Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Curcuminoids Solutions
2.2. Analysis of Curcuminoid Compositions by HPLC
2.3. DPPH Radical Scavenging Assay
2.4. Cell Culture
2.5. Cell Morphological Analysis
2.6. WST-1 Assay for Cell Viability
2.7. Quantitative Analysis of Cell Apoptosis by Flow Cytometry
2.8. Analysis of Cell Cycle by Flow Cytometry
2.9. Wound Healing Assay
2.10. Cytokine ELISA
2.11. Statistical Analysis
3. Results
3.1. HPLC Analysis of Total Curcuminoids
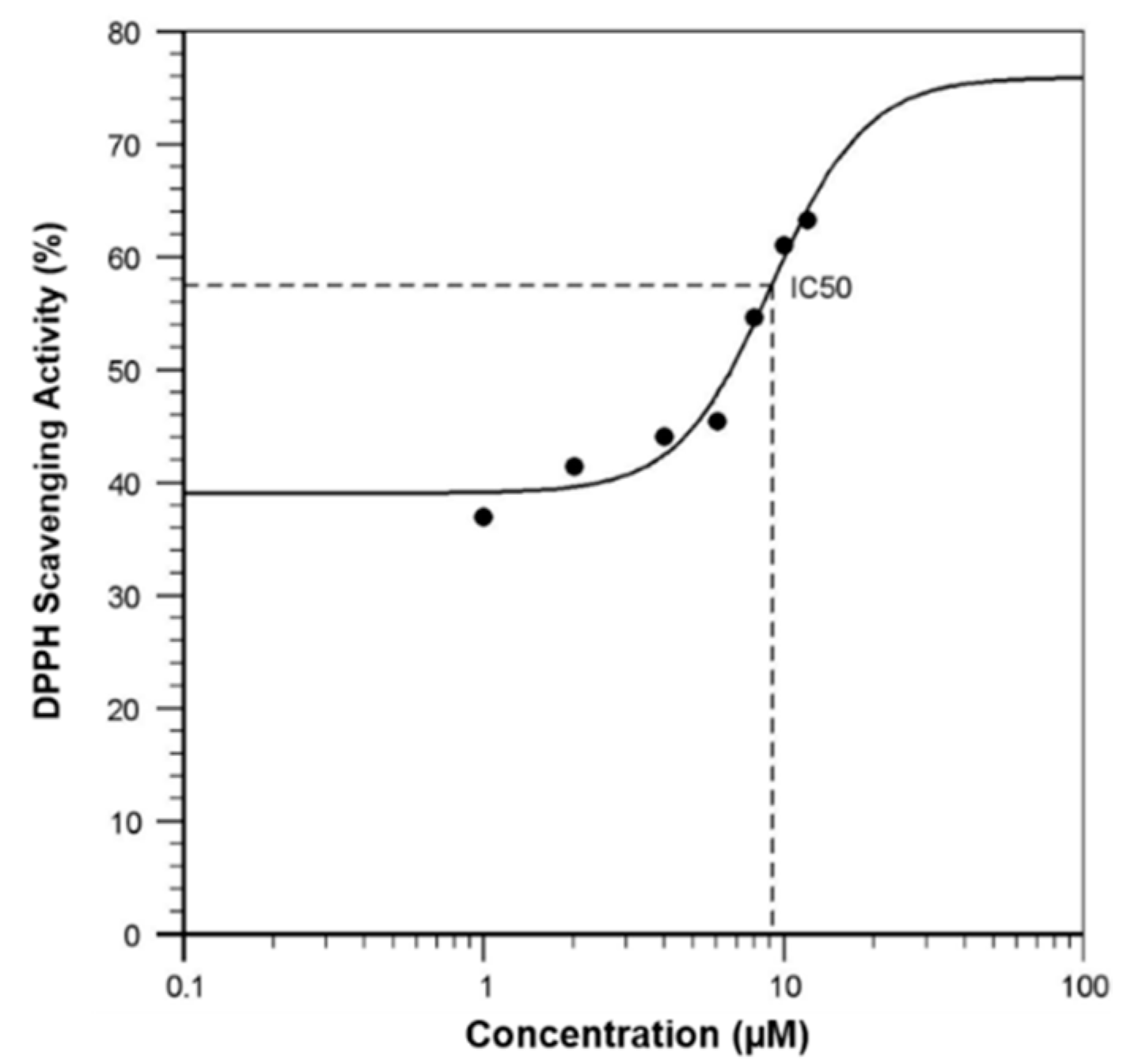
3.2. Free Radical Scavenging Activity of Curcuminoids in a Cell-Free System
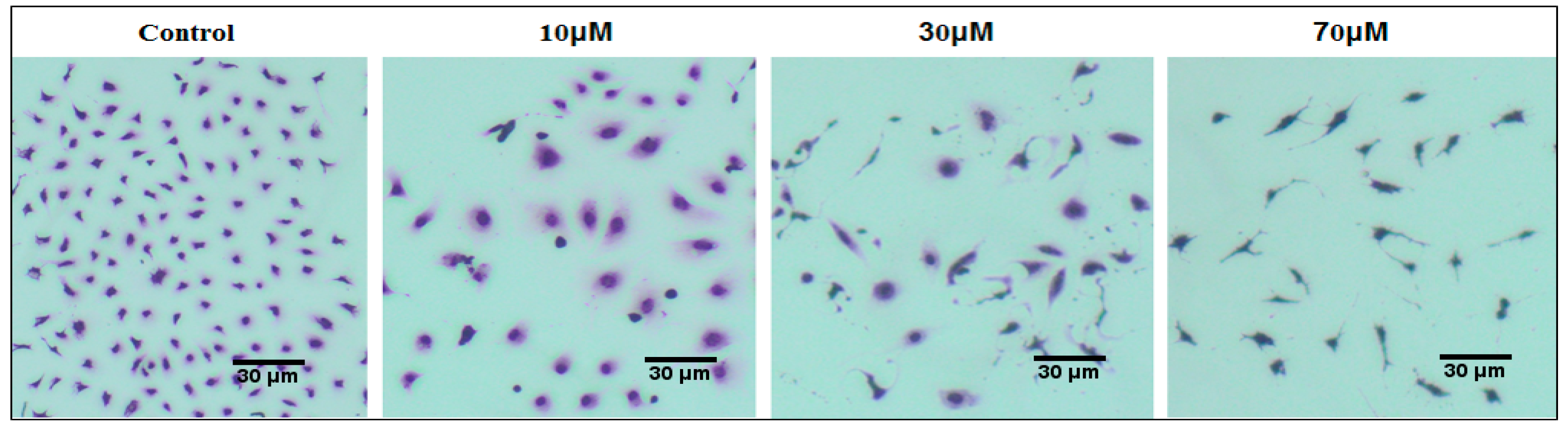
3.3. Effect of Curcuminoids on SKOV-3 Cell Morphology
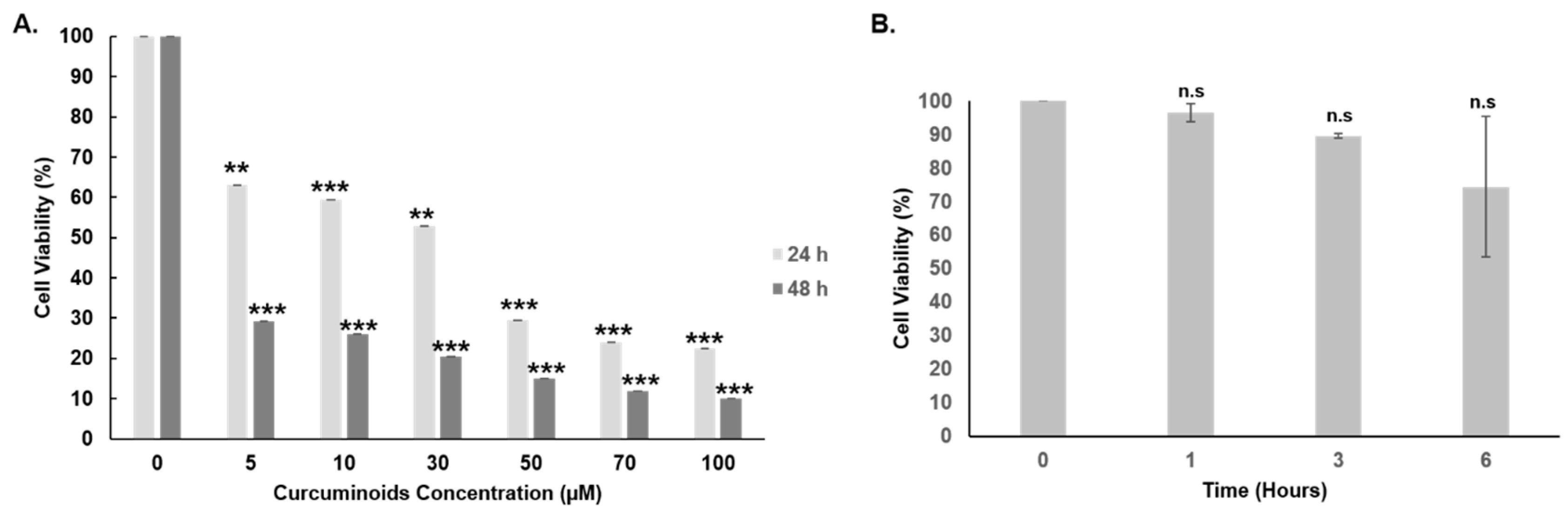
3.4. Cytotoxicity of Curcuminoids against SKOV-3 Cells
3.5. Curcuminoids Induce Apoptosis in SKOV-3 Cells
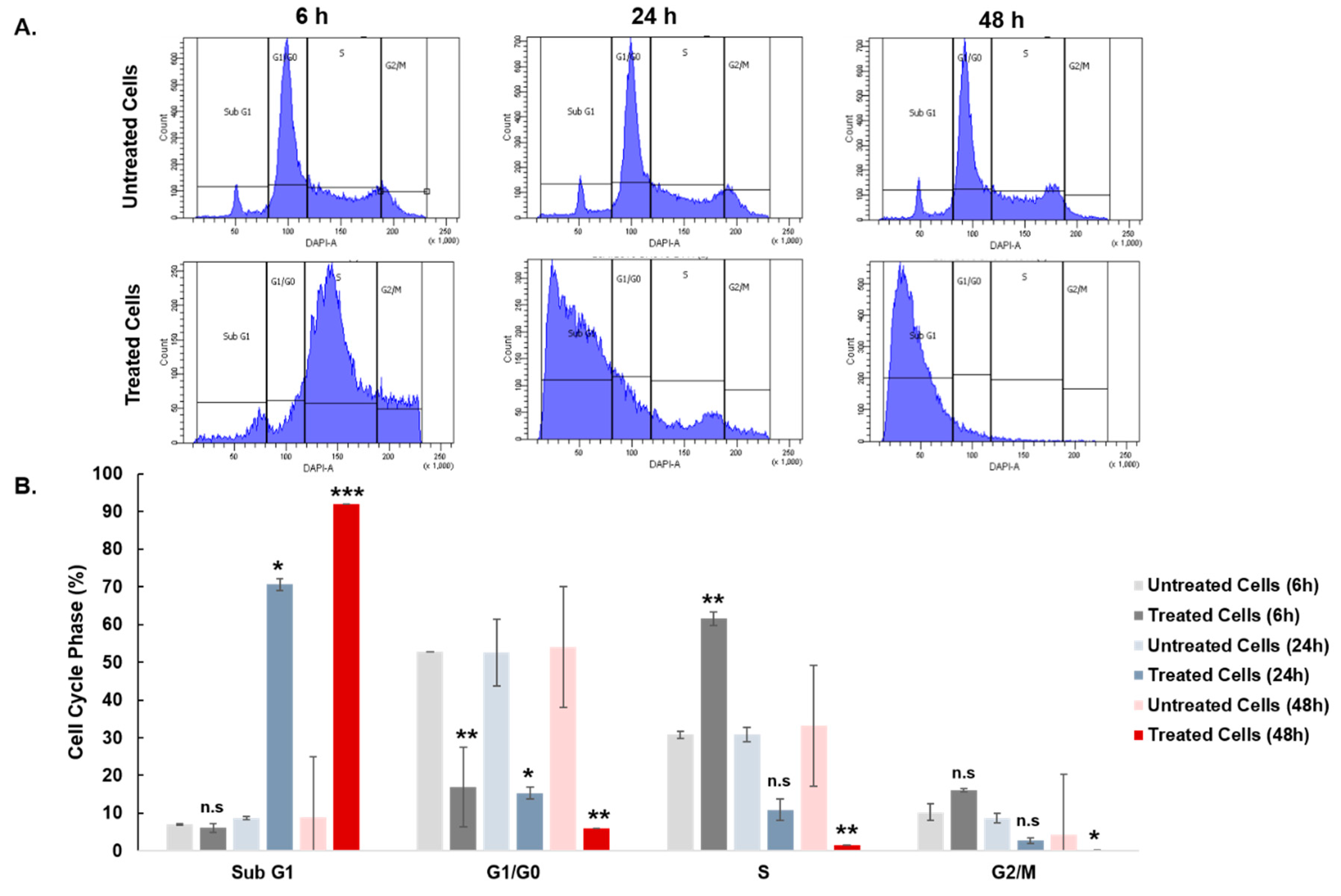
3.6. Effect of Curcuminoids on SKOV-3 Cell Cycle Distribution
3.7. Curcuminoids Impair Wound Healing in SKOV-3 Cells
3.8. Effect of Curcuminoids on TNF-α and IL-10 Secretion in SKOV-3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Traditional. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalap, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Shishodia, S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of curcumin in cancer therapy. Curr. Probl. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef] [PubMed]
- Arulkumar, A.; Ramanchandran, K.; Paramasivam, S.; Palanivel, R.; Rameshthangam, P.; Manuel, J. Effects of turmeric (Curcuma longa) on shelf life extension and biogenic amine control of cuttlefish (Sepia brevimana) during chilled storage. J. Food 2017, 3, 441–444. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Armstrong, D. Relapsed ovarian cancer: Challenges and management strategies for a chronic disease. Oncologist 2002, 7, 20–28. [Google Scholar] [CrossRef]
- Markman, M. Pharmaceutical management of ovarian cancer: Current status. Drugs 2008, 68, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Quest Graph™ IC50 Calculator. AAT Bioquest, Inc. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 7 August 2019).
- Venter, C.; Niesler, C.U. Rapid quantification of cellular proliferation and migration using ImageJ. BioTechniques 2019, 66, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Siegert, A.; Denkert, C.; Köbel, M.; Hauptmann, S. Interleukin-10 in serous ovarian carcinoma cell lines. Cancer Immunol. Immunother. 2001, 50, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data. Antioxidants 2018, 7, 35. [Google Scholar] [CrossRef]
- Singh, U.; Barik, A.; Singh, B.G.; Priyadarsini, K.I. Reactions of reactive oxygen species (ROS) with curcumin analogues: Structure-activity relationship. Free Radic. Res. 2011, 45, 317–325. [Google Scholar] [CrossRef]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen ((1)O(2)) quencher. Biochem. Biophys. Res. Commun. 2002, 1, 62–66. [Google Scholar] [CrossRef]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Abas, F.; Hui, L.S.; Ahmad, S.; Stanslas, J.; Israf, D.A.; Shaari, K.; Lajis, N.H. Biological Evaluation of Curcumin and Related Diarylheptanoids. Z. Naturforsch. 2006, 61, 625–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akter, J.; Hossain, M.A.; Takara, K.; ZahorulIslam, M.; Hou, D.X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp. Biochem. Physiol. Part C Toxicol. Pharm. 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Yang, L.; Wang, Y. Cancerous inhibitor of protein phosphatase 2A regulates cisplatin resistance in ovarian cancer. Oncol. Lett. 2019, 17, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Díaz Osterman, C.J.; Gonda, A.; Stiff, T.; Sigaran, U.; Valenzuela, M.M.A.; Bennit, H.R.F.; Moyron, R.B.; Khan, S.; Wall, N.R. Curcumin Induces Pancreatic Adenocarcinoma Cell Death Via Reduction of the Inhibitors of Apoptosis. Pancreas 2016, 45, 101–109. [Google Scholar] [CrossRef]
- Chen, H.W.; Lee, J.Y.; Huang, J.Y.; Wang, C.C.; Chen, W.J.; Su, S.F.; Huang, C.W.; Ho, C.C.; Chen, J.J.; Tsai, M.F.; et al. Curcumin Inhibits Lung Cancer Cell Invasion and Metastasis through the Tumor Suppressor HLJ1. Cancer Res. 2008, 68, 7428–7438. [Google Scholar] [CrossRef]
- Guan, F.; Ding, Y.; Zhang, Y.; Zhou, Y.; Li, M.; Wang, C. Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS ONE 2016, 11, e0146553. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Jia, Z.; Gao, Y.; Zhao, C.; Yao, Y. Curcumin inhibits bladder cancer progression via regulation of β-catenin expression. Tumor Biol. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Ampasavate, C.; Sung, B.; Aggarwal, B.B.; Limtrakul, P. Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur. J. Pharm. Sci. 2010, 627, 8–15. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, A.; Zhao, Z.; Shen, Y.; Wang, S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol. Rep. 2012, 28, 85–90. [Google Scholar] [PubMed]
- Pei, H.; Yang, Y.; Cui, L.; Yang, J.; Li, X.; Yang, Y.; Duan, H. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci. Rep. 2016, 6, 28773. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Yang, H.P.; Zhou, X.D. Role of Wnt Inhibitory Factor-1 in Inhibition of Bisdemethoxycurcumin Mediated Epithelial-to-Mesenchymal Transition in Highly Metastatic Lung Cancer 95D Cells. Chin. Med J. 2015, 128, 1376. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.L.; Blobe, G.C. Role of transforming growth factor beta in human cancer. J. Clin. Oncol. 2005, 23, 2078–2093. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Ucisik, M.H.; Küpcü, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013, 11, 37. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effect of Curcumin on Breast Cancer Cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef]
- Shi, M.; Cai, Q.; Yao, L.; Mao, Y.; Ming, Y.; Ouyang, G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol. Int. 2006, 30, 221–226. [Google Scholar] [CrossRef]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P.; Durand-Fontanier, S.; Delage, C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Ramezani, M.; Hatamipour, M.; Sahebkar, A. Promising anti-tumor properties of bisdemethoxycurcumin: A naturally occurring curcumin analogue. J. Cell. Physiol. 2018, 233, 880–887. [Google Scholar] [CrossRef]
- Liao, C.L.; Chu, Y.L.; Lin, H.Y. Bisdemethoxycurcumin suppresses migration and invasion of human cervical cancer HeLa cells via inhibition of NF-ĸB, MMP-2 and -9 pathways. Anticancer Res. 2018, 38, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Huang, A.C.; Tang, N.Y.; Liu, H.C.; Liao, C.L.; Ji, B.C.; Chou, Y.C.; Yang, M.D.; Lu, H.F.; Chung, J.G. Bisdemethoxycurcumin-induced S phase arrest through the inhibition of cyclin A and E and induction of apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathways in human lung cancer NCI H460 cells. Environ. Toxicol. 2016, 31, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Mattson, M.P.; Rattan, S. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxic. 2019, 129, 399–404. [Google Scholar] [CrossRef]
- Aldajani, W.A.; Salazar, F.; Sewell, H.F. Expression and regulation of immune-modulatory enzyme Indoleamine 2,3-dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget 2016, 7, 57606–57617. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Langley, R.R.; Fidler, I.J. Interleukin-6, Secreted by Human Ovarian Carcinoma Cells, Is a Potent Proangiogenic Cytokine. Cancer Res. 2005, 65, 10794–10800. [Google Scholar] [CrossRef]
- Nounamo, B.; Liem, J.; Cannon, M.; Liu, J. Myxoma Virus Optimizes Cisplatin for the Treatment of Ovarian Cancer In Vitro and in a Syngeneic Murine Dissemination Model. Mol. Therapy: Oncol. 2017, 6, 90–99. [Google Scholar] [CrossRef]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef]
- Mustea, A.; Braicu, E.I.; Koensgen, D.; Sehouli, J. Monitoring of IL-10 in the serum of patients with advanced ovarian cancer: Results from a prospective pilot-study. Cytokines 2009, 45, 8–11. [Google Scholar] [CrossRef]
- Kulbe, H.; Thompson, R.; Wilson, J.L.; Robinson, S.; Hagemann, T.; Fatah, R.; Gould, D.; Ayhan, A.; Balkwill, F. The Inflammatory Cytokine Tumor Necrosis Factor-α Generates an Autocrine Tumor-Promoting Network in Epithelial Ovarian Cancer Cells. Cancer Res. 2007, 67, 585–592. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Grimshaw, M.J.; Kulbe, H.; Wilson, J.L.; Wilbanks, G.D.; Burke, F.; Balkwill, F.R. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol. Cancer Ther. 2006, 5, 382–390. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Cicero, R.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Damiati, S.; Scheberl, A.; Zayni, S.; Damiati, S.A.; Schuster, B.; Kompella, U.B. Albumin-Bound Nanodiscs as Delivery Vehicle Candidates: Development and Characterization. Biophys. Chem. 2019, 251, 106178. [Google Scholar] [CrossRef]
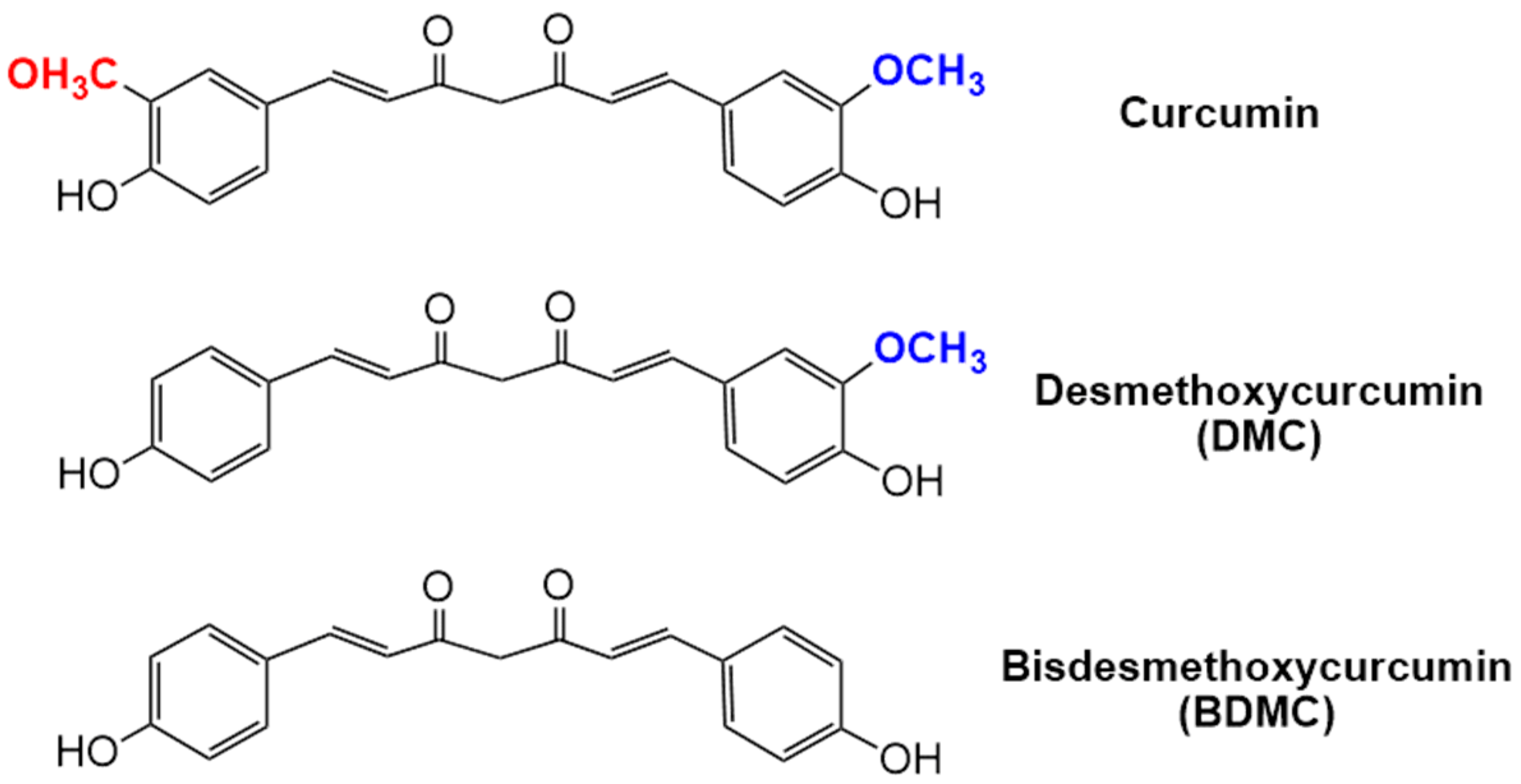
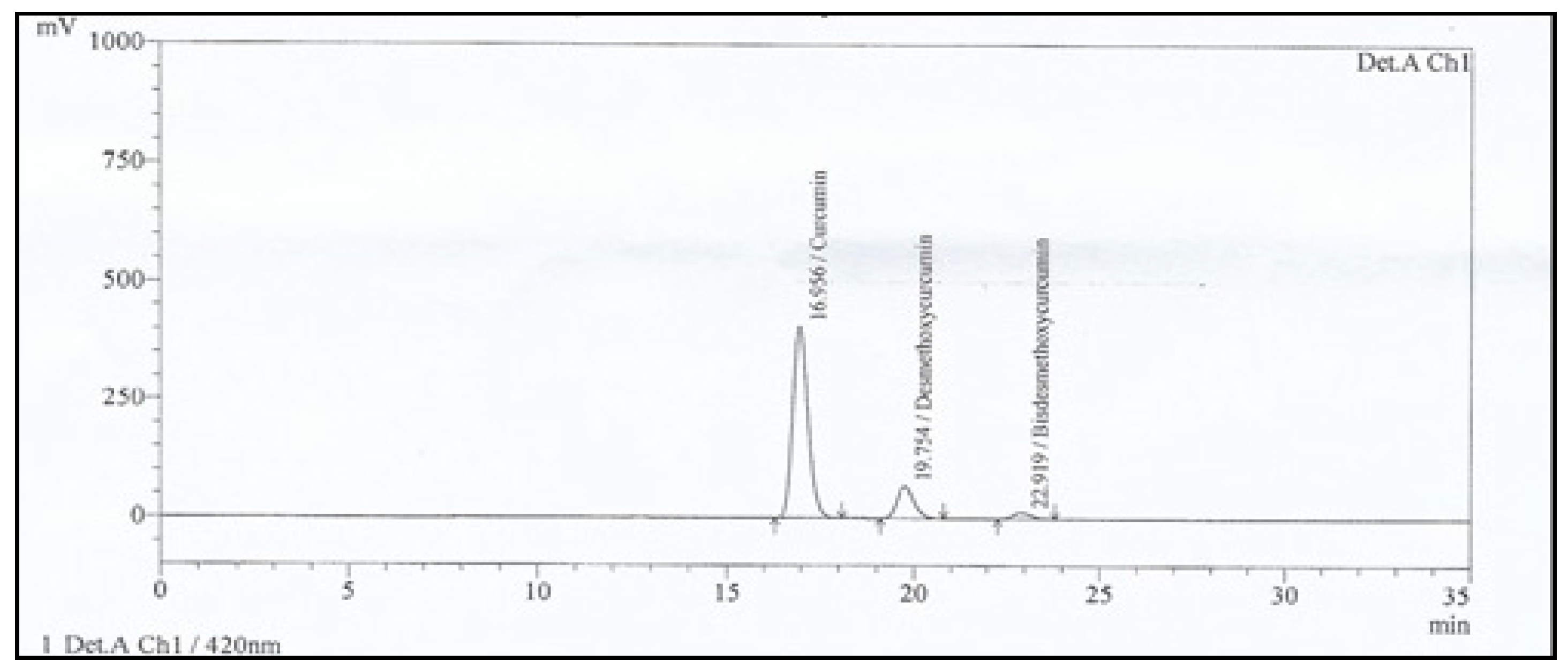


| Parameters | Curcuminoids USP Standard | Curcuminoids Sample | ||||
|---|---|---|---|---|---|---|
| Curcumin | DMC | BDMC | Curcumin | DMC | BDMC | |
| Area (%) | 74.857 | 20.146 | 4.997 | 81.755 | 15.161 | 3.084 |
| Retention Time (min) | 16.68 | 19.41 | 22.48 | 16.96 | 19.75 | 22.92 |
| Tailing Factor | 1.231 | 1.183 | 1.145 | 1.241 | 1.195 | 1.163 |
| Resolution | 0.000 | 3.631 | 3.716 | 0.000 | 3.635 | 3.711 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almosa, H.; Alqriqri, M.; Denetiu, I.; Baghdadi, M.A.; Alkhaled, M.; Alhosin, M.; Aldajani, W.A.; Zamzami, M.; Ucisik, M.H.; Damiati, S. Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3. Sci. Pharm. 2020, 88, 11. https://doi.org/10.3390/scipharm88010011
Almosa H, Alqriqri M, Denetiu I, Baghdadi MA, Alkhaled M, Alhosin M, Aldajani WA, Zamzami M, Ucisik MH, Damiati S. Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3. Scientia Pharmaceutica. 2020; 88(1):11. https://doi.org/10.3390/scipharm88010011
Chicago/Turabian StyleAlmosa, Heba, Mihal Alqriqri, Iuliana Denetiu, Mohammed A. Baghdadi, Mohammed Alkhaled, Mahmoud Alhosin, Wejdan A. Aldajani, Mazin Zamzami, Mehmet H. Ucisik, and Samar Damiati. 2020. "Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3" Scientia Pharmaceutica 88, no. 1: 11. https://doi.org/10.3390/scipharm88010011
APA StyleAlmosa, H., Alqriqri, M., Denetiu, I., Baghdadi, M. A., Alkhaled, M., Alhosin, M., Aldajani, W. A., Zamzami, M., Ucisik, M. H., & Damiati, S. (2020). Cytotoxicity of Standardized Curcuminoids Mixture against Epithelial Ovarian Cancer Cell Line SKOV-3. Scientia Pharmaceutica, 88(1), 11. https://doi.org/10.3390/scipharm88010011





