Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity
Abstract
1. Introduction
2. Results and Discussion
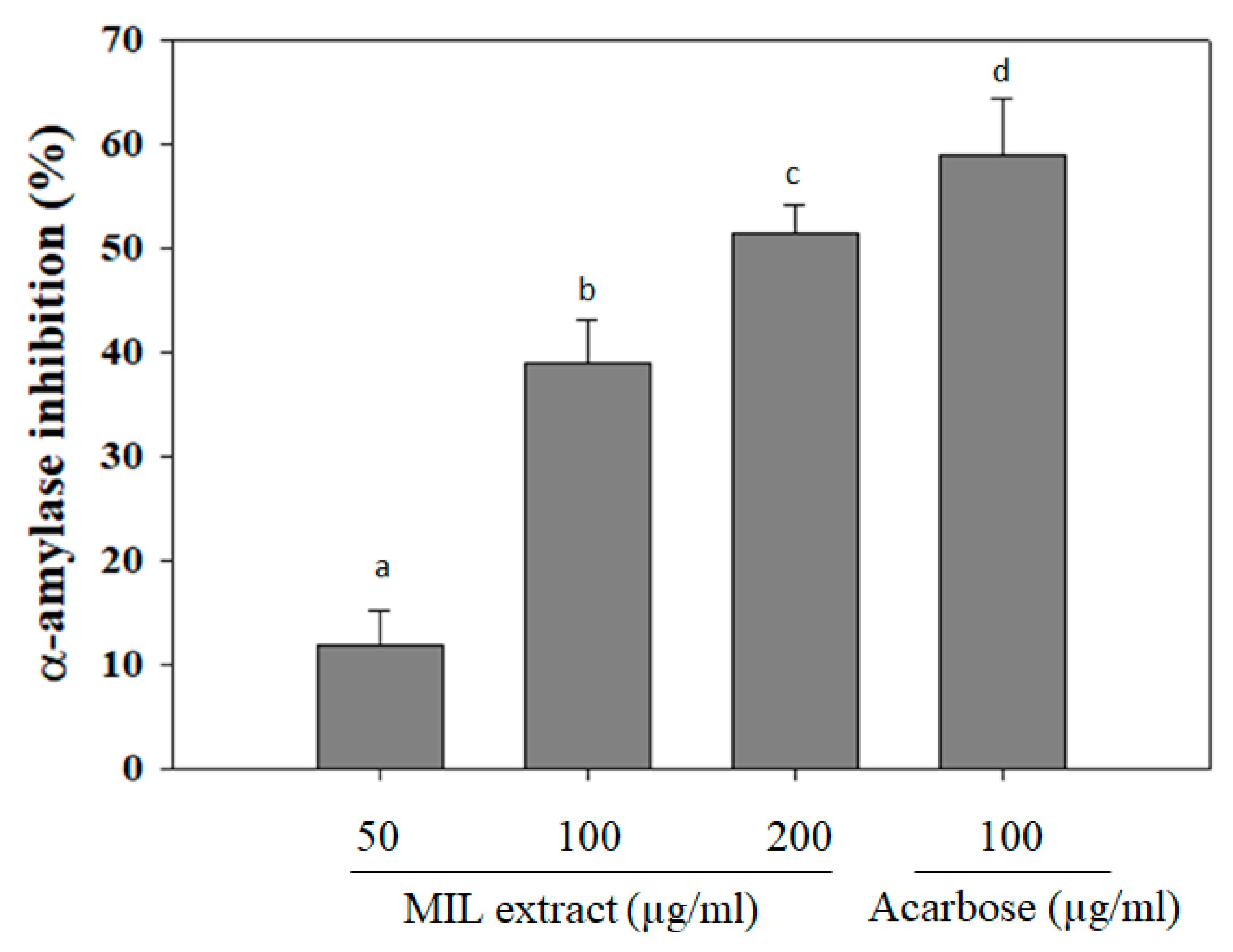
2.1. Alpha-Amylase Inhibitory Activity
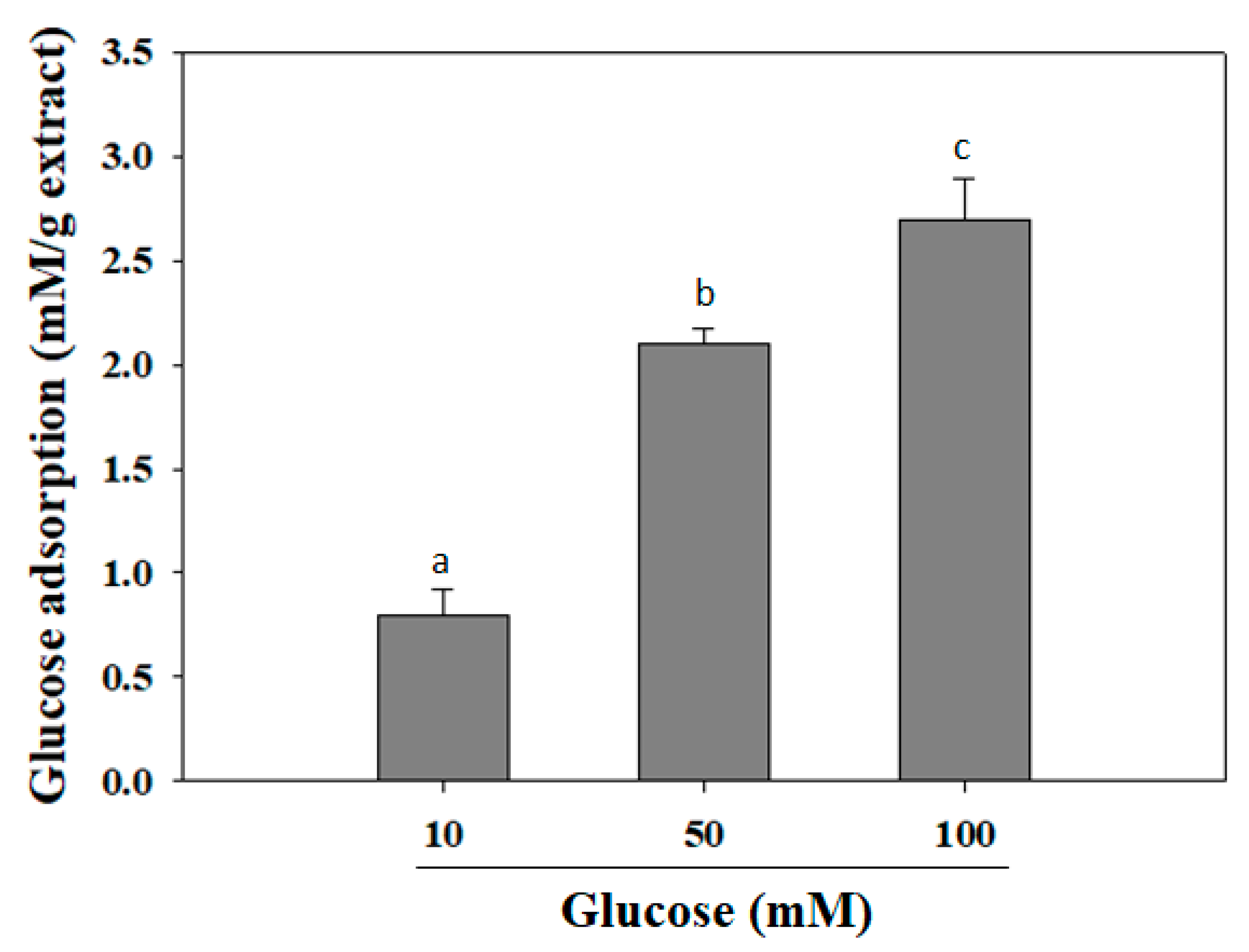
2.2. Glucose Adsorption Capacity
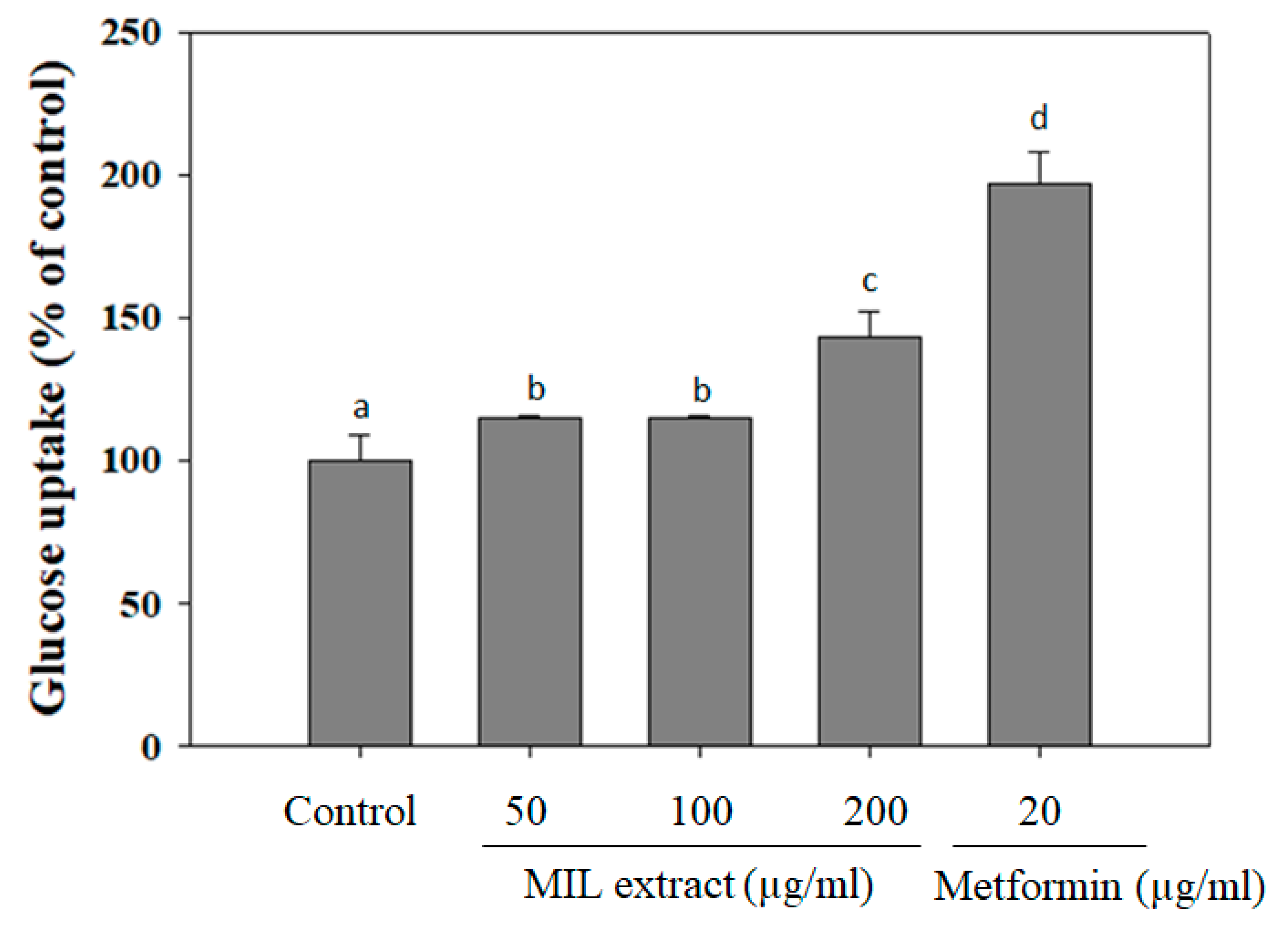
2.3. The Effect MIL Extract on Glucose Uptake
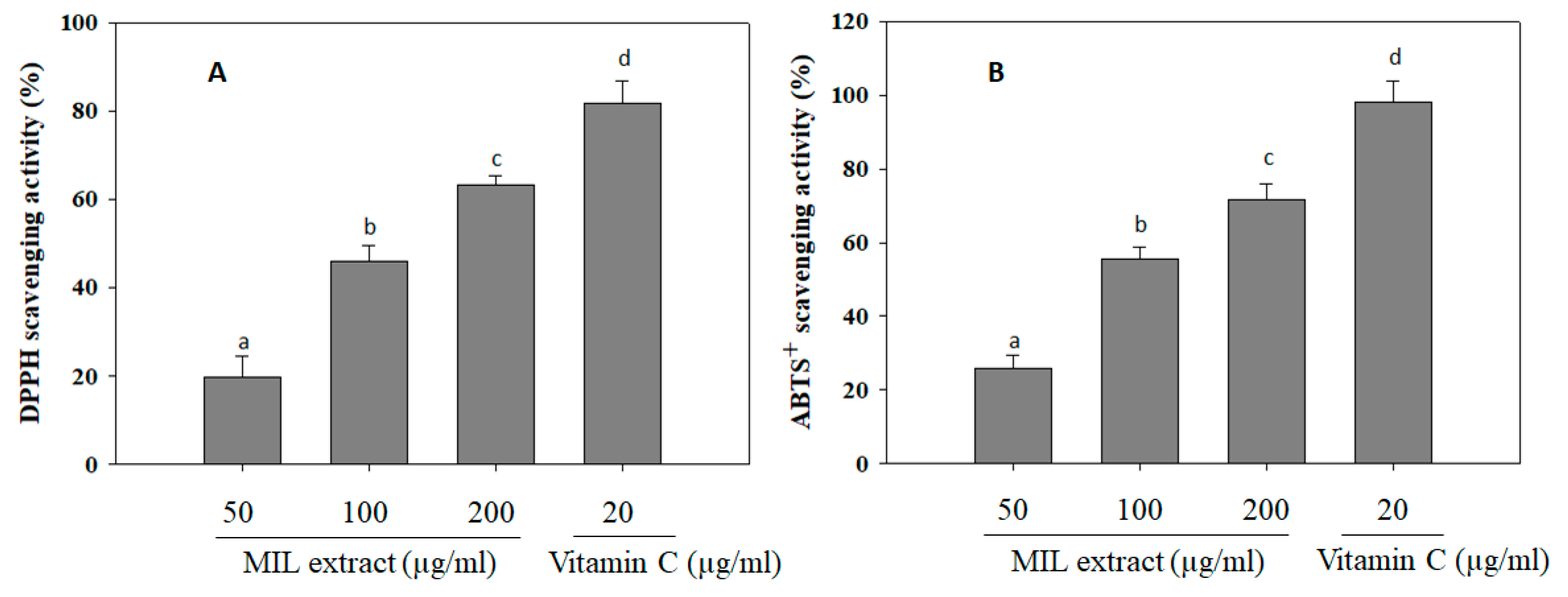
2.4. The Free Radical Scavenging Activities
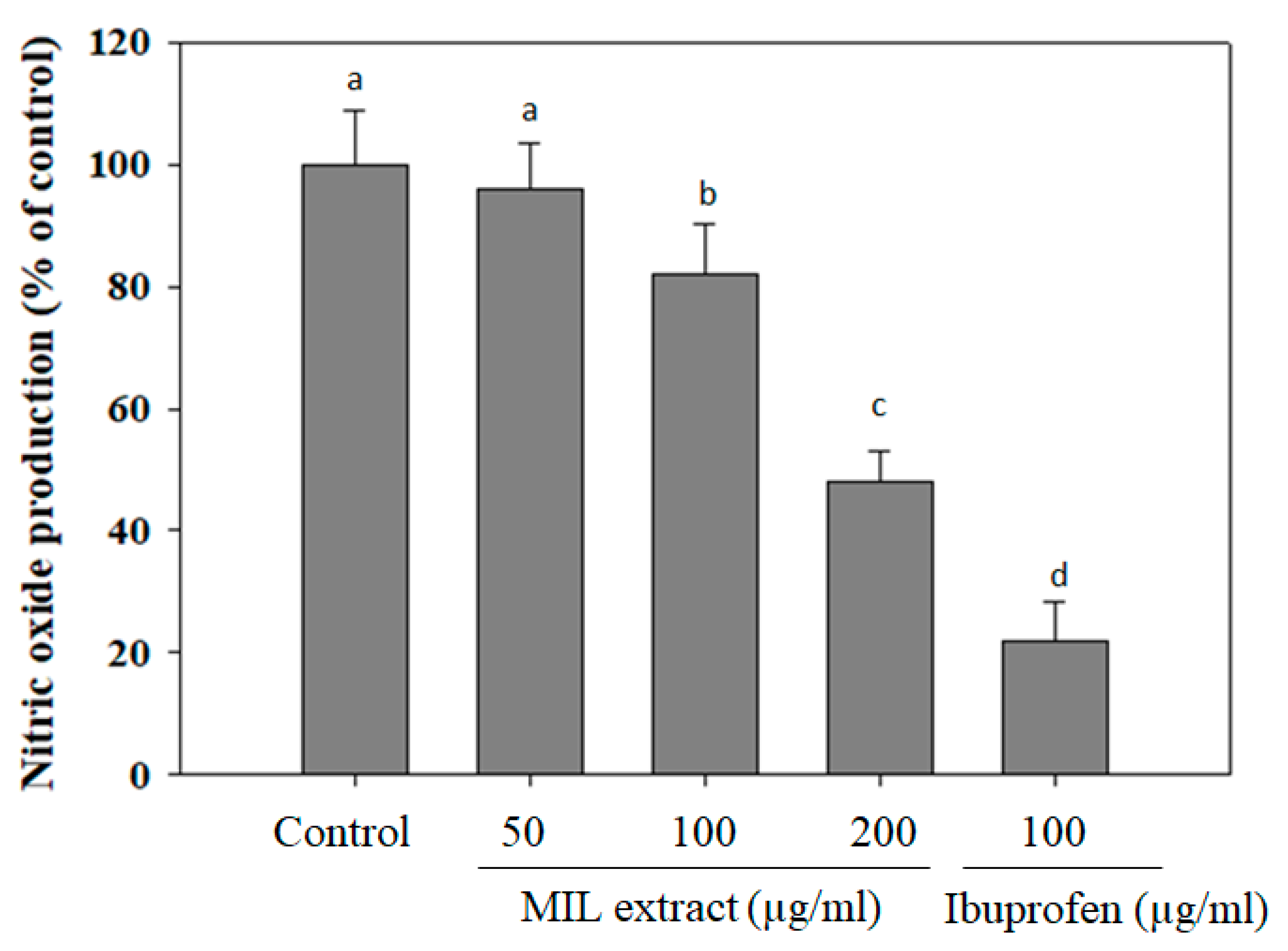
2.5. The Inhibitory Effect of MIL Extract on Nitric Oxide (NO) Production
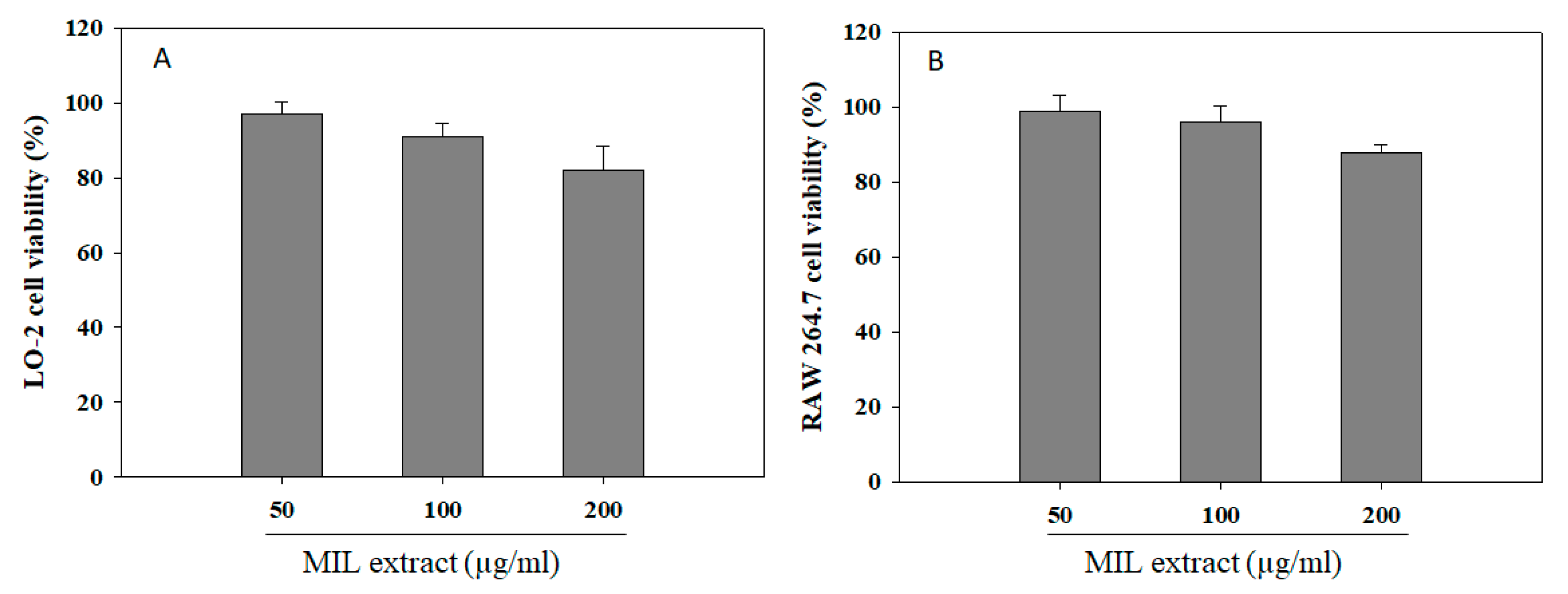
2.6. Effect of Extract on Cell Viability
3. Materials and Methods
3.1. Materials
3.2. Extraction
3.3. Alpha-Amylase Inhibitory Assay
3.4. Determination of Glucose Adsorption Capacity
3.5. Glucose Uptake in Hepatic LO-2 Cells
3.6. 1,1-Diphenyl-2-Picryl-Hydrazyl Assay
3.7. 2,2-Azinobis-3-Ethyl Benzothiazoline-6-Sulfonic Acid (ABTS) Assay
3.8. Nitric Oxide Production Assay
3.9. Cell Viability Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Guillausseau, P.J.; Meas, T.; Virally, M.; Laloi-Michelin, M.; Médeau, V.; Kevorkian, J.P. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab. 2008, 34, S43–S48. [Google Scholar] [CrossRef]
- Medina Escobar, P.; Moser, M.; Risch, L.; Risch, M.; Nydegger, U.E.; Stanga, Z. Impaired glucose metabolism and type 2 diabetes in apparently healthy senior citizens. Swiss Med. Wkly. 2015, 145, w14209. [Google Scholar]
- White, N.H. Long-term outcomes in youth with diabetes mellitus. Pediatr. Clin. N. Am. 2015, 62, 889–909. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid. Based Complement. Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.A.; Patel, M.B.; Patel, R.J.; Parmar, P.K. Mangifera Indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchards recently planted in sicilian rural areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Kumolosasi, E.; Ibrahim, S.A.; Shukri, S.A.; Ahmad, W. Immunostimulant activity of standardised extracts of Mangifera indica leaf and Curcuma domestica rhizome in mice. Trop. J. Pharm. Res. 2018, 17, 77–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, Z.; Liu, E.; Shi, P.; Han, L.; Guo, L.; Gao, X.; Wang, T. Acute and long-term toxicity of mango leaves extract in mice and rats. Evid. Based Complement. Altern. Med. 2014, 2014, 691574. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Dwivedi, S.K.; Swarup, D. Hypoglycaemic potential of Mangifera indica leaves in rats. Int. J. Pharm. 1997, 35, 130–133. [Google Scholar] [CrossRef]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A. Antihyperglycaemic effect of Mangifera indica in rat. Phytother. Res. 1999, 13, 504–507. [Google Scholar] [CrossRef]
- Wadood, N.; Wadood, A.; Shah, S.A. Effect of Mangifera indica on blood glucose and total lipid levels of normal and alloxan diabetic rabbits. Planta Med. 1992, 58, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N. A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes. Comp. Clin. Path. 2016, 25, 1253–1264. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. J. Cell Biochem. 2019, 120, 425–438. [Google Scholar] [CrossRef]
- Kato, E.; Kushibiki, N.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Astilbe thunbergii reduces postprandial hyperglycemia in a type 2 diabetes rat model via pancreatic alpha-amylase inhibition by highly condensed procyanidins. Biosci. Biotechnol. Biochem. 2017, 81, 1699–1705. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef]
- Poojari, S.; Porika, R.; Mamidala, E. Phytochemical analysis and in vitro antidiabetic activities of Physalis Angulata fruit extracts. Natl. J. Integr. Res. Med. 2014, 5, 34–38. [Google Scholar]
- Agarwal, P.; Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. J. Med. Health Sci. 2016, 5, 1–8. [Google Scholar]
- Fang, W.; Wei, C.; Dong, Y.; Tang, X.; Zu, Y.; Chen, Q. The effect on gut microbiota structure of primarily diagnosed type 2 diabetes patients intervened by sancai lianmei particle and acarbose: A randomized controlled trial. J. Clin. Trials 2016, 6, 270. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin. J. Integr. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Perry, J.R.; Ying, W. A review of physiological effects of soluble and insoluble dietary fibers. J. Nutr. Food Sci. 2016, 6, 476. [Google Scholar]
- Bisoi, P.C.; Sahoo, G.; Mishra, S.K.; Das, C.; Das, K.L. Hypoglycemic Effects of Insoluble Fiber Rich Fraction of Different Cereals and Millets. J. Food Process. Technol. 2012, 3, 191. [Google Scholar]
- Tripathy, D.; Carlsson, M.; Almgren, P.; Isomaa, B.; Taskinen, M.R.; Tuomi, T.; Groop, L.C. Insulin secretion and insulin sensitivity in relation to glucose tolerance: Lessons from the Botnia Study. Diabetes 2000, 49, 975–980. [Google Scholar] [CrossRef]
- Schinner, S.; Scherbaum, W.A.; Bornstein, S.R.; Barthel, A. Molecular mechanisms of insulin resistance. Diabet. Med. 2005, 22, 674–682. [Google Scholar] [CrossRef]
- Xia, E.Q.; Zhu, S.S.; He, M.J.; Luo, F.; Fu, C.; Zou, T.B. Marine peptides as potential agents for the management of type 2 diabetes mellitus—A prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef]
- Sagbo, I.J.; van de Venter, M.; Koekemoer, T.; Bradley, G. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. J. Evid. Based Complement. Altern. Med. 2018, 2018, 4170372. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Rao, P.S.; Kalva, S.; Yerramilli, A.; Mamidi, S. Free radicals and tissue damage: Role of antioxidants. Free Rad. Antiox. 2011, 1, 2–7. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ojiako, O.A.; Ogbuji, A.C. Oxidative stress in diabetes mellitus. Int. J. Biol. Chem. 2015, 9, 92–109. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones 2013, 45, 141–147. [Google Scholar]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam Repub. Iran. 2017, 31, 134. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef]
- Pal, P.; Joseph, S.B.; Lucas, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar]
- Adela, R.; Nethi, S.K.; Bagul, P.K.; Barui, A.K.; Mattapally, S.; Kuncha, M.; Patra, C.R.; Reddy, P.N.; Banerjee, S.K. Hyperglycaemia enhances nitric oxide production in diabetes: A study from south indian patients. PLoS ONE 2015, 10, e0125270. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Shimoda, H.; Shan, S.J.; Tanaka, J.; Seki, A.; Seo, J.W.; Kasajima, N.; Tamura, S.; Ke, Y.; Murakami, N. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J. Med. Food 2010, 13, 156–162. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [PubMed]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Edmondson, J.M.; Armstrang, L.S.; Martiner, A.O. A rapid and simple MTT-basedspectrophotometric assay for determining drug sensitivity in monolayercultures. J. Tissue Cult. Methods 1998, 11, 15–17. [Google Scholar] [CrossRef]
- Bale, S.S.; Vernetti, L.; Senutovitch, N.; Jindal, R.; Hegde, M.; Gough, A.; McCarty, W.J.; Bakan, A.; Bhushan, A.; Shun, T.Y.; et al. In vitro platforms for evaluating liver toxicity. Exp. Biol. Med. 2014, 239, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Descotes, J. Immunotoxicity of pharmaceuticals: An introduction. Drug Inf. J. 1996, 30, 271–274. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, D.P.; Singh, M.; Maurya, S.; Srivastava, J.S.; Singh, R.B.; Singh, S.P. Characterization of phenolic compounds in some Indian mango cultivars. Int. J. Food Sci. Nutr. 2004, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Salomon, S.; Sevilla, I.; Betancourt, R.; Romero, A.; Nuevas-Paz, L.; Acosta-Esquijarosa, J. Extraction of mangiferin from Mangifera indica L. leaves using microwaveassisted technique. Emir. J. Food Agric. 2014, 26, 616–622. [Google Scholar] [CrossRef]
- Kanwal, Q.; Hussain, I.; Latif Siddiqui, H.; Javaid, A. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res. 2010, 24, 1907–1914. [Google Scholar] [CrossRef]
- Gebara, S.S.; de Oliveira Ferreira, W.; Ré-Poppi, N.; Simionatto, E.; Carasek, E. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chem. 2011, 127, 689–693. [Google Scholar] [CrossRef]
- Bhutkar, M.A.; Bhise, S.B. In vitro assay of alpha amylase inhibitory activity of some indigenous plants. Int. J. Chem. Sci. 2012, 10, 457–462. [Google Scholar]
- Ou, S.; Kwok, K.C.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Van de Venter, M.; Roux, S.; Bungu, L.C.; Louw, J.; Crouch, N.R.; Grace, O.M.; Maharaj, V.; Pillay, P.; Sewnarian, P.; Bhagwandin, N.; et al. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J. Ethnopharmacol. 2008, 119, 81–86. [Google Scholar] [CrossRef]
- Vo, T.S.; Le, P.U.; Ngo, D.H. The increased gamma-aminobutyric acid content by optimizing fermentation conditions of bacteria from kimchi and investigation of its biological activities. EurAsian J. BioSci. 2018, 12, 369–376. [Google Scholar]
- Vo, T.S.; Le, P.U.; Ngo, D.H. Free radical scavenging and anti-proliferative activities of avocado (Persea americana Mill.) seed extract. Asian Pac. J. Trop. Biomed. 2019, 9, 91–97. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, D.-H.; Ngo, D.-N.; Vo, T.T.N.; Vo, T.S. Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity. Sci. Pharm. 2019, 87, 13. https://doi.org/10.3390/scipharm87020013
Ngo D-H, Ngo D-N, Vo TTN, Vo TS. Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity. Scientia Pharmaceutica. 2019; 87(2):13. https://doi.org/10.3390/scipharm87020013
Chicago/Turabian StyleNgo, Dai-Hung, Dai-Nghiep Ngo, Thi Thanh Nhan Vo, and Thanh Sang Vo. 2019. "Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity" Scientia Pharmaceutica 87, no. 2: 13. https://doi.org/10.3390/scipharm87020013
APA StyleNgo, D.-H., Ngo, D.-N., Vo, T. T. N., & Vo, T. S. (2019). Mechanism of Action of Mangifera indica Leaves for Anti-Diabetic Activity. Scientia Pharmaceutica, 87(2), 13. https://doi.org/10.3390/scipharm87020013




