary pharmacokinetic parameters, which reflect total drug exposure (i.e., Cmax and AUC0–∞), between nations provoke questions concerning the appropriate therapeutic dose of betahistine to be administered to patients with Ménière’s disease in order to obtain optimal effects (i.e., maximum clinical effect and minimum adverse effects). Further clinical pharmacokinetic trials by administering betahistine tablets to patients with Ménaière’s disease are suggested to confirm the results obtained from the current investigation.