The Optimization of Gel Preparations Using the Active Compounds of Arabica Coffee Ground Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Tools and Materials
2.2. Research Design
2.3. Formulation of Gel Preparations
2.4. Characterization of Gel Preparations
2.4.1. Acidity Degree (pH) Test
2.4.2. Spreadability Test
2.4.3. Viscosity Test
2.4.4. Total Phenolic Test
3. Results and Discussion
3.1. Prediction of Optimum Formulation
3.2. Organoleptic
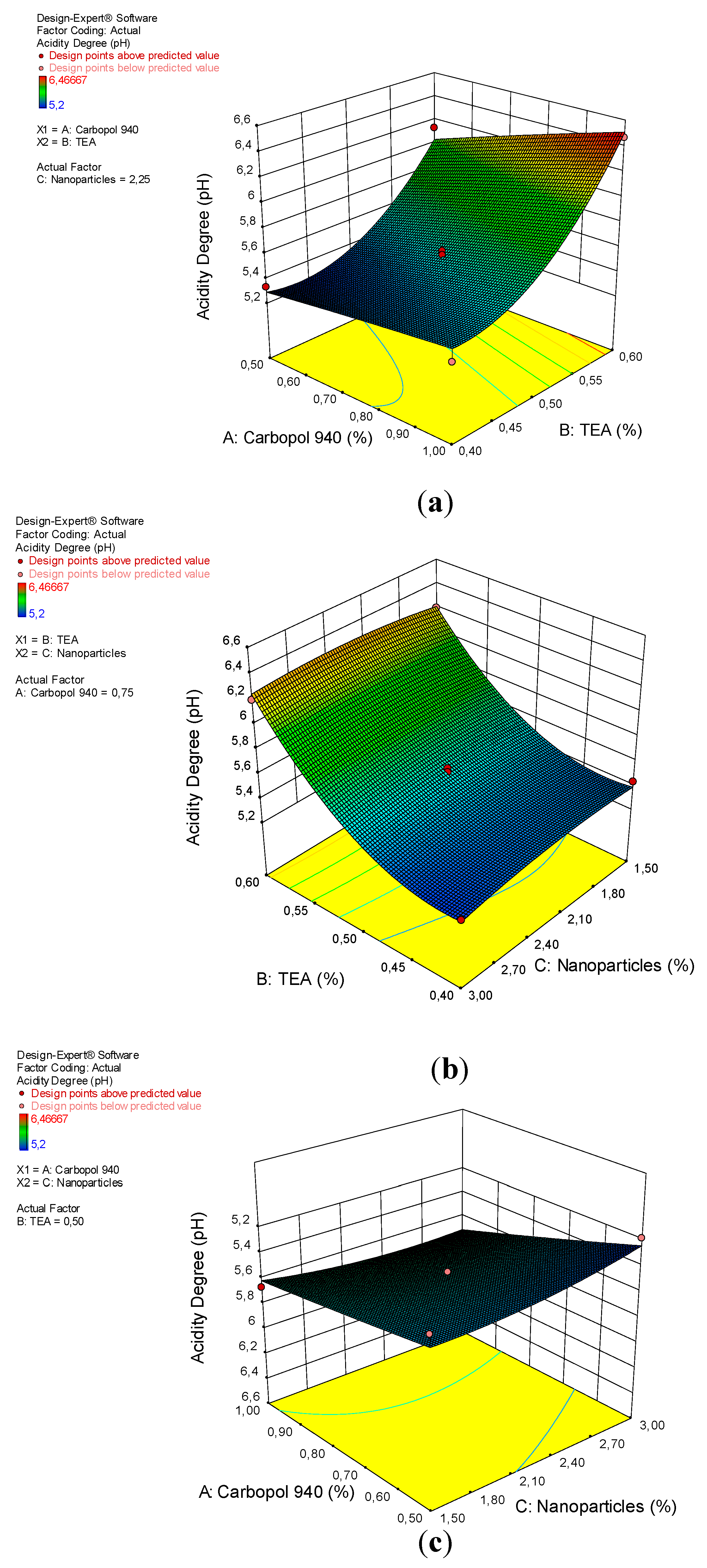
3.3. Acidity Degree
3.4. Spreadibility
3.5. Viscosity
3.6. Total Phenolic
3.7. Optimization of Gel Preparations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohanraj, V.J.; Chen, Y. Nanoparticles – A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Ariningsih, E. Prospects of Nanotechnology Application in Agriculture and Food Processing in Indonesia. Forum Penelit. Agro Ekon. 2016, 34, 1–20. [Google Scholar] [CrossRef]
- Sudibyo, A.; Hutajulu, T.F. Potensi penerapan polimer nanokomposit dalam kemasan pangan. J. Kim. dan Kemasan 2013, 35, 6–19. [Google Scholar] [CrossRef]
- Berekaa, M.M. Review Article Nanotechnology in Food Industry ; Advances in Food processing, Packaging and Food Safety. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 345–357. [Google Scholar]
- Liu, Y.; Niu, T.; Zhang, L.; Yang, J. Review on nano-drugs. Nat. Sci. 2010, 2, 41–48. [Google Scholar] [CrossRef]
- Astruc, D. Introduction to Nanomedicine. Molecule 2016, 21, 4. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Sawasdee, K.; Choksawad, P.; Pimcharoen, S.; Prapainop, K. Development of Size-Tunable Polymeric Nanoparticles for Drug Delivery Applications. Glob. Heal. Manag. J. 2017, 1, 31–36. [Google Scholar] [CrossRef]
- Prasetyo, Y.A.; Abdassah, M.; Rusdiana, T. Preparation and Characterization of Glucosamine Nanoparticle by Ionic Gelation Method Using Chitosan and Alginate. Indones. J. Pharm. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Hanutami, N.B.; Budiman, A. Review Artikel : Penggunaan Teknologi Nano pada Formulasi Obat Herbal. Farmaka 2017, 15, 29–41. [Google Scholar]
- Cruz, R.; Gomes, T.; Ferreie, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva, L.F.S.; Correa, A.P.F.; Bach, E.; Brandelli, A. Total Polyphenols, Antioxidant, Antimicrobial and Allelopathic Activities of Spend Coffee Ground Aqueous Extract. Waste and Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent co ff ee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, H.; Jeong, I.; Hong, S.; Oh, M.; Yoon, M. Contents of chlorogenic acids and caffeine in various coffee-related products. J. Adv. Res. 2019, 17, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Petrik, S.; Obruča, S.; Benešová, P.; Márová, I. Bioconversion of spent coffee grounds into carotenoids and other valuable metabolites by selected red yeast strains. Biochem. Eng. J. 2014, 90, 307–315. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. N. Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Page, J.C.; Arruda, N.P.; Freitas, S.P. Crude ethanolic extract from spent coffee grounds : Volatile and functional properties. Waste Manag. 2017, 69, 463–469. [Google Scholar] [CrossRef]
- Passos, C.P.; Moreira, A.S.P.; Domingues, M.R.M.; Evtuguin, D.V.; Coimbra, M.A. Sequential microwave superheated water extraction of mannans from spent coffee grounds. Carbohydr. Polym. 2014, 103, 333–338. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Tongcumpou, C. Industrial Crops & Products Sequential extraction and reactive extraction processing of spent co ff ee grounds : An alternative approach for pretreatment of biodiesel feedstocks and biodiesel production. Ind. Crop. Prod. 2018, 117, 359–365. [Google Scholar]
- Nurman, S.; Lamona, A.; Nasir, M. Optimization and Characterization of N-Hexane Extracts of Arabica Coffee Ground ( Coffea arabica L.) from Gayo Plateau as Source of Natural Antioxidant. In Proceedings of the International Conference on Education, Science and Technology (ICON-EST 2018) at Serambi Mekkah University, Banda Aceh, Indonesia, 17–18th October 2018; 2019; pp. 1–9. [Google Scholar]
- Palupi, N.W.; Praptiningsih, Y. Oxidized Tapioca Starch As an Alginate Substitute for Encapsulation of Antioxidant from Coffee Residue. Agric. Agric. Sci. Procedia 2016, 9, 304–308. [Google Scholar] [CrossRef]
- Cheong, M.W.; Tong, K.H.; Ong, J.J.M.; Liu, S.Q.; Curran, P.; Yu, B. Volatile composition and antioxidant capacity of Arabica coffee. Food Res. Int. 2013, 51, 388–396. [Google Scholar] [CrossRef]
- Kasman, B.B.S.; Saputri, F.A. Artikel Review: Formulasi dan Evaluasi Sediaan Gel dari Ekstrak Seledri Apium graveolens Linn Sebagai Anti-Inflamasi. Farmaka 2018, 16, 63–71. [Google Scholar]
- Ulfa, M.; Hendrarti, W.; Muhram, P.N. Formulasi Gel Ekstrak Daun Kelor ( Moringa oleifera Lam.) Sebagai Anti Inflamasi Topikal Pada Tikus ( Rattus novergicus ). J. Pharm. Med. Sci. 2016, 1, 30–35. [Google Scholar]
- Oramahi, H. Optimasi dengan RSM dan Rancangan Percobaan (Aplikasi dengan SPSS dan SAS), 1st ed.; Gava Media: Yogyakarta, Indonesia, 2016. [Google Scholar]
- Getachew, A.T.; Cho, Y.J.; Chun, B.S. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef]
- Tekindal, M.A.; Bayrak, H.; Ozkaya, B.; Genc, Y. Box- Behnken Experimental Design in Factorial Experiments: The Importance of Bread For Nutrition and Health. Turkish J. F. Crop. 2012, 17, 115–123. [Google Scholar]
- Megha, M.A.; Unnma, U.; Rameshpathy, M.; Karikalan, K.; Vickram, S.; Kumar, S.V.; Sridharan, B. Formulation of nano-encapsulated poly-herbal ointment for anti- inflammation. Der Pharm. Lett. 2013, 5, 164–170. [Google Scholar]
- Ruiz-ruiz, J.C.; Matus-basto, A.J.; Acereto-escoffié, P.; Segura-Campos, M.R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric. Immunol. 2017, 28, 1424–1437. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases : A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Lamona, A.; Nurman, S. Studi Awal Komponen Minyak Biji Kopi Arabika ( Coffea arabica L.) dari Kabupaten Bener Meriah, Provinsi Aeh Menggunakan GC-MS. Agriovet 2018, 1, 61–72. [Google Scholar]
- Nurman, S.; Yulia, R. The Potency of Gayo Arabica Coffee Powder Oil ( Coffea arabica L.) as a Source of Natural Antioxidants. J. Food, Agric. Environ. 2019, 17, 59–64. [Google Scholar]
- Złotek, U.; Szymanowska, U.; Pecio, L.; Kozachok, S.; Jakubczyk, A. Antioxidative and Potentially Anti-inflammatory Activity of Phenolics from Lovage Leaves Levisticum officinale Koch Elicited with Jasmonic Acid and Yeast Extract. Molecules 2019, 24, 2–12. [Google Scholar]
- Irianto, H.E.; Muljanah, I. Proses dan Aplikasi Nanopartikel Kitosan Sebagai Penghantar Obat. Squalen 2011, 6, 1–8. [Google Scholar]
- Yulia, R.; Meilina, H.; Adisalamun, A.; Darmadi, D. Aplikasi Metode Advance Oxidation Process (AOP) Fenton pada Pengolahan Limbah Cair Pabrik Kelapa Sawit Application of Advanced Oxidation Process (AOP) Fenton on Palm Oil Mill Effluent Treatment. J. Rekayasa Kim. dan Lingkung. 2016, 11, 1–9. [Google Scholar] [CrossRef][Green Version]
- Saryanti, D.; Zulfa, I.N. Optimization Carbopol And Glycerol As Basis Of Hand Gel Antiseptics Extract Ethanol Ceremai Leaf (Phyllantus Acidus (L.) Skeels) With Simplex Lattice Design. J. Pharm. Sci. Clin. Res. 2017, 02, 35–43. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press and American Pharmacists Association: London, UK; Washington, DC, USA, 2009. [Google Scholar]
- Danimayostu, A.A.; Shofiana, N.M.; Permatasari, D. Pengaruh Penggunaan Pati Kentang (Solanum tuberosum) Termodifikasi Asetilasi- Oksidasi sebagai Gelling agent terhadap Stabilitas Gel Natrium Diklofenak. Phamaceutical J. Indones. 2017, 3, 25–32. [Google Scholar] [CrossRef]
- Pristiana, D.Y.; Susanti, S.; Nurwantoro, N. Antioksidan dan Kadar Fenol Berbagai Ekstrak Daun Kopi (Coffea sp.): Potensi Aplikasi Bahan Alami untuk Fortifikasi Pangan. J. Apl. Teknol. Pangan 2017, 6, 89–92. [Google Scholar]
- Gómez-Ruiz, J.Á.; Ames, J.M.; Leake, D.S. Antioxidant activity and protective effects of green and dark coffee components against human low density lipoprotein oxidation. Eur. Food Res. Technol. 2008, 227, 1017–1024. [Google Scholar] [CrossRef]
- García, R.L.P.; Biasetto, C.R.; Araujo, A.R.; Luiz, V.; Bianchi, D. Enhanced Extraction of Phenolic Compounds from Coffee Industry’s Residues Through Solid State Fermentation by Penicillium Purpurogenum. Food Sci. Technol. 2015, 35, 704–711. [Google Scholar] [CrossRef]
- Shang, Y.F.; Xu, J.L.; Lee, W.J.; Um, B.H. Antioxidative Polyphenolics Obtained from Spent Coffee Grounds by Pressurized Liquid Extraction. South African J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
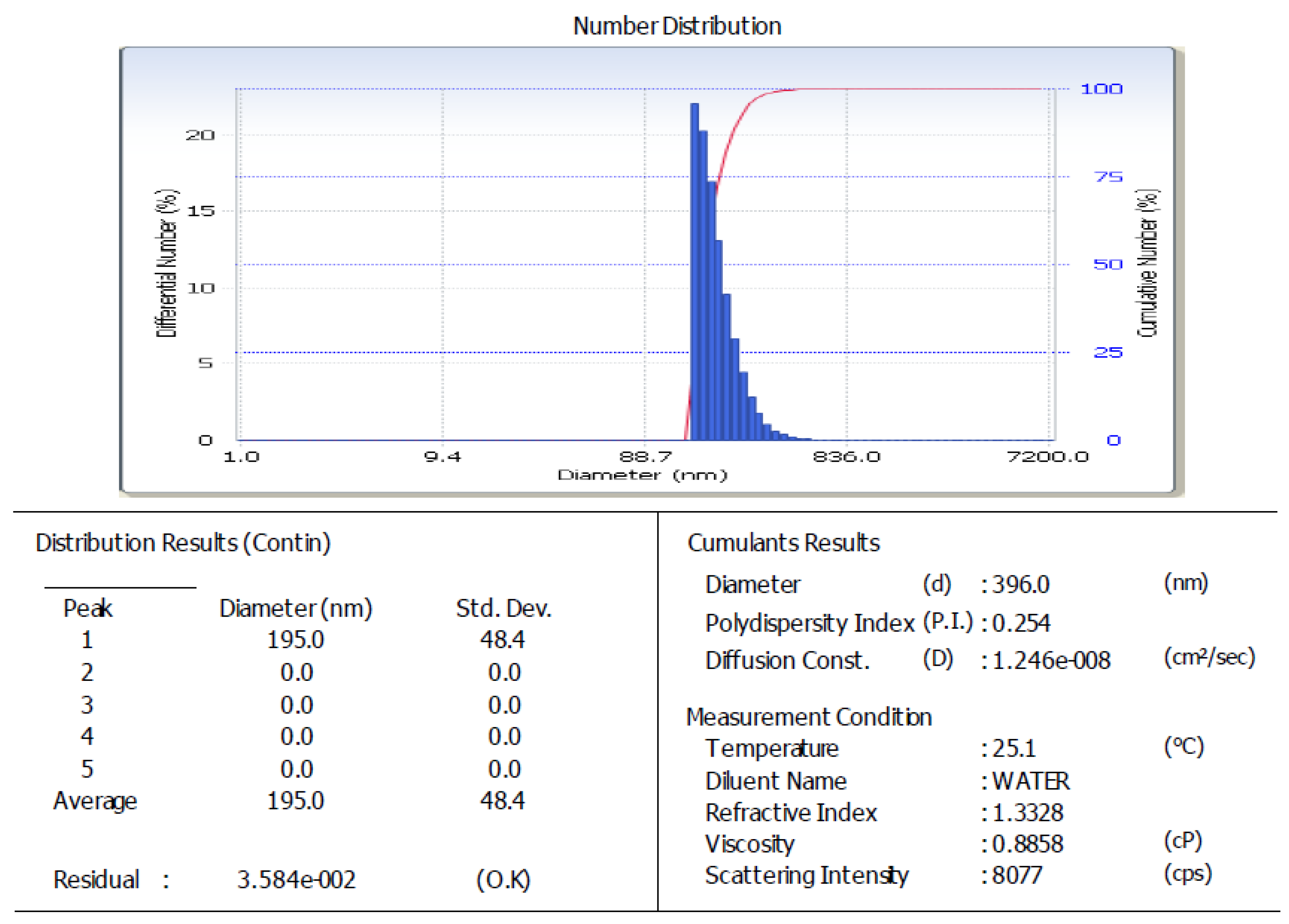
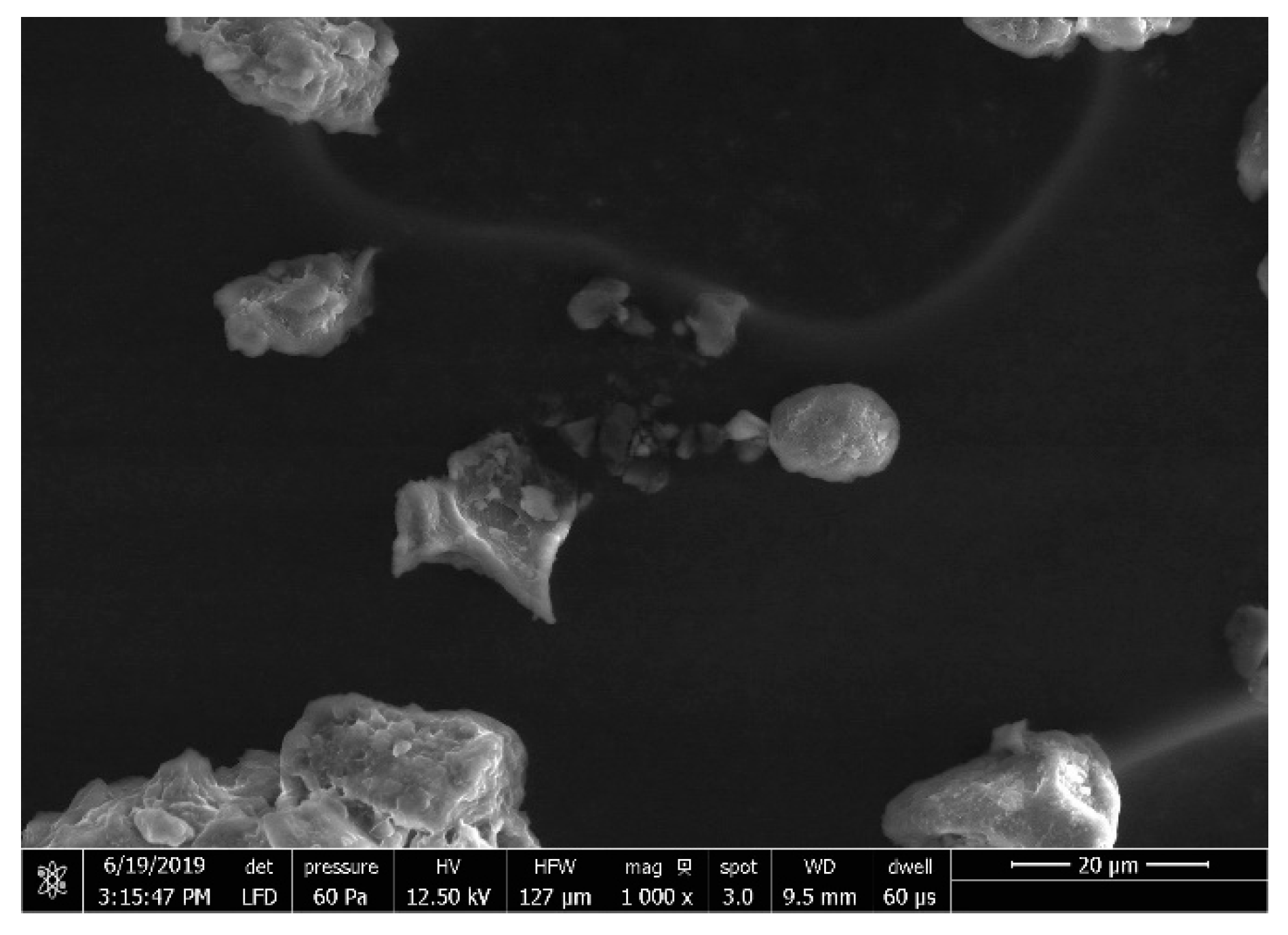
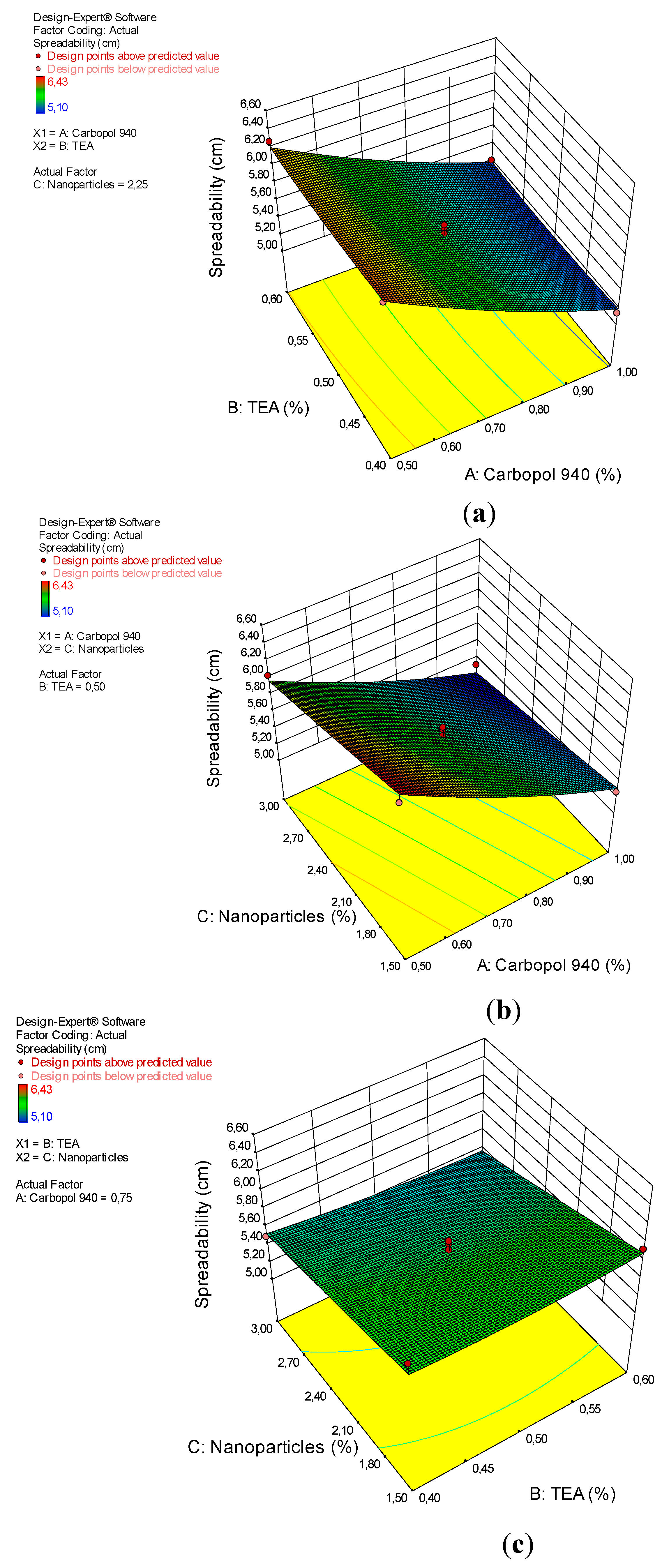
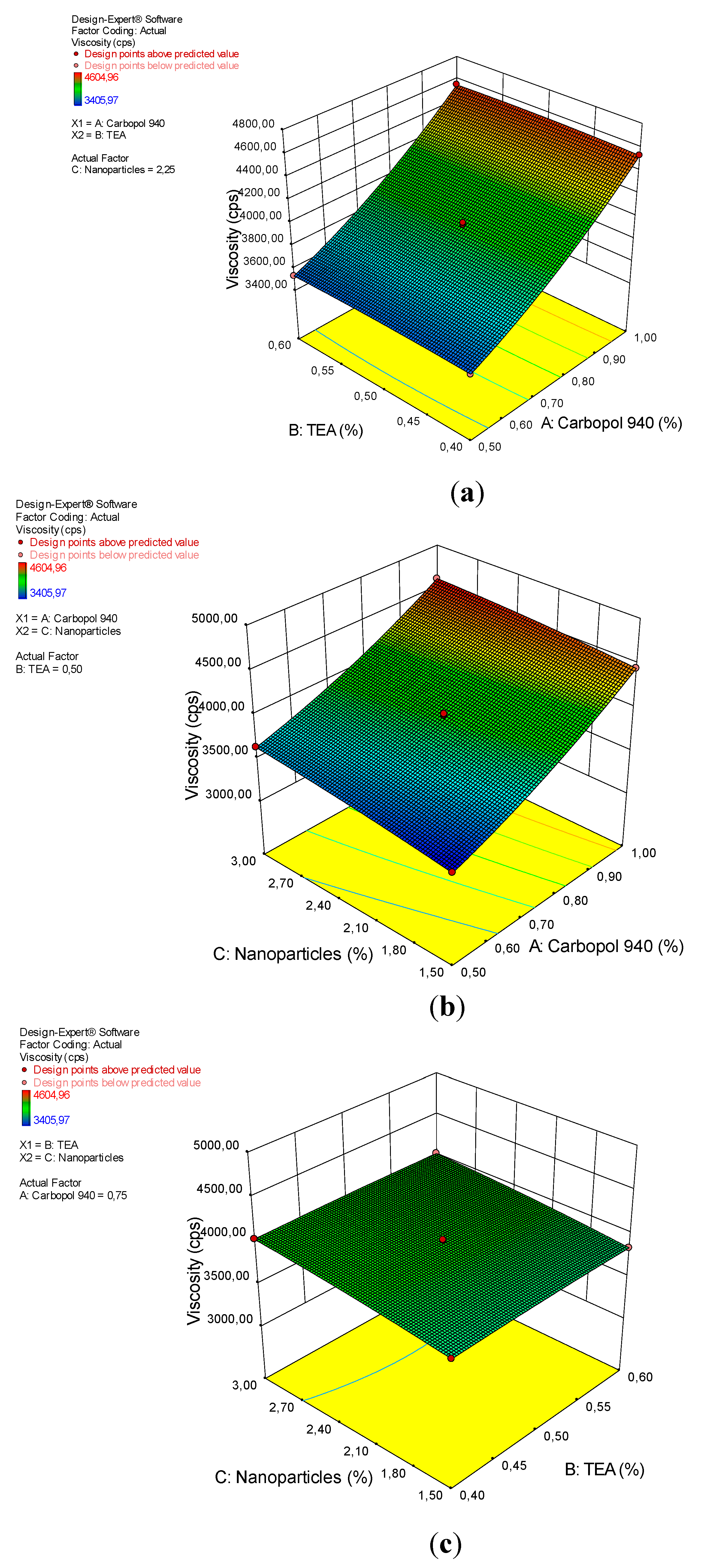
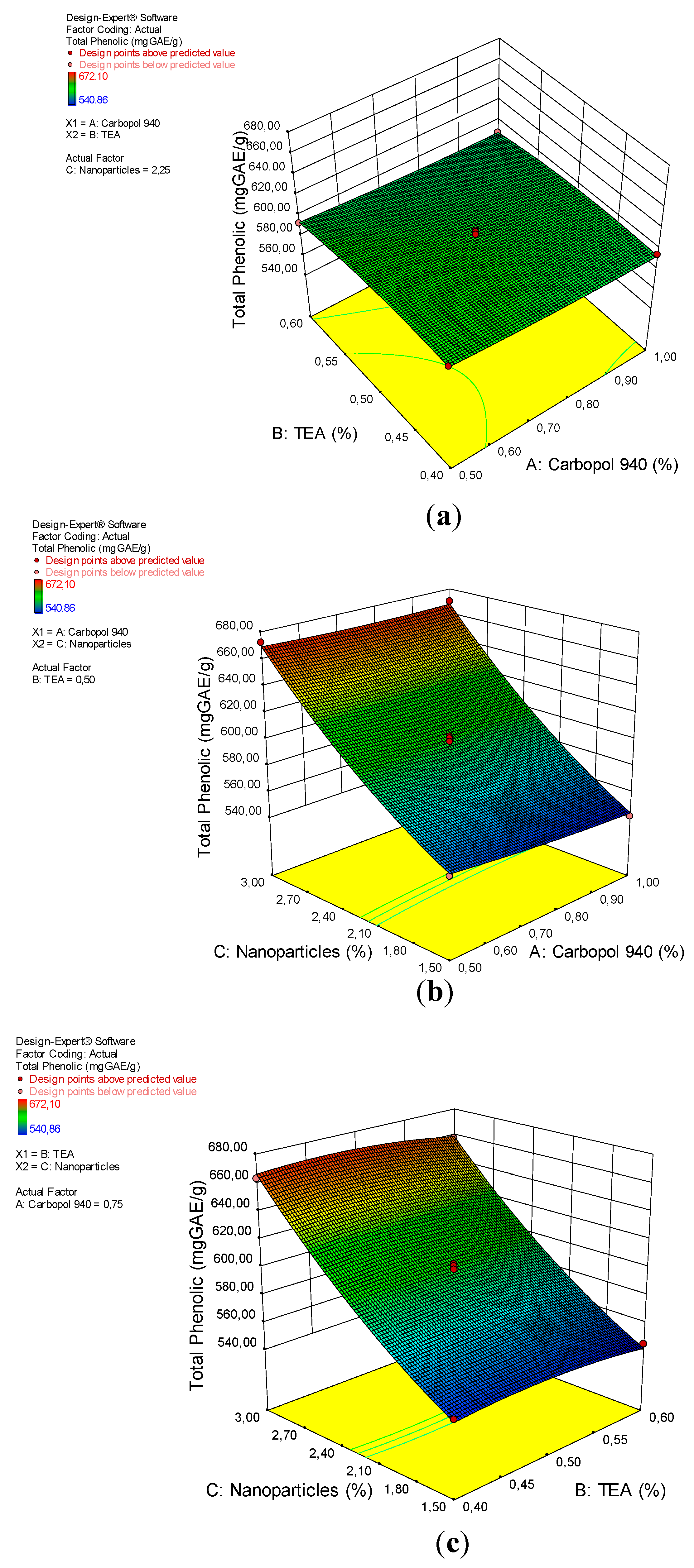
| Factor | Parameters | Levels | ||
|---|---|---|---|---|
| Low (-) | Medium (0) | High (+) | ||
| x1 | Carbopol 940 (%) | 0.50 | 0.75 | 1.00 |
| x2 | TEA (%) | 0.40 | 0.50 | 0.60 |
| x3 | Nanoparticles (%) | 1.50 | 2.25 | 3.00 |
| Run | Factor 1 A: Carbopol 940 % | Factor 2 B: TEA % | Factor 3 C: Nanoparticles % | Response 1 Acidity Degree pH | Response 2 Spreadability cm | Response 3 Viscosity cps | Response 4 Total Phenolic µgGAE/g |
|---|---|---|---|---|---|---|---|
| 1 | 1.00 | 0.50 | 1.50 | 5.67 | 5.27 | 4455.05 | 540.86 |
| 2 | 0.75 | 0.50 | 2.25 | 5.50 | 5.50 | 3965.05 | 590.17 |
| 3 | 1.00 | 0.40 | 2.25 | 5.37 | 5.13 | 4535.08 | 595.41 |
| 4 | 0.50 | 0.40 | 2.25 | 5.33 | 6.33 | 3523.39 | 602.42 |
| 5 | 0.50 | 0.50 | 3.00 | 5.20 | 6.03 | 3641.82 | 672.10 |
| 6 | 0.75 | 0.60 | 1.50 | 6.20 | 5.93 | 3845.48 | 543.83 |
| 7 | 0.75 | 0.40 | 1.50 | 5.43 | 6.00 | 3823.57 | 548.10 |
| 8 | 0.75 | 0.50 | 2.25 | 5.60 | 5.60 | 3942.74 | 601.13 |
| 9 | 0.75 | 0.50 | 2.25 | 5.60 | 5.63 | 3979.20 | 602.51 |
| 10 | 0.75 | 0.50 | 2.25 | 5.50 | 5.70 | 3956.10 | 597.44 |
| 11 | 0.75 | 0.40 | 3.00 | 5.30 | 5.50 | 4033.90 | 663.14 |
| 12 | 0.75 | 0.60 | 3.00 | 6.20 | 5.27 | 4027.11 | 657.02 |
| 13 | 0.50 | 0.50 | 1.50 | 5.33 | 6.43 | 3405.97 | 553.75 |
| 14 | 0.75 | 0.50 | 2.25 | 5.57 | 5.73 | 3981.97 | 598.54 |
| 15 | 1.00 | 0.50 | 3.00 | 5.80 | 5.10 | 4604.96 | 669.76 |
| 16 | 0.50 | 0.60 | 2.25 | 6.13 | 6.27 | 3532.03 | 593.45 |
| 17 | 1.00 | 0.60 | 2.25 | 6.47 | 5.20 | 4549.12 | 588.52 |
| Response | Source | Std.Dev | R-Square | Adj R-Square | Pred R-Square | Adeq Precisior | PRESS |
|---|---|---|---|---|---|---|---|
| Acidity Degree | Linear | 0.180 | 0.8064 | 0.7617 | 0.6231 | 13.741 | 0.84 |
| 2FI | 0.200 | 0.8264 | 0.7222 | 0.2259 | 9.621 | 1.73 | |
| Quadratic | 0.092 | 0.9734 | 0.9393 | 0.6412 | 17.753 | 0.80 | |
| Cubic | 0.051 | 0.9954 | 0.9817 | - | 28.653 | - | |
| Spreadability | Linear | 0.100 | 0.9508 | 0.9395 | 0.9124 | 29.942 | 0.26 |
| 2FI | 0.110 | 0.9594 | 0.9350 | 0.8551 | 21.849 | 0.42 | |
| Quadratic | 0.120 | 0.9681 | 0.9270 | 0.6542 | 17.245 | 1.01 | |
| Cubic | 0.091 | 0.9886 | 0.9542 | - | 16.703 | - | |
| Viscosity | Linear | 50.380 | 0.9847 | 0.9811 | 0.9696 | 49.298 | 65,402.03 |
| 2FI | 55.620 | 0.9856 | 0.9770 | 0.9332 | 33.755 | 143,700.00 | |
| Quadratic | 12.600 | 0.9995 | 0.9988 | 0.9989 | 124.683 | 2403.64 | |
| Cubic | 16.310 | 0.9995 | 0.9980 | - | 84.042 | - | |
| Total Phenolic | Linear | 7.040 | 0.9778 | 0.9727 | 0.9583 | 36.788 | 1214.05 |
| Content | 2FI | 7.840 | 0.9789 | 0.9662 | 0.9107 | 25.565 | 2596.65 |
| Quadratic | 4.510 | 0.9951 | 0.9888 | 0.9674 | 37.169 | 948.55 | |
| Cubic | 4.800 | 0.9968 | 0.9873 | - | 31.288 | - |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 2.18 | 9 | 0.24 | 28.52 | 0.0001 | significant |
| A-Carbopol 940 | 0.21 | 1 | 0.21 | 24.90 | 0.0016 | |
| B-TEA | 1.59 | 1 | 1.59 | 187.43 | <0.0001 | |
| C-Nanopartikel | 2.222 × 10−3 | 1 | 2.222 × 10−3 | 0.26 | 0.6245 | |
| AB | 0.022 | 1 | 0.022 | 2.65 | 0.1474 | |
| AC | 0.018 | 1 | 0.018 | 2.10 | 0.1910 | |
| BC | 4.444 × 10−3 | 1 | 4.444 × 10−3 | 0.52 | 0.4927 | |
| A2 | 1.433 × 10−4 | 1 | 1.433 × 10−4 | 0.017 | 0.9003 | |
| B2 | 0.32 | 1 | 0.32 | 38.22 | 0.0005 | |
| C2 | 9.500 × 10−3 | 1 | 9.500 × 10−3 | 1.12 | 0.3251 | |
| Residual | 0.059 | 7 | 8.484 × 10−3 | |||
| Lack of Fit | 0.049 | 3 | 0.016 | 6.41 | 0.0523 | not significant |
| Pure Error | 0.010 | 4 | 2.556 × 10−3 | |||
| Cor Total | 2.24 | 16 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 2.82 | 9 | 0.31 | 23.57 | 0.0002 | significant |
| A-Carbopol 940 | 2.38 | 1 | 2.38 | 179.29 | < 0.0001 | |
| B-TEA | 0.011 | 1 | 0.011 | 0.85 | 0.3882 | |
| C-Nanoparticles | 0.38 | 1 | 0.38 | 28.25 | 0.0011 | |
| AB | 4.444 × 10−3 | 1 | 4.444 × 10−3 | 0.33 | 0.5812 | |
| AC | 0.014 | 1 | 0.014 | 1.02 | 0.3453 | |
| BC | 6.944 × 10−3 | 1 | 6.944 × 10−3 | 0.52 | 0.4933 | |
| A2 | 0.019 | 1 | 0.019 | 1.41 | 0.2741 | |
| B2 | 4.678 × 10−3 | 1 | 4.678 × 10−3 | 0.35 | 0.5717 | |
| C2 | 2.924 × 10−4 | 1 | 2.924 × 10−4 | 0.022 | 0.8863 | |
| Residual | 0.093 | 7 | 0.013 | |||
| Lack of Fit | 0.060 | 3 | 0.020 | 2.39 | 0.2096 | not significant |
| Pure Error | 0.033 | 4 | 8.333 × 10−3 | |||
| Cor Total | 2.91 | 16 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 2.149 × 106 | 9 | 2.388 × 105 | 1504.50 | < 0.0001 | significant |
| A-Carbopol 940 | 2.041 × 106 | 1 | 2.041 × 106 | 12,862.08 | < 0.0001 | |
| B-TEA | 178.67 | 1 | 178.67 | 1.13 | 0.3239 | |
| C-Nanopartikel | 75,606.37 | 1 | 75,606.37 | 476.41 | < 0.0001 | |
| AB | 7.28 | 1 | 7.28 | 0.046 | 0.8365 | |
| AC | 1846.35 | 1 | 1846.35 | 11.63 | 0.0113 | |
| BC | 205.87 | 1 | 205.87 | 1.30 | 0.2922 | |
| A2 | 28,424.39 | 1 | 28,424.39 | 179.11 | < 0.0001 | |
| B2 | 634.39 | 1 | 634.39 | 4.00 | 0.0857 | |
| C2 | 1722.34 | 1 | 1722.34 | 10.85 | 0.0132 | |
| Residual | 1110.90 | 7 | 158.70 | |||
| Lack of Fit | 46.26 | 3 | 15.42 | 0.058 | 0.9793 | not significant |
| Pure Error | 1064.64 | 4 | 266.16 | |||
| Cor Total | 2.150 × 106 | 16 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 28,940.33 | 9 | 3215.59 | 158.15 | < 0.0001 | significant |
| A-Carbopol 940 | 92.27 | 1 | 92.27 | 4.54 | 0.0706 | |
| B-TEA | 86.11 | 1 | 86.11 | 4.24 | 0.0786 | |
| C-Nanopartikel | 28,259.62 | 1 | 28,259.62 | 1389.87 | < 0.0001 | |
| AB | 1.09 | 1 | 1.09 | 0.054 | 0.8233 | |
| AC | 27.78 | 1 | 27.78 | 1.37 | 0.2807 | |
| BC | 0.84 | 1 | 0.84 | 0.042 | 0.8443 | |
| A2 | 10.02 | 1 | 10.02 | 0.49 | 0.5053 | |
| B2 | 87.24 | 1 | 87.24 | 4.29 | 0.0771 | |
| C2 | 389.40 | 1 | 389.40 | 19.15 | 0.0032 | |
| Residual | 142.33 | 7 | 20.33 | |||
| Lack of Fit | 50.30 | 3 | 16.77 | 0.73 | 0.5862 | not significant |
| Pure Error | 92.03 | 4 | 23.01 | |||
| Cor Total | 29,082.65 | 16 |
| No | Carbopol 940 | TEA | Nanoparticles | Acidity Degree | Spreadability | Viscosity | Total Phenolic | Desirability | w/o Intervals |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.577 | 0.467 | 3.000 | 5.216 | 5.831 | 3746.158 | 669.138 | 0.981 | 0.993 |
| 2 | 0.575 | 0.468 | 3.000 | 5.217 | 5.826 | 3743.249 | 669.151 | 0.981 | 0.994 |
| 3 | 0.580 | 0.467 | 3.000 | 5.220 | 5.836 | 3750.518 | 669.098 | 0.981 | 0.993 |
| 4 | 0.577 | 0.468 | 3.000 | 5.219 | 5.840 | 3745.906 | 669.128 | 0.981 | 0.993 |
| 5 | 0.584 | 0.467 | 3.000 | 5.224 | 5.839 | 3757.318 | 669.044 | 0.981 | 0.993 |
| 6 | 0.582 | 0.466 | 3.000 | 5.220 | 5.832 | 3754.542 | 669.075 | 0.981 | 0.993 |
| 7 | 0.578 | 0.466 | 3.000 | 5.214 | 5.811 | 3747.527 | 669.138 | 0.981 | 0.993 |
| 8 | 0.569 | 0.468 | 3.000 | 5.212 | 5.850 | 3734.244 | 669.227 | 0.981 | 0.994 |
| 9 | 0.587 | 0.466 | 3.000 | 5.223 | 5.817 | 3760.642 | 669.027 | 0.981 | 0.993 |
| 10 | 0.582 | 0.470 | 3.000 | 5.228 | 5.805 | 3753.348 | 669.046 | 0.981 | 0.993 |
| 11 | 0.584 | 0.465 | 3.000 | 5.218 | 5.788 | 3756.707 | 669.069 | 0.981 | 0.993 |
| 12 | 0.591 | 0.467 | 3.000 | 5.229 | 5.792 | 3767.803 | 668.965 | 0.981 | 0.993 |
| 13 | 0.579 | 0.464 | 3.000 | 5.211 | 5.875 | 3749.066 | 669.142 | 0.981 | 0.993 |
| 14 | 0.599 | 0.467 | 3.000 | 5.235 | 5.853 | 3779.763 | 668.878 | 0.981 | 0.993 |
| 15 | 0.574 | 0.473 | 3.000 | 5.229 | 5.885 | 3741.851 | 669.094 | 0.981 | 0.993 |
| 16 | 0.578 | 0.474 | 3.000 | 5.236 | 5.772 | 3748.470 | 669.020 | 0.980 | 0.993 |
| 17 | 0.549 | 0.468 | 3.000 | 5.194 | 5.888 | 3705.619 | 669.495 | 0.980 | 0.994 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurman, S.; Yulia, R.; Irmayanti; Noor, E.; Candra Sunarti, T. The Optimization of Gel Preparations Using the Active Compounds of Arabica Coffee Ground Nanoparticles. Sci. Pharm. 2019, 87, 32. https://doi.org/10.3390/scipharm87040032
Nurman S, Yulia R, Irmayanti, Noor E, Candra Sunarti T. The Optimization of Gel Preparations Using the Active Compounds of Arabica Coffee Ground Nanoparticles. Scientia Pharmaceutica. 2019; 87(4):32. https://doi.org/10.3390/scipharm87040032
Chicago/Turabian StyleNurman, Salfauqi, Ruka Yulia, Irmayanti, Erliza Noor, and Titi Candra Sunarti. 2019. "The Optimization of Gel Preparations Using the Active Compounds of Arabica Coffee Ground Nanoparticles" Scientia Pharmaceutica 87, no. 4: 32. https://doi.org/10.3390/scipharm87040032
APA StyleNurman, S., Yulia, R., Irmayanti, Noor, E., & Candra Sunarti, T. (2019). The Optimization of Gel Preparations Using the Active Compounds of Arabica Coffee Ground Nanoparticles. Scientia Pharmaceutica, 87(4), 32. https://doi.org/10.3390/scipharm87040032