Abstract
In order to identify new regularities of the “structure–analgesic activity” relationship in the series of 2,1-benzothiazine derivatives, the synthesis of methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate and a group of its analogs substituted in the benzene moiety of the molecule, as well as their mono-and diammonium salts, was performed with tris(hydroxymethyl)aminomethane. The algorithm was proposed; it allows for uniquely solving the question of the nature of the substituent and its true position in the benzothiazine core based on the complex use of NMR (1H and 13C) and mass spectrometry data. Using single-crystal X-ray diffraction analysis it was proven that salt formation first passes through the cyclic sulfamide group and only then through the 4-hydroxyl group, and is always accompanied by a significant conformational rearrangement of the molecule. Based on the results of pharmacological tests it was found that modification of the benzene moiety of the molecule can be used as a method for enhancing the analgesic properties of the class of compounds studied. The presence of a substitute in position 7 is particularly effective, regardless of its nature. A comparative analysis of the analgesic activity of the initial esters and their mono- and diammonium salts convincingly showed that the common belief about a direct relationship between the solubility of a substance and the level of its biological effect is not always true. As it turned out, increasing the solubility in water can lead to a variety of consequences: From a significant increase in analgesia to its complete elimination. It was suggested that the analgesic activity of the compounds studied is determined not by solubility, but by the molecular conformations formed during their obtainment.
1. Introduction
Methyl esters of numerous carboxylic acids are very common in wildlife. The flora of our planet is especially rich in them. The purposes for which plants produce such compounds are very diverse and sometimes not always clear. However, this did not prevent a person from successfully using these gifts of nature for medicinal purposes since ancient times. For example, essential oils containing large amounts of methyl benzoate (I, R = H, Figure 1), methyl anthranilate (I, R = NH2), methyl salicylate (I, R = OH) or their chemically more complex derivatives have proven to be effective external analgesics and anti-inflammatory agents [1,2,3,4]. Another methyl ester—cocaine—has long been known to humans (Figure 1). If this natural substance did not have an extremely undesirable ability to cause drug addiction, it could take a worthy place in the range of modern pain killers. The characteristic property of cocaine, which is quite rare among analgesics, to cause a powerful surface local anesthesia is very popular in such areas of medicine as nasal and lacrimal duct surgery [5].
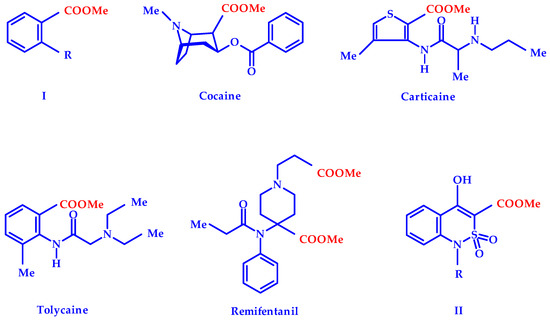
Figure 1.
Natural and synthetic methyl esters with marked analgesic properties [1,2,3,4,5,6,7,8,9,10,11].
Methyl carboxylates are also widely represented in the list of synthetic pharmaceuticals [6,7]. They can be found in different pharmacological groups, including numbing agents: Local anesthetics carticaine and tolycaine, analgesic remifentanil (Figure 1) and others. Conducting a targeted search for new pain control agents in the series of derivatives of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acids, a high analgesic effect in methyl esters of general formula II was previously repeatedly noted [8]. It has been convincingly shown in numerous examples that the biological properties of esters II can be significantly affected by chemical modification of the R substituent at a cyclic nitrogen atom [8,9,10,11]. The bioisosteric replacement of a hydroxyl in position 4 of these compounds on the 4-methyl group was studied in detail [12]. At the same time, an equally interesting and promising fragment of the substances studied—the benzene moiety of the benzothiazine bicycle—remained completely unaffected. We made an attempt to fill this gap in this study.
2. Materials and Methods
2.1. Chemistry
1Н- and 13С-NMR (proton and carbon nuclear magnetic resonance) spectra were obtained on a Varian Mercury-400 (Varian Inc., Palo Alto, CA, USA) instrument (400 and 100 MHz, respectively) in hexadeuterodimethyl sulfoxide (DMSO-d6) with tetramethylsilane as internal standard. The chemical shift values were recorded on a δ scale and the coupling constants (J) in hertz. The following abbreviations were used in reporting spectra: s = singlet, d = doublet, t = triplet. The electron impact mass spectra (EI-MS) were recorded on a Varian 1200 L (Varian Inc., Walnut Creek, CA, USA) mass spectrometer with complete scanning in the m/z range from 35 to 700 and direct sample inlet. The electron impact ionization was at 70 eV. Melting points were determined in a capillary using Electrothermal IA9100X1 (Bibby Scientific Limited, Stone, UK) digital melting point apparatus. The elemental analysis was performed on a Euro Vector EA-3000 (Eurovector SPA, Redavalle, Italy) microanalyzer. In the synthesis of methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4) described in this article, the commercial substituted 2-aminobenzoic acids (1) or their methyl esters (2) of Aldrich company (St. Louis, MO, USA) were used.
2.2. General Procedure for the Synthesis of Methyl Anthranilates (2a–p)
To the solution of the corresponding anthranilic acid (1) (10.0 g) in anhydrous methyl alcohol (30 mL), the concentrated sulfuric acid (10 mL) is carefully added, after that it is refluxed on a sand bath at a temperature of 80 °C for 20 h. The reflux condenser is changed to a distillation one, and the excess of methyl alcohol is removed from the reaction mixture at reduced pressure. First cold water (40 mL) is added to the residue, then Na2CO3 to рН 8. The isolated crystals of ester 2 are filtered, washed with cold water and dried in the air. If ester 2 is released as an oily liquid, then one proceeds as follows: The reaction mixture is treated with CH2Cl2 (3 × 20 mL). Organic extracts are combined, after that the solvent is removed (at reduced pressure at the end). It is not necessary to dry organic extracts since water residues are easily removed in the form of azeotrope with CH2Cl2.
In this way, methyl anthranilates (2) are obtained with yields of 85–92%. They are used in further syntheses without additional purification. As a rule, after the isolation of esters 2, a certain amount of the original anthranilic acids (1) remains in aqueous solutions of Na2CO3. Taking into account their high cost it is advisable to acidify these solutions with HCl to pH ≈ 3, after that filter the precipitates of acids (1), dry and use further as necessary.
2.3. General Procedure for the Synthesis of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates (4a–p)
Methyl (chlorosulfonyl) acetate (1.90 g, 0.011 mol) is added dropwise, with stirring, to the solution of the corresponding methyl anthranilate 2 (0.010 mol) and triethylamine (1.54 mL, 0.011 mol) in CH2Cl2 (20 mL) and cooled (−5 to 0 °C). In 10 h, water (50 mL) is added to the reaction mixture; then it is acidified to pH 4 with 1 N HCl and mixed thoroughly. The organic layer is separated, dried over anhydrous CaCl2, and the solvent is distilled (at reduced pressure at the end). The resulting anilide 3 is subjected to heterocyclization without purification. The solution of sodium methylate in anhydrous methanol (from metallic Sodium (0.69 g, 0.030 mol) and absolute methanol (20 mL)) is added and the mixture is boiled and stored for 15 h at room temperature. The reaction mixture is diluted with cold water and acidified with 1N HCl to pH 4. The solid ester 4 is filtered, separated, washed with water, and dried in the air. It is crystallized from methanol. If there is a need to purify esters 4 with activated charcoal, only their brands that do not contain impurities of iron salts should be used. Otherwise, the final products acquire a stable yellow and even red color, which is further very difficult to get rid of.
Methyl 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4a). The yield was 2.39 g (94%); colorless crystals; melting point (mp) 189–191 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.23 (br. s, 2H, 4-OH + SO2NН), 7.94 (d, 1Н, J = 8.1 Hz, Н-5), 7.67 (t, 1Н, J = 7.6 Hz, Н-7), 7.26 (t, 1Н, J = 7.7 Hz, Н-6), 7.11 (d, 1Н, J = 8.3 Hz, Н-8), 3.90 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 167.3 (4-С-ОН), 166.3 (С=О), 140.9, 135.5, 126.9, 123.7, 119.1, 117.6, 105.4, 53.8 (ОCH3). Mass spectrum (MS) (m/z, %): 255 [M]+ (60.4), 222 [M−СH3OH]+ (100). This was analytically calculated (Anal. Calcd.) for C10H9NO5S: C, 47.06; H, 3.55; N, 5.49; S 12.56%. We found: C, 46.99; H, 3.50; N, 5.53; S 12.61%.
Methyl 6-Fluoro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4b). The yield was 2.46 g (90%); colorless crystals; m.p. 213–215 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.28 (br. s, 2H, 4-OH + SO2NН), 7.66 (dd, 1Н, 3JHF = 9.1 Hz, 4J = 2.6 Hz, Н-5), 7.55 (dd, 1Н, 3JHF = 9.7 Hz, J = 8.7 Hz, Н-7), 7.17 (dd, 1Н, J = 8.9 Hz, 4JHF 4.6 Hz, Н-8), 3.91 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.7 (4-C-OH), 166.1 (С=О), 158.0 (d, JC-F 239.8 Hz, С-6), 135.8, 123.2 (d, 2JC-F 23.6 Hz, С-5), 120.5 (d, 3JC-F 7.8 Hz, С-8), 117.3 (d, 3JC-F 7.9 Hz, С-4a), 112.3 (d, 2JC-F 24.6 Hz, С-7), 106.8 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 273 [MH]+ (62.1), 241 [M−СH3OH]+ (100), 121 (68.8), 95 (12.6). The Anal. Calcd. was for C10H8FNO5S: C, 43.96; H, 2.95; N, 5.13; S 11.73%. We found: C, 44.05; H, 3.03; N, 5.06; S 11.78%.
Methyl 7-Fluoro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4c). The yield was 2.51 g (92%); colorless crystals; m.p. 193–195 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.31 (br. s, 2H, 4-OH + SO2NН), 7.98 (dd, 1Н, J = 7.6 Hz, 4JHF = 6.6 Hz, Н-5), 7.10 (dd, 1Н, 3JHF = 8.7 Hz, J = 8.7 Hz, Н-6), 6.89 (d, 1Н, 3JHF = 9.7 Hz, Н-8), 3.92 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 167.3 (4-C-OH), 164.8 (С=О), 152.4 (d, JC-F 241.5 Hz, С-7), 141.4 (d, 3JC-F 12.9 Hz, С-8a), 130.2 (d, 3JC-F 11.3 Hz, С-5), 112.5, 111.5 (d, 2JC–F 22.9 Hz, С-6), 105.0 (C-3), 104.6 (d, 2JC-F 25.6 Hz, С-8), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 273 [M]+ (37.4), 241 [M−СH3OH]+ (100), 149 (26.4). The Anal. Calcd. was for C10H8FNO5S: C, 43.96; H, 2.95; N, 5.13; S 11.73%. We found: C, 44.02; H, 2.92; N, 5.05; S 11.67%.
Methyl 6,7-Difluoro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4d). The yield was 2.53 g (87%); colorless crystals; m.p. 206–208 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 11.10 (br. s, 2H, 4-OH + SO2NН), 7.91 (dd, 1Н, 3JHF = 9.3 Hz, 4JHF = 9.0 Hz, Н-5), 7.14 (dd, 1Н, 3JHF = 6.9 Hz, 4JHF = 6.8 Hz, Н-8), 3.92 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.6 (4-C-OH), 165.7 (С=О), 153.9 (d, JC-F 253.1 Hz, С-6), 146.3 (d, JC-F 246.8 Hz, С-7), 137.0, 115.4 (d, 2JC-F 19.8 Hz, С-5), 112.7, 107.5 (d, 2JC-F 20.4 Hz, С-8), 106.1 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 291 [M]+ (43.5), 259 [M−СH3OH]+ (100), 155 (39.4), 139 (49.3), 112 (15.6). The Anal. Calcd. was for C10H7F2NO5S: C, 41.24; H, 2.42; N, 4.81; S 11.01%. We found: C, 41.31; H, 2.48; N, 4.73; S 10.93%.
Methyl 5-Chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4e). The yield was 2.43 g (84%); colorless crystals; m.p. 191–193 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.37 (br. s, 2H, 4-OH + SO2NН), 7.58 (t, 1Н, J = 7.9 Hz, Н-7), 7.34 (d, 1Н, J = 7.5 Hz, Н-6), 7.13 (d, 1Н, J = 7.5 Hz, Н-8), 3.93 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 168.7 (4-C-OH), 167.4 (С=О), 141.6, 135.2, 133.5, 127.2, 118.8, 118.3, 107.3 (C-3), 54.1 (OCH3). Mass spectrum (MS) (m/z, %): 289/291 [M]+ (40.7/11.5), 257/259 [M−СH3OH]+ (100/32.0), 153/155 (81.3/33.0), 126 (38.1). The Anal. Calcd. was for C10H8ClNO5S: C, 41.46; H, 2.78; N, 4.83; S 11.07%. We found: C, 41.54; H, 2.83; N, 4.75; S 10.98%.
Methyl 6-Chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4f). The yield was 2.57 g (89%); colorless crystals; m.p. 215–217 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.15 (br. s, 2H, 4-OH + SO2NН), 7.89 (s, Н-5), 7.71 (d, 1Н, J = 8.4 Hz, Н-7), 7.17 (d, 1Н, J = 8.8 Hz, Н-6), 3.93 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.6 (4-C-OH), 165.8 (С=О), 138.1, 135.2, 127.4, 125.9, 120.5, 117.2, 106.7 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 289/291 [M]+ (37.1/11.7), 257/259 [M−СH3OH]+ (100/36.6), 137 (25.3). The Anal. Calcd. was for C10H8ClNO5S: C, 41.46; H, 2.78; N, 4.83; S 11.07%. We found: C, 41.51; H, 2.85; N, 4.87; S 11.00%.
Methyl 7-Chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4g). The yield was 2.69 g (93%); colorless crystals; m.p. 230–232 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.20 (br. s, 2H, 4-OH + SO2NН), 7.95 (d, 1Н, J = 8.7 Hz, Н-5), 7.32 (d, 1Н, J = 9.0 Hz, Н-6), 7.17 (s, 1Н, Н-8), 3.94 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 167.0 (4-C-OH), 166.6 (С=О), 140.3, 139.8, 128.9, 123.7, 117.7, 114.6, 105.9 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 289/291 [M]+ (37.2/10.2), 257/259 [M−СH3OH]+ (100/28.9), 165 (13.1). The Anal. Calcd. was for C10H8ClNO5S: C, 41.46; H, 2.78; N, 4.83; S 11.07%. We found: C, 41.40; H, 2.72; N, 4.77; S 11.13%.
Methyl 8-Chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4h). The yield was 2.54 g (88%); colorless crystals; m.p. 247–249 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 11.35 (br. s, 2H, 4-OH + SO2NН), 7.95 (d, 1Н, J = 7.9 Hz, Н-5), 7.84 (d, 1Н, J = 7.8 Hz, Н-7), 7.36 (t, 1Н, J = 8.0 Hz, Н-6), 3.95 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.7 (4-C-OH), 166.5 (С=О), 135.9, 135.2, 126.0, 125.3, 124.9, 120.4, 107.7 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 289/291 [M]+ (40.0/11.1), 257/259 [M−СH3OH]+ (100/32.6), 137 (18.7). The Anal. Calcd. was for C10H8ClNO5S: C, 41.46; H, 2.78; N, 4.83; S 11.07%. We found: C, 41.54; H, 2.83; N, 4.75; S 10.98%.
Methyl 6-Bromo-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4i). The yield was 3.13 g (94%); colorless crystals; m.p. 240–242 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 11.60 (br. s, 2H, 4-OH + SO2NН), 7.98 (s, 1Н, Н-5), 7.79 (d, 1Н, J = 8.1 Hz, Н-7), 7.08 (d, 1Н, J = 8.7 Hz, Н-8), 3.91 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.6 (4-C-OH), 165.8 (С=О), 138.4, 137.9, 128.8, 120.7, 117.5, 115.0, 106.6 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 333/335 [M]+ (37.4/36.1), 301/303 [M−СH3OH]+ (92.0/100), 197/199 (43.8/41.5). The Anal. Calcd. was for C10H8BrNO5S: C, 35.95; H, 2.41; N, 4.19; S 9.60%. We found: C, 36.04; H, 2.49; N, 4.11; S 9.52%.
Methyl 7-Bromo-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4j). The yield was 3.06 g (92%); colorless crystals; m.p. 248–250 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.04 (br. s, 2H, 4-OH + SO2NН), 7.83 (d, 1Н, J = 8.7 Hz, Н-5), 7.43 (d, 1Н, J = 8.7 Hz, Н-6), 7.29 (s, 1Н, Н-8), 3.91 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.9 (4-C-OH), 166.7 (С=О), 140.3, 128.8, 128.7, 126.5, 120.6, 114.9, 106.1 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 333/335 [M]+ (44.0/46.6), 301/303 [M−СH3OH]+ (93.3/100), 197/199 (25.2/20.4). The Anal. Calcd. was for C10H8BrNO5S: C, 35.95; H, 2.41; N, 4.19; S 9.60%. We found: C, 36.01; H, 2.46; N, 4.24; S 9.54%.
Methyl 6,8-Dibromo-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4k). The yield was 2.32 g (81%); colorless crystals; m.p. 157–159 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.00 (br. s, 2H, 4-OH + SO2NН), 7.87 (s, 1Н, Н-5), 7.85 (s, Н-7), 3.85 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.9 (4-C-OH), 166.2 (С=О), 147.6, 139.5, 132.1, 130.0, 112.4, 111.3, 105.6 (C-3), 53.0 (OCH3). Mass spectrum (MS) (m/z, %): 411/413/415 [M]+ (4.1/7.1/3.3), 379/381/383 [M−СH3OH]+ (14.1/27.2/16.4), 307/309/311 (41.4/88.7/43.6), 275/277/279 (50.1/100/53.8). The Anal. Calcd. was for C10H7Br2NO5S: C, 29.08; H, 1.71; N, 3.39; S 7.76%. We found: C, 29.15; H, 1.80; N, 3.32; S 7.68%.
Methyl 4-Hydroxy-6-iodo-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4l). The yield was 3.39 g (89%); colorless crystals; m.p. 208–210 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.27 (br. s, 2H, 4-OH + SO2NН), 8.15 (s, 1Н, Н-5), 7.92 (d, 1Н, J = 7.4 Hz, Н-7), 6.93 (d, 1Н, J = 8.6 Hz, Н-8), 3.90 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 166.7 (4-C-OH), 165.9 (С=О), 150.8, 143.4, 139.6, 138.8, 134.6, 120.6, 106.4 (C-3), 53.9 (OCH3). Mass spectrum (MS) (m/z, %): 381 [M]+ (2.5), 349 [M−СH3OH]+ (100). The Anal. Calcd. was for C10H8INO5S: C, 31.51; H, 2.12; N, 3.67; S 8.41%. We found: C, 31.44; H, 2.06; N, 3.60; S 8.35%.
Methyl 4-Hydroxy-6-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4m). The yield was 2.50 g (93%); colorless crystals; m.p. 200–202 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.30 (br. s, 2H, 4-OH + SO2NН), 7.72 (s, 1Н, Н-5), 7.47 (d, 1Н, J = 7.6 Hz, Н-7), 7.06 (d, 1Н, J = 8.0 Hz, Н-8), 3.94 (s, 3Н, OCH3), 2.34 (s, 3Н, 6-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 168.1 (4-C-OH), 167.6 (С=О), 137.2, 136.5, 132.7, 126.3, 118.6, 115.7, 105.7 (C-3), 53.8 (OCH3), 20.9 (6-CH3). Mass spectrum (MS) (m/z, %): 269 [M]+ (36.6), 237 [M−СH3OH]+ (100), 133 (28.1). The Anal. Calcd. was for C11H11NO5S: C, 49.07; H, 4.12; N, 5.20; S 11.91%. We found: C, 49.15; H, 4.20; N, 5.14; S 11.83%.
Methyl 4-Hydroxy-6-methoxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4n). The yield was 2.59 g (91%); colorless crystals; m.p. 207–209 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.03 (br. s, 1H, NН), 7.36 (s, 1Н, Н-5), 7.31 (d, 1Н, J = 8.8 Hz, Н-7), 7.12 (d, 1Н, J = 8.8 Hz, Н-8), 3.94 (s, 3Н, OCH3), 3.81 (s, 3Н, 6-OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 167.6 (4-C-OH), 167.5 (С=О), 155.5, 133.1, 123.8, 120.7, 117.1, 108.7, 106.1 (C-3), 56.3 (6-OCH3), 53.8 (OCH3). Mass spectrum (MS) (m/z, %): 285 [M]+ (46.4), 253 [M−СH3OH]+ (76.0), 162 (32.6), 134 (100), 106 (37.5). The Anal. Calcd. was for C11H11NO6S: C, 46.31; H, 3.89; N, 4.91; S 11.24%. We found: C, 46.24; H, 3.95; N, 4.98; S 11.15%.
Methyl 4-Hydroxy-7-methoxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4o). The yield was 2.56 g (90%); colorless crystals; m.p. 215–217 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.44 (br. s, 1H, NН), 7.86 (d, 1Н, J = 9.1 Hz, Н-5), 6.86 (d, 1Н, J = 8.9 Hz, Н-6), 6.60 (s, 1Н, Н-8), 3.93 (s, 3Н, OCH3), 3.85 (s, 3Н, 7-OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 168.4 (4-C-OH), 168.0 (С=О), 165.1, 141.7, 128.9, 111.6, 108.6, 103.1, 101.6 (C-3), 56.5 (7-OCH3), 53.7 (OCH3). Mass spectrum (MS) (m/z, %): 285 [M]+ (34.5), 253 [M−СH3OH]+ (100), 162 (10.8). The Anal. Calcd. was for C11H11NO6S: C, 46.31; H, 3.89; N, 4.91; S 11.24%. We found: C, 46.25; H, 3.87; N, 4.86; S 11.17%.
Methyl 6,7-Dimethoxy-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4p). The yield was 2.93 g (93%); colorless crystals; m.p. 266–268 °C (methanol); 1H-NMR (400 MHz, DMSO-d6): δ 12.28 (br. s, 1H, NН), 7.27 (s, 1Н, Н-5), 6.63 (s, 1Н, Н-8), 3.90 (s, 3Н, OCH3), 3.85 (s, 3Н, OCH3), 3.79 (s, 3Н, OCH3). 13C-NMR (100 MHz, DMSO-d6): δ 168.5 (4-C-OH), 168.2 (С=О), 155.8, 145.8, 135.7, 107.7, 107.2, 103.1, 101.3 (C-3), 56.9 (OCH3), 56.7 (OCH3), 53.7 (OCH3). Mass spectrum (MS) (m/z, %): 315 [M]+ (51.1), 283 [M−СH3OH]+ (100), 164 (35.8). The Anal. Calcd. was for C12H13NO7S: C, 45.71; H, 4.16; N, 4.44; S 10.17%. We found: C, 45.63; H, 4.08; N, 4.50; S 10.11%.
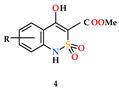
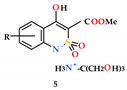
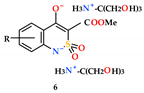
2.4. General Procedure for the Synthesis of Mono-and Disubstituted Salts of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates and Tris(hydroxymethyl)aminomethane (5–6)
In a 25 mL glass bottle, 0.002 mol (accurate weight) of the corresponding methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4a–p) and 0.2423 g (0.002 mol) (accurate weight) of tris(hydroxymethyl)aminomethane are placed. Aqueous solutions of salts 5a–p, having a yellow color, are prepared immediately before the pharmacological tests. To do this, 20 mL of sterile water is added to each bottle and mixed thoroughly.
Disubstituted salts 6a–p are prepared in a similar way using a double amount of tris(hydroxymethyl)aminomethane in relation to esters 4a–p.
Monosubstituted Salt of Methyl 4-Hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate and Tris(hydroxymethyl)aminomethane monohydrate (5a). The aqueous solution of salt 5а (see the previous example) is left at room temperature for slow evaporation of the solvent. Gradually, crystals of the monohydrate of salt 5а are formed in the solution. They are filtered and used for X-ray diffraction studies. Yellow crystals; m.p. 92–94 °C.
2.5. X-ray Structural Analysis of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate (4a)
The crystals of ester 4a (C10H9NO5S) were monoclinic, colorless. At 20 °C: a 9.733 (2), b 7.611 (2), c 15.345 (3) Å; β 103.57 (2)°; V 1104.9 (4) Å3, Z 4, space group P21/c, dcalc 1.534 g/cm3, µ(MoKα) 0.302 mm−1, F(000) 528. The unit cell parameters and intensities of 10,193 reflections (3203 independent reflections, Rint = 0.113) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited, Oxford, UK) using MoKα radiation, a charge coupled device (CCD) detector, graphite monochromator and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry, Göttingen, Germany) [13]. The positions of the hydrogen atoms were found from the electron density difference map and refined using isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.125 for 3129 reflections (R1 0.047 for 1988 reflections with F > 4σ (F), S 0.955). The final atomic coordinates, and crystallographic data for molecule of ester 4a have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (Fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition numbers CCDC 1981599 [14].
2.6. X-ray Structural Analysis of Mono-Substituted Salt of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Tris(hydroxymethyl)aminomethane Monohydrate (5a)
The crystals of salt 5a (C10H8N-O5S · C4H12N+O3·H2O) were orthorhombic, yellow. At 20 °C: a 23.2983 (8), b 6.8659 (2), c 21.3660 (7) Å; V 3417.8(2) Å3, Z 2, space group Pca21, dcalc 1.533 g/cm3, µ(MoKα) 0.243 mm−1, F(000) 1664. The unit cell parameters and intensities of 58,839 reflections (6010 independent reflections, Rint = 0.96) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 50°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [13]. The positions of the hydrogen atoms were found from the electron density difference map and refined using the “rider” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for protonated amino groups, hydroxyl groups, methyl groups, and n = 1.2 for the other hydrogen atoms). The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.276 for 5952 reflections (R1 0.111 for 4791 reflections with F > 4σ (F), S 1.140). The final atomic coordinates and crystallographic data for molecule of salt 5a were deposited with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (Fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition numbers CCDC 1981600 [15].
2.7. Pharmacology
Analgesic Test
All biological experiments were carried out in full accord with the European Convention on the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and the Ukrainian Law No. 3447-IV “On protection of animals from severe treatment” [16] (project ID 3410U14, approved 15 October 2015).
In this study, male Wistar rats (190–220 g) were obtained from vivarium of the Institute of Pharmacology and Toxicology (Kyiv, Ukraine). All animals received standard food for rodents and water. They were acclimatized within 10 days. One day before the experiments, the animals were transferred to the scientific laboratory for adaptation. All the time they were maintained at 20–22 °C, 40–60% relative humidity and 12/12 h (light/dark) cycle.
The analgesic properties of esters 4 and salts 5, 6 were studied compared to Meloxicam (Boehringer Ingelheim, Ingelheim am Rhein, Germany) and Xefocam (Takeda Austria GmbH, Linz, Austria), being similar by the structure on the model of the thermal tail-flick procedure in white rats (Tail Immersion Test) [17], allowing for judgement to be made about the central effect on the nociceptive system. For this purpose, the rat’s tail tip was immersed in a water bath heated to 54 °С, and the latent period of the tail withdrawal (immersion) expressed in seconds was determined. The analgesic effect (in %) was assessed by the change of the latent period in 1 h after introduction of the test substances and reference drugs compared to the baseline level taken as a control. Ten experimental animals were involved to obtain statistically reliable results (the significance level of the confidence interval accepted in this work was p ≤ 0.05) in testing each of esters 4, salts 5, 6 and reference drugs. Esters 4a–p and Meloxicam were introduced orally in the form of fine aqueous suspensions stabilized with Tween-80 in the dose of 20 mg/kg. Salts 5a–p, 6a–p and Xefocam were introduced orally in the form of aqueous solutions in the dose of 20 mg/kg (in terms of the active ingredient).
3. Results and Discussion
3.1. Chemistry
Methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates theoretically substituted in the benzene moiety of the molecule can be obtained using two synthetic schemes. The fundamental difference between them is at what stage the corresponding substituent is introduced—before the formation of the benzothiazine bicycle or after it. At first glance, the chemical modification of ready-made benzothiazine looks more attractive. However, upon closer analysis, it turns out that the practical implementation of such a path is fraught with very serious difficulties. The presence of several potential reaction centers in a single molecule at once significantly complicates all sorts of chemical transformations with similar objects—whether it is alkylation, halogenation, nitration, etc. As a rule, such reactions do not always take place unambiguously and in the right direction (see, for example, alkylation of 4-hydroxy-2,2-dioxo-2,1-benzothiazine [18] or bromination of structurally similar 4-hydroxy-2-oxo-quinolines [19,20,21]). As a result, mixtures of isomeric products are formed, and they require not only labor-intensive separation, but also an unquestionable determination of the structure of each of their components.
Taking these factors into account we decided to use a simpler and more reliable scheme, that is, to make a synthesis of commercially available anthranilic acids (1) or methyl anthranilates (2) with already known substituents and their exact location in the benzene core (Scheme 1). Sulfonylation with methyl chlorosulfonyl acetate and the subsequent treatment of intermediate anilides 3 with sodium methylate solution in anhydrous methanol (other alcohols should not be used due to easy partial transesterification [8]) gives the target methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4a–p) with high yields and purity.
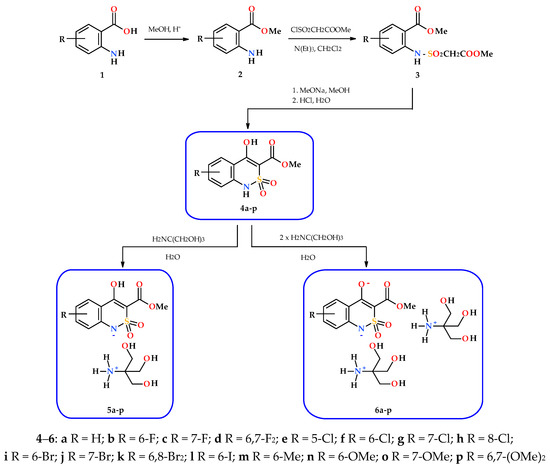
Scheme 1.
The synthesis of methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4), and the mono-(5) and di-(6) substituted salts with tris(hydroxymethyl)aminomethane.
After crystallization from methanol, all esters 4a–p synthesized are colorless crystalline substances with clear melting points (see Section 2). At room temperature, they are readily soluble in DMSO, soluble in ethyl acetate, slightly soluble in alcohols and insoluble in hexane and water.
Complex application of NMR spectroscopy of NMR 1Н and 13С allows for confirming the structure of the esters 4a–p obtained with a high level of confidence. At the same time, it should be taken into account the fact that in all 1H-NMR spectra, without exception, aromatic protons are manifested by well-resolved signals of multiplicity corresponding to the chemical surrounding and intensity of 1H each in the following order: H-5, H-7, H-6 and H-8 (in the direction from a weak field to a strong one). It is clear that, in the substituted esters 4b–p, one or two of these signals are missing, and the remaining ones have slightly different values of chemical shifts and multiplicity, but their sequence remains unchanged. Using this simple algorithm, the position of the substituent in the moiety of the molecule can be easily determined and thus isomeric products can be confidently distinguished. For example, 5-Cl-ester 4e and its 8-Cl-substituted isomer 4h in the aromatic region of NMR 1Н spectra give a similar picture of one triplet and two doublets (Figure 2).
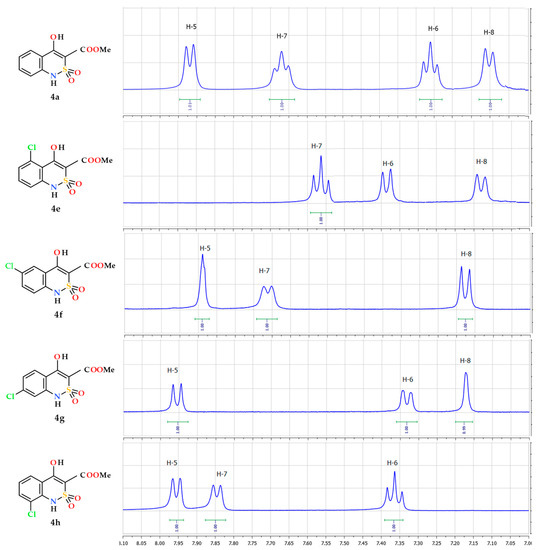
Figure 2.
Fragments of 1H-NMR spectra (the signals of aromatic protons) of ester 4a and of its chloro-substituted analogues 4e–4h.
Theoretically, such a set of signals does not contradict any of the structures mentioned. However, the order in which they follow each other: t d d and d d t, according to the above algorithm, uniquely indicates the presence of a substituent in position 5 and 8, respectively. A similar approach to the analysis of externally similar NMR 1Н spectra of 6- and 7-Cl-substituted esters 4f and 4g (Figure 2), their bromine-(4i,j) or methoxy-substituted (4n,o) analogs allows us to reliably identify each of the isomers.
In general, NMR spectroscopy (1Н and 13С) gives excellent and unambiguous results when determining the structure of fluoro-, methyl- and methoxy-substituted methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4). In the case of chlorine-, bromine- and iodine-substituted analogs, only the position of the substituents in the benzene moiety of the molecule is reliably determined, but not their nature. The cause is quite simple—atoms of chlorine, bromine and iodine do not have a magnetic moment, and do not affect the multiplicity of signals of carbon atoms bound to them and neighboring protons at all. Therefore, conventional NMR spectroscopy is not able to confidently distinguish between the same type of chlorine, bromine and iodine-substituted derivatives (for example, esters 4f, 4i and 4l). Of course, one can try to apply special NMR techniques (NOESY, HMQC, HMBC, etc.), but it is much easier and more reliable to solve such analytical problems using mass spectrometry.
In fact, all esters 4a–p appeared to be rather stable substances capable of producing medium-intensive peaks of molecular ions in the mass spectra registered during electron impact ionization. In this way, another characteristic of each test sample—its molecular weight—is determined. The information is certainly important and useful, especially for identification of structurally similar chlorine-, bromine- and iodine-substituted esters 4f, 4i and 4l.
The mass spectrometric behavior of all methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4a–p) obtained by us was very similar regardless of the substituents in the benzene moiety of the molecule. In all cases, the initially formed molecular ions undergo the primary ketene-type destruction, which proceeds with the typical CO-OAlk bond break for lower alkyl esters of carboxylic acids (Scheme 2).
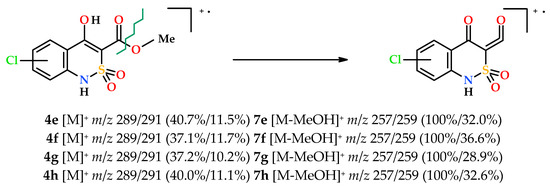
Scheme 2.
The primary fragmentation of the chloro-substituted esters 4e–h molecular ions.
Unfortunately, the same behavior is not always favorable for solving structural problems. In particular, the mass spectra of chlorine-substituted esters 4e–h (Scheme 2) are so similar even in details (m/z values, multiplicity and intensity of peaks of molecular ions, primary fragment ketenes 7e–h, and other fragment cation radicals) that it is not possible to distinguish one isomer from another. However, knowing that the substituent is a chlorine atom and comparing these data with NMR spectra determination of the true structure of each isomers does not cause difficulties.
3.2. The Molecular and Crystal Structure Study
As we have shown repeatedly before, the spatial structure o of 4-R-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acids derivatives can significantly affect their biological properties [22,23,24,25,26]. On the other hand, it is well known that salt formation by 4-ОН group is accompanied by drastic changes of 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazines crystal molecular conformations [18,27]. Hence, it is interesting along with esters 4a–p to include their salts in the range of the objects studied—both mono- and disubstituted ones since the salt formation by each of the acid groups of esters 4a–p must make a specific contribution to changing their structure.
Highly water-soluble mono-substituted salts 5a–p were obtained by mixing equimolar amounts of the corresponding esters 4a–p and tris(hydroxymethyl)aminomethane in water (Scheme 1). Initially formed aqueous solutions of salts 5a–p of an intensely yellow color after sterilization were used directly in biological experiments. However, as shown in the example of salt 5a, if necessary, they can be isolated as light yellow crystals and characterized (see Section 2). Disubstituted salts 6a–p were synthesized in a similar way, but using a double excess and tris(hydroxymethyl)-aminomethane. In this case, the isolation of salts 6a–p in pure form is also possible although we unfortunately failed to obtain crystals suitable for single-crystal X-ray diffraction studies.
According to our research, the partially saturated heterocycle in the bicyclic fragment of ester 4а is located in the half-chair conformation (the puckering parameters [28] are: S = 0.55, Θ = 44.8°, Ψ = 29.1°). Deviations of N(1) and S(1) atoms from the mean square plane of the remaining atoms of the cycle are −0.22 and 0.39 Å, respectively (Figure 3). The nitrogen atom has a pyramidal configuration (the sum of the valence angles centered on it is 345°). The S(1)‒O(4) bond is axial, and the S(1)‒O(5) bond is equatorial (torsion angles O(4)‒S(1)‒C(8)‒C(7) 85.8(2)° and O(5)‒S(1)‒C(8)‒C(7)‒142.7(1)°). The hydroxyl group forms not only a strong intramolecular hydrogen bond with the carbonyl group of the ester substituent (O(1)‒H(1O) … O(2): H … O 1.61 Å, O‒H … O 151°), but also a weaker intermolecular hydrogen bond O(1)‒H(1O) … O(2)’: (1 − x, 2 − y, 1 − z; H … O 2.29 Å, O‒H … O 116°). The formation of the hydrogen bifurcation bond leads to a shortening of the O(1)‒С(7) bond to 1.328 (2) Å compared to the mean value [29] of 1.362 Å and to an elongation of the C(7)‒С(8) bond to 1.376 (2) Å (the mean value is 1.326 Å), while the C(9)‒О(2) 1.211 (2) Å bond is almost not deformed (the mean value is 1.210 Å). Probably, the participation of the hydroxyl group in the intermolecular hydrogen bond also contributes to some reversal of the carbonyl group relative to the endocyclic double bond (torsion angle С(7)‒С(8)‒С(9)‒О(2) 12.3(3)°). The methyl group of the ester substituent is in the ар-conformation with respect to the С(8)‒С(9) bond (torsion angle С(10)‒О(3)‒С(9)‒С(8) 176.8(2)°). A shortened intramolecular contact Н(5)…О(1) 2.37 Å was also found in the molecule the ester 4а with the sum of the van der Waals radii [30] of 2.46 Å.
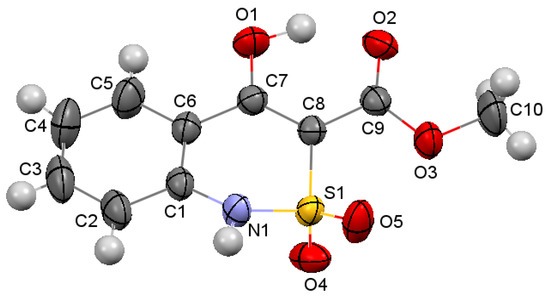
Figure 3.
The molecular structure of ester 4a according to X-ray diffraction data. The atoms represented by thermal vibration ellipsoids of 50% probability.
In the crystal the molecules of ester 4а form chains along the crystallographic direction [010] (Figure 4, left) due to the formation of the intermolecular hydrogen bond N(1)‒H(1N) … O(4)’: (1 − x, y − 0.5, 1.5 − z; H … O 2.17 Å, N‒H … O 171°). The neighboring chains are connected by weaker hydrogen bonds O(1)‒H(1O) … O(2)’: (1 − x, 2 − y, 1 − z; H … O 2.29 Å, O‒H … O 116°), it leads to the formation of the layers parallel to the crystallographic plane (0 1 1) (Figure 4, on right). It should be noted that only one oxygen atom of the sulfo group participates in the formation of an intermolecular hydrogen bond as a proton acceptor.
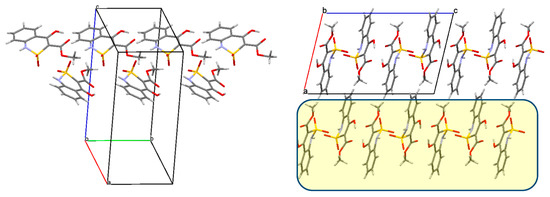
Figure 4.
(Left) a chain of the molecules of ester 4a along the crystallographic direction [0 1 0]; (right) packing of the molecules of ester 4a, projection along the crystallographic direction [0 1 0], the layers parallel to the crystallographic plane (0 1 1) are marked in yellow.
The X-ray diffraction study of compound 5а (Figure 5) showed that it is a salt of an organic anion with protonated tris (hydroxymethyl) aminomethane and exists in the crystal as a monohydrate. In the independent part of the unit cell there are two anion molecules (A and B), two cation molecules (A and B), and two water molecules (А and В). It was clearly found that the negative charge of the anion is localized on the deprotonated nitrogen atom of the sulfamide fragment, which is further indicated by the participation of this nitrogen atom in the intermolecular hydrogen bond as a proton acceptor. It indicates that the cyclic sulfamide group of methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates (4a–p) has higher acidic properties than the 4-hydroxyl group, and the salt formation takes place exactly on it. The positive charge of the cation is localized on the nitrogen atom of the protonated amino group of tris(hydroxymethyl)aminomethane (hydrogen atoms were identified objectively from the difference synthesis of electron density).
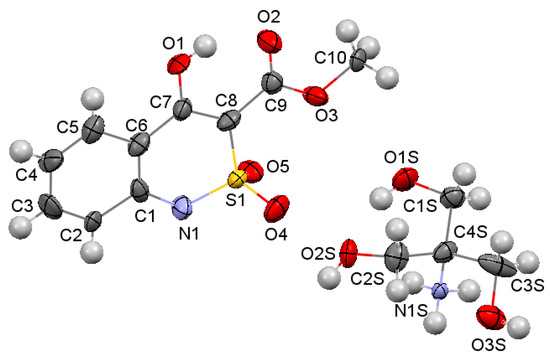
Figure 5.
The molecular structure of salt 5a according to X-ray diffraction data. The atoms represented by thermal vibration ellipsoids of 50% probability.
As expected, the salt formation introduced drastic conformational changes in the range of compounds studied. Thus, in contrast to ester 4а described above the benzothiazine fragment of its salt 5а becomes flat with an accuracy of 0.03 Å in anion А and 0.02 Å in anion В. The ester substituent in both anions is coplanar to the bicycle plane, it is facilitated by the formation of an intramolecular hydrogen bond О(1)‒Н … О(2): (Н … О 1.76 Å, О‒Н … О 148°) in anion А and (Н … О 1.82 Å, О‒Н … О 144°) in anion В. The methyl group is in ар-conformation with respect to the С(8)‒С(9) bond (torsion angles С(7)‒С(8)‒С(9)‒О(2) 2(1)° in anion А and −2(1)° in anion В; С(8)‒С(9)‒О(3)‒С(10) −176.0(6)° in А and 173.7(6)° in В).
In addition, the salt formation leads to a significant increase in the number of the intermolecular hydrogen bonds. In this case, hydrogen bonds, in which the proton acceptor is the nitrogen atom of the benzothiazine fragment (N(1Sa)‒H(3NS) … N(1b) and N(1Sb)‒H(5NS) … N(1a)), can be considered as charge-assisted hydrogen bonds. But the analysis of the geometric characteristics of the intermolecular hydrogen bonds shows that all interactions selected are almost equal (Table 1).

Table 1.
Intermolecular hydrogen bonds in the structure of salt 5a and their geometric characteristics.
A more thorough analysis of the crystal structure of salt 5a allows us to separate the layers parallel to the crystallographic plane (1 1 0), within which the molecules of organic cations and anions are connected to each other and through the bridging water molecules by the intermolecular hydrogen bonds mentioned (Figure 6). It is worth noting that in contrast to ester 4а in its salt 5а, both atoms of the sulfo group are proton acceptors in the intermolecular hydrogen bonds, and it can contribute to the benzothiazine fragment flattening.
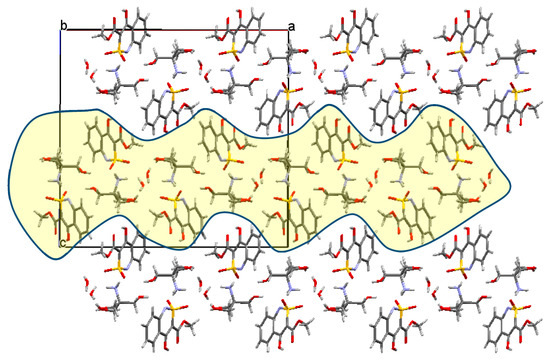
Figure 6.
Packing of molecules in the structure of salt 5a, projection along the crystallographic direction [0 1 0], the layers are marked in yellow.
3.3. Evaluation of the Analgesic Activity
Our pharmacological tests convincingly showed that the chemical modification of the benzene moiety of the molecule of methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate can be considered as a very effective means of enhancing the analgesic activity of the base structure (Table 2). Thus, if the unsubstituted ester 4а is not of any interest as an analgesic, then its analogs with fluorine atoms in positions 6 or 7 (esters 4b or 4c) are almost as active as Meloxicam. At the same time, the simultaneous presence of two fluorine atoms in the same positions in the benzene core (ester 4d) completely deprives the molecule of analgesic properties. The same pattern is observed in the case of methoxy derivatives 4n–p, with the only difference that here the 6-monosubstituted ester 4n belongs to the medium-level analgesics, but not the high-level ones.

Table 2.
The analgesic activity of esters 4, their mono-(5) and di-(6) ammonium salts with tris(hydroxymethyl)aminomethane on the “tail-flick” model in rats.
The group of mono-chlorine-substituted derivatives 4e–h should be particularly noted if only because in this case it was possible to obtain and test all theoretically possible isomers. This revealed a clear relationship between the level of activity and the position of the chlorine atom: 7-Cl > 5-Cl >> 8-Cl > 6-Cl. A similar structural and biological regularity when a 7-substituted isomer significantly exceeds its 6-substituted analog in analgesic properties is demonstrated by all other derivatives.
Interesting and in some ways even unexpected results were obtained in pharmacological tests of mono-and disubstituted salts 5 and 6. As it turned out, the statement, which is widely accepted in scientific circles and often accepted as an axiom, that the biological activity of a substance is directly proportional to its solubility is not always true. For example, the transition from ester 4а to its water-soluble form 5а is indeed accompanied by a powerful increase in analgesic properties (Table 2). However, the disubstituted salt 6а, which solubility in water is even higher, is completely untenable as an analgesic. Similar “inconsistencies” are observed in the vast majority of examples. The only exceptions are two samples: 6-and 7-chlorine substituted esters 4f and 4g. Only in these two cases, the increase in water solubility due to the formation of mono-and disubstituted salts is accompanied by an increase in analgesic properties or at least their preservation at a sufficiently high level. If we also take into account the fact that one of these substances in the water-insoluble acid form 4f does not have activity, then only methyl 7-chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (4g) can be recommended as the leader structure of all substances studied.
The decrease in the analgesic effect of the same substance during the transition from its acid form to the salt form was noted by us earlier [27]. Moreover, it was already experimentally proven that such chemical modifications were accompanied by significant conformational rearrangements and eventually by a decrease in activity. Ester 4а and its salt with tris(hydroxymethyl)-aminomethane 5а described in this article once again clearly confirm the assumptions made earlier. However, the activity has changed in the opposite direction—it is significantly increased. Nevertheless, in general, it is not the direction (growth or decline) of pharmacological effects that is important here, but the fact that they are caused by changes in molecular conformations. It is obvious that the other examples, although indirectly, also show in favor of the fact that the strength of the analgesic activity of a substance is determined not by solubility, but by its molecular conformation. Only an experiment can show which conformation is active and which is not. Therefore, it is not surprising that some of the substances presented—esters 4d,h,k,l,p and their salts—retain approximately the same level of activity regardless of what form they are in. Some initially highly effective analgesics—esters 4b,c,e,j,o—lose their activity after conversion to water-soluble forms. The reverse, or mixed version, is also possible. For example, esters 4a,i from virtually inactive substances turn into very powerful analgesics of mono-salts 5a,i. But after salt formation by the second acid group—disubstituted salts 6a,i—and, as a result, after another conformational change they again are completely inactivated.
4. Conclusions
An effective scheme of obtaining has been proposed for the synthesis of a new group of potential analgesics methyl 4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate and its analogs substituted in the benzene moiety of the molecule. To identify structural and biological regularities, their water-soluble mono- and disubstituted salts with tris(hydroxymethyl)-aminomethane were obtained on the basis of all esters. Elemental analysis, NMR spectroscopy (1Н and 13С) and mass spectrometry were used to confirm the structure of the compounds synthesized. It was shown how the spectral data obtained can uniquely determine the nature and position of the substituent in the benzothiazine core. The X-ray diffraction study of one of the esters and its monoammonium salt with tris(hydroxymethyl)-aminomethane was conducted. It was proven that salt formation passes through the cyclic sulfamide group, this leads to a significant increase in the number of intermolecular hydrogen bonds, changes in the crystal packaging and, ultimately, to a conformational rearrangement of the molecule. Pharmacological tests of all compounds synthesized for the presence of analgesic properties were performed. The studies were conducted on the model of the thermal tail-flick procedure (Tail Immersion Test) in white rats using the oral dose of 20 mg/kg; the reference drugs were Meloxicam and Xefocam. It was found that the introduction of substituents in the benzene moiety of the molecule (especially in position 7) is an effective method of enhancing the analgesic activity of 2λ6,1-benzothiazine-3-carboxylic acids derivatives. It was experimentally determined that, contrary to popular belief, increasing the solubility of a substance does not always have a positive effect on the strength of its biological effect. Specific examples demonstrate that good solubility can not only enhance the analgesic properties of the compounds studied, but also significantly reduce them, or even have no effect at all. Since chemical modification aimed at changing the solubility is always accompanied by a rearrangement of molecular conformations, there is every reason to consider this factor as the main cause for determining the level of the analgesic activity. According to the results of the studies conducted only one compound—methyl 7-chloro-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate—was selected from the whole group for further research as a promising analgesic; it demonstrates powerful analgesia in all forms regardless of their solubility.
Author Contributions
The synthesis of the compounds presented in this work and analysis of their characteristics were performed by I.V.U., and L.A.P. Single crystal X-ray diffraction structural studies were performed by S.V.S. Mass spectrometric studies were performed by L.V.S. The pharmacological studies were conducted by T.V.A., I.I.T. and A.A.D. The manuscript was written by I.V.U., L.A.P. and S.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Candidate of Chemistry Magda D. Tsapko (Taras Shevchenko National University, Kiev, Ukraine) for her help in registration of NMR spectra of the compounds synthesized.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choudhary, M.I.; Naheed, N.; Abbaskhan, A.; Musharraf, S.G.; Siddiqui, H.; Atta-ur-Rahman. Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 2008, 69, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.; Moore, R.A.; Edwards, J.E.; McQuay, H.J.; Derry, S.; Wiffen, P.J. Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ 2004, 328, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Tramèr, M.R. It’s not just about rubbing—Topical capsaicin and topical salicylates may be useful as adjuvants to conventional pain treatment. BMJ 2004, 328, 998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Negwer, M. Organische Arzneimittel und Ihre Synonyma; Akademie: Berlin, Germany, 1978. [Google Scholar]
- Dwyer, C.; Sowerby, L.; Rotenberg, B.W. Is cocaine a safe topical agent for use during endoscopic sinus surgery? Laryngoscope 2016, 126, 1721–1723. [Google Scholar] [CrossRef]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 5th ed.; Thieme: Stuttgart, Germany, 2008. [Google Scholar]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P. 2,1-Benzothiazine 2,2-dioxides. 1. Synthesis, structure, and analgesic activity of 1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid esters. Chem. Heterocycl. Compd. 2013, 49, 1378–1383. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Bereznyakova, N.L. Effect of bromination on the pharmacological properties of methyl 1-allyl-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2015, 49, 519–522. [Google Scholar] [CrossRef]
- Azotla-Cruz, L.; Shishkina, S.; Ukrainets, I.; Lijanova, I.; Likhanova, N. Crystal structure of methyl 1-allyl-4-methyl-1H-benzo[c][1,2]thiazine-3-carboxylate 2,2-dioxide. Acta Crystallogr. E Crystallogr. Commun. 2016, 72, 1574–1576. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Sim, G.; Grinevich, L.A. Synthesis and molecular structure of ethyl-4-hydroxy-1-phenyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2017, 51, 482–485. [Google Scholar] [CrossRef]
- Azotla-Cruz, l.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New synthesis, structure and analgesic properties of methyl 1-R-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates. Sci. Pharm. 2017, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1981599. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 February 2020).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1981600. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 February 2020).
- Ukrainian Law No. 3447-IV. On Protection of Animals from Severe Treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 4 August 2017).
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1014–1016. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Shishkina, S.V.; Petrushova, L.A.; Sim, G. 2,1-Benzothiazine 2,2-dioxides. 9. Alkylation of methyl 4-hydroxy-1-methyl-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylate with ethyl iodide. Chem. Heterocycl. Compd. 2014, 50, 1741–1747. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Taran, S.G.; Evtifeeva, O.A.; Gorokhova, O.V.; Filimonova, N.I.; Turov, A.V. 4-Hydroxy-2-quinolones. 26. Bromination of 3-substituted 2-oxo-4-hydroxyquinolones. Chem. Heterocycl. Compd. 1995, 31, 176–179. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Jaradat, N.A.; Bevz, O.V.; Turov, A.V. 4-Hydroxy-2-quinolones. 204. Synthesis, bromination, and analgetic properties of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid arylalkylamides. Chem. Heterocycl. Compd. 2012, 48, 1347–1356. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Chernenok, I.N.; Shishkina, S.V.; Parshikov, V.A. 4-Hydroxy-2-quinolones. 220. Bromination of ethyl 7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxylate. Chem. Heterocycl. Compd. 2013, 49, 1665–1669. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. C Struct. Chem. 2016, 72, 411–415. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Levandovskiy, I.A.; Ukrainets, I.V.; Sidorenko, L.V.; Grinevich, L.A.; Yanchuk, I.B. Polymorphic modifications of a 1H-pyrrolo[3,2,1-ij]quinoline-5-carboxamide possessing strong diuretic properties. Acta Crystallogr. C Struct. Chem. 2018, 74, 1759–1767. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Voloshchuk, N.I.; Malchenko, O.V.; Shishkina, S.V.; Grinevich, L.A.; Grynenko, V.V.; Sim, G. Molecular conformations and biological activity of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Sci. Pharm. 2018, 86, 50. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Burian, A.A.; Baumer, V.N.; Shishkina, S.V.; Sidorenko, L.V.; Tugaibei, I.A.; Voloshchuk, N.I.; Bondarenko, P.S. Synthesis, crystal structure and biological activity of ethyl 4-methyl-2,2-dioxo- 1H-2λ6,1-benzothiazine-3-carboxylate polymorphic modifications. Sci. Pharm. 2018, 86, 21. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Ukrainets, I.V.; Vashchenko, O.V.; Voloshchuk, N.I.; Bondarenko, P.S.; Petrushova, L.A.; Sim, G. Biological properties of two enantiomorphic forms of N-(2,6-dimethylphenyl)-4-hydroxy- 2,2-dioxo-1H -2λ6,1benzothiazine-3-carboxamide, a structural analogue of piroxicam. Acta Crystallogr. C Struct. Chem. 2020, 76, 69–74. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).