The Anti-Inflammatory Activity of Nigella sativa Balm Sticks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Balm Stick Preparation
2.3. Animal
2.4. Acute Anti-Inflammatory Test (Carrageenan-Induced Paw Oedema)
2.5. Sub-Acute Anti-Inflammatory Test (Granuloma Pouch Method)
2.5.1. Leucocyte Count
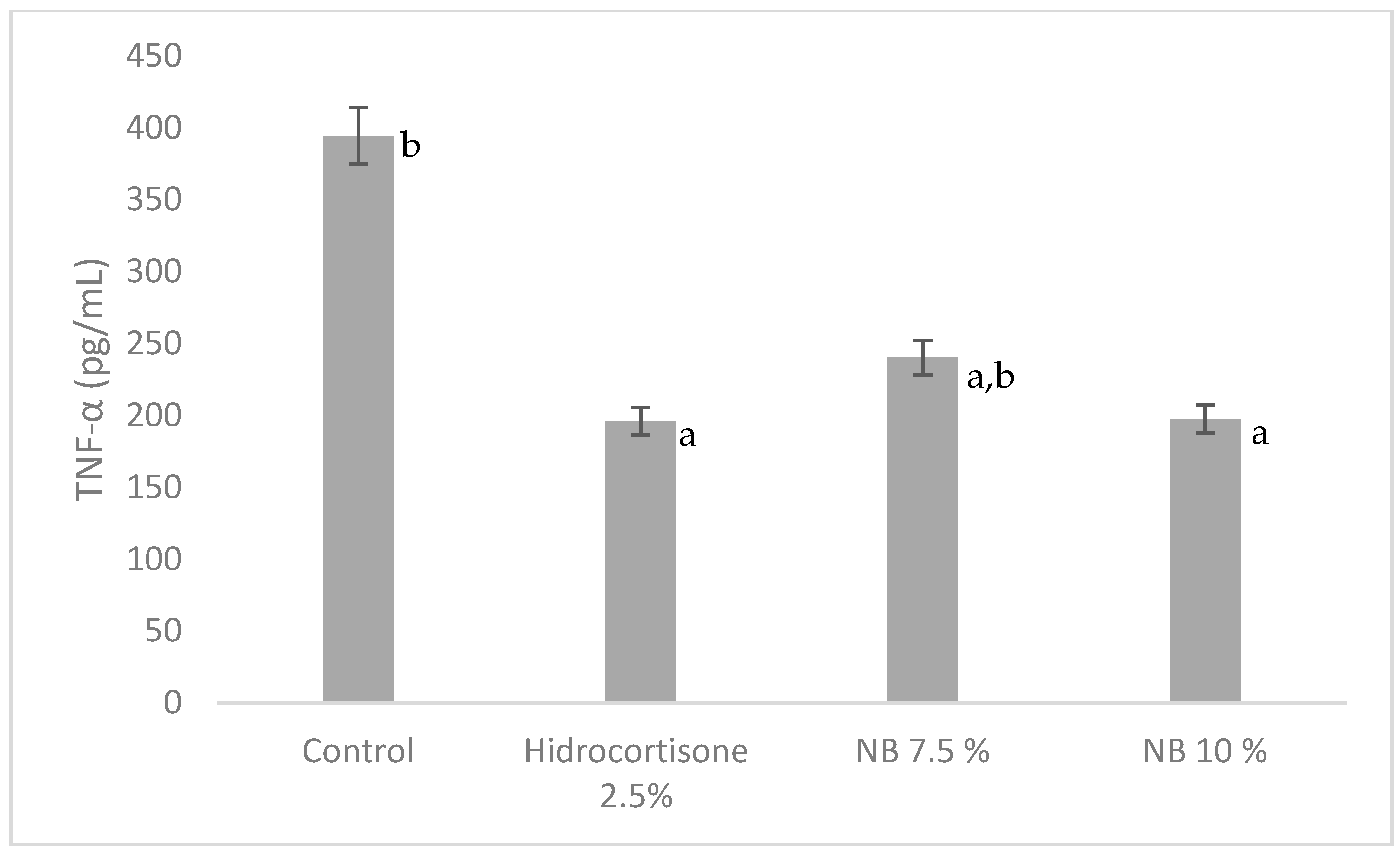
2.5.2. Determination of TNF-α
2.6. Data Analysis
3. Results
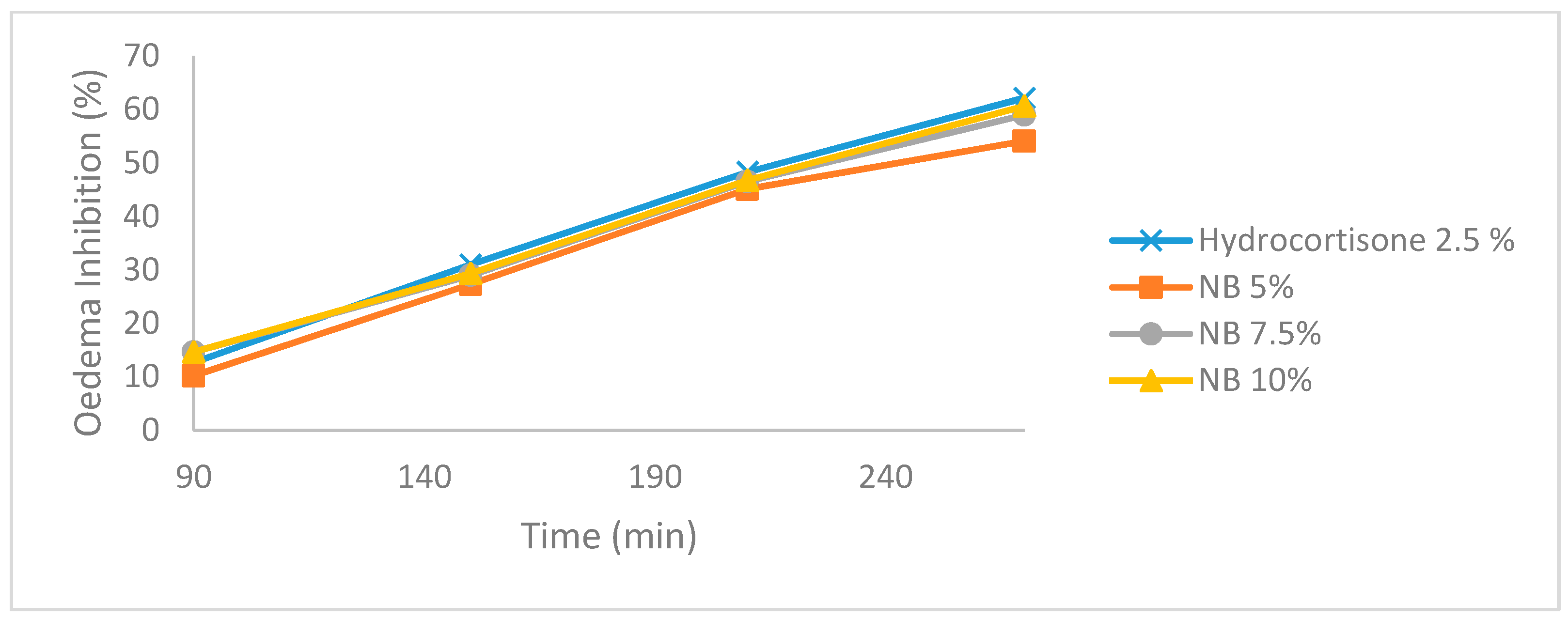
3.1. Carrageenan-Induced Paw Edema
3.2. Sub-Scute Inflammation (Granuloma Pouch)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gilani, A.H.; Jabeen, Q.; Khan, M.A.U. A review of medicinal uses and pharmacological activities of Nigella sativa. Pak. J. Biol. Sci. 2004, 4, 441–451. [Google Scholar]
- Duncker, S.C.; Philippe, D.; Martin-Paschoud, C.; Moser, M.; Mercenier, A.; Nutten, S. Nigella sativa (Black cumin) seed extract alleviates symptoms of allergic diarrhea in mice, involving opioid receptors. PLoS ONE 2012, 7, e39841. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Beg, Z.H. Mitigating role of thymoquinone rich fractions from Nigella sativa oil and its constituents, thymoquinone and limonene on lipidemic-oxidative injury in rats. SpringerPlus 2014, 3, 316. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical characteristics of Nigella seed (Nigella sativa L.) oil as affected by different extraxtion methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Assiri, A.M.A.; Alzohairy, A.M.; Oraby, H.F. Health-promoting value and food applications of black cumin essential oil: An overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Nazmul, H.; Siddiqui, S.A.; Rashid, M.A.; Mahmud, A.Z.; Rahman, M.S.; Rahman, A. The black seed Nigella sativa linnaeus: A study of the antioxidant activity of the essential oil and extacts. J. Nat. Sci. Sustain. Technol. 2010, 7, 37–41. [Google Scholar]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Khaldi, T.; Chekchaki, N.; Boumendjel, M.; Taibi, F.; Abdellaoui, M.; Messarah, M.; Boumendjel, A. Ameliorating effects of Nigella sativa oil on aggravation of inflammation, oxidative stress and cytotoxicity induced by smokeless tobacco extract in an allergic asthma model in Wistar rats. Allergol. Immunopathol. (Madrid) 2018, 46, 472–481. [Google Scholar] [CrossRef]
- Alam, M.; Galav, V. Anti-inflammatory effect and toxicological evaluation of thymoquinone (volatile oil of black seed) on adjuvant-induced arthritis in Wistar rat. Indian J. Life Sci. 2013, 2, 17–22. [Google Scholar]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent Thymoquinone: An overview on the black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. Sci. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Tekeoglu, I.; Dogan, A.; Demiralp, L. Retracted: Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phyther. Res. 2006, 20, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Tuna, H.I.; Babadag, B.; Ozkaraman, A.; Balci Alparslan, G. Investigation of the effect of black cumin oil on pain in osteoarthritis geriatric individuals. Complement. Ther. Clin. Pract. 2018, 31, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J. The effectiveness of Nigella sativa, methotrexate and their combination in the treatment of moderate to severe psoriasis. J. Clin. Exp. Investig. 2014, 5, 521–528. [Google Scholar] [CrossRef]
- Yaman, I.; Durmus, A.S.; Ceribasi, S.; Yaman, M. Effects of Nigella sativa and silver sulfadiazine on burn wound healing in rats. Int. J. Vet. Biomed. Sci. 2010, 55, 619–624. [Google Scholar] [CrossRef]
- Badri, W.; El Asbahani, A.; Miladi, K.; Baraket, A.; Agusti, G.; Nazari, Q.A.; Errachid, A.; Fessi, H.; Elaissari, A. Poly (ε-caprolactone) nanoparticles loaded with indomethacin and Nigella sativa L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Technol. 2018, 46, 234–242. [Google Scholar] [CrossRef]
- Pise, H.N.; Padwal, S.L. Evaluation of anti-inflammatory activity of Nigella sativa: An experimental study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 707–711. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. (Praha) 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Alabi, A.; Ajayi, A.M.; Olooto, W.E.; Emegoakor, C.; Oladunjoye, O.; Obikoya, Y. Antinociceptive and anti-inflammatory properties of a polyherbal extract of Plumbago zeylinica and Capsicum frutescens in rodents. Afr. J. Biomed. Res. 2017, 20, 277–285. [Google Scholar]
- Ajayi, A.M.; Ologe, M.O.; Ben-Azu, B.; Okhale, S.E.; Adzu, B.; Ademowo, O.G. Ocimum gratissimum Linn. Leaf extract inhibits free radical generation and suppressed inflammation in carrageenan-induced inflammation models in rats. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 531–541. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.Q.; Wyatt, P.S.; Bourdon, D.M.; Marino, M.H.; Manning, P.T.; Currie, M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996, 118, 829–838. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Singer, M. Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Cavaillon, J.-M., Singer, M., Eds.; Wiley-VCH: Weinheim, Germany, 2018; Volume 1,2, ISBN 9783527338993. [Google Scholar]
- Vogel, H.G. Drug Discovery and Evaluation Pharmacological Assays; Springer: Berlin, Germany, 2002; ISBN 3540423966. [Google Scholar]
- Menezes, L.D.; Gomes, G.O.; Antônio, J.; Neto, S.; De Mesquita, L.; Ferreira, V.M.; Valero, M. Evaluation of anti-inflammatory and antinociceptive activities of the Austroplenckia populnea extract in topical formulations. Afr. J. Pharm. 2014, 8, 1180–1185. [Google Scholar] [CrossRef]
- Haj-allahyari, S. Anti-inflammatory effect of alcoholic extract of Nigella sativa L. on bovine fibroblast-like synoviocyte and THP-1. Int. J. Contemp. Res. Rev. 2018, 9, 20181–20191. [Google Scholar] [CrossRef]

| No. | Materials | NB 5% (%) | NB 7.5% (%) | NB 10% (%) | Control (%) |
|---|---|---|---|---|---|
| 1 | Nigella sativa oil | 5 | 7.5 | 10 | - |
| 2 | Cera alba | 30 | 30 | 30 | 30 |
| 3 | Adeps lanae | 10 | 10 | 10 | 10 |
| 4 | Setil alcohol | 10 | 10 | 10 | 10 |
| 5 | Butyl Hydroxytoluene | 0.1 | 0.1 | 0.1 | 0.1 |
| 6 | VCO | ad 100 | ad 100 | ad 100 | ad 100 |
| Groups | Edema percentage (%) | ||||
|---|---|---|---|---|---|
| At 30 min | At 90 min | At 150 min | At 210 min | At 270 min | |
| Control | 110 ± 13.69 | 135 ± 13.69 | 170 ± 27.39 | 205 ± 13.69 | 210 ± 11.18 |
| Hydrocortisone 2.5% | 96 ± 8.94 | 96 ± 8.94 a | 77 ± 14.40 a | 58 ± 16.05 a | 10 ± 13.69 a |
| NB 5% | 92 ± 10.95 b | 92 ± 10.95 a,b | 74 ± 16.36 a,b | 51 ± 14.32 a,b | 28 ± 12.55 a |
| NB 7.5% | 100 ± 0 | 100 ± 0 a,b | 85 ± 13.69 a,b | 65 ± 13.69 a,b | 25 ± 0 a,b |
| NB 10% | 96 ± 8.94 b | 96 ± 8.94 a,b | 82 ± 17.54 a,b | 58 ± 16.05 a,b | 15 ± 13.69 a,b |
| Groups | Exudate Volume (mL) | Leucocytes Count (mm3) |
|---|---|---|
| Control | 4.3 ± 0.83 b | 52,525 ± 9787.87 b |
| Hydrocortisone 2.5% | 0.62 ± 0.26 a | 27500 ± 1388.64 a |
| NB 5% | 3.8 ± 0.40 a,b | 41816.67 ± 483.39 a,b |
| NB 7.5% | 1.4 ± 0.68 a | 35641.66 ± 638.29 a,b |
| NB 10% | 0.9 ± 0.49 a | 29650 ± 463.68 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwita, L.P.; Yati, K.; Gantini, S.N. The Anti-Inflammatory Activity of Nigella sativa Balm Sticks. Sci. Pharm. 2019, 87, 3. https://doi.org/10.3390/scipharm87010003
Dwita LP, Yati K, Gantini SN. The Anti-Inflammatory Activity of Nigella sativa Balm Sticks. Scientia Pharmaceutica. 2019; 87(1):3. https://doi.org/10.3390/scipharm87010003
Chicago/Turabian StyleDwita, Lusi Putri, Kori Yati, and Sri Nevi Gantini. 2019. "The Anti-Inflammatory Activity of Nigella sativa Balm Sticks" Scientia Pharmaceutica 87, no. 1: 3. https://doi.org/10.3390/scipharm87010003
APA StyleDwita, L. P., Yati, K., & Gantini, S. N. (2019). The Anti-Inflammatory Activity of Nigella sativa Balm Sticks. Scientia Pharmaceutica, 87(1), 3. https://doi.org/10.3390/scipharm87010003




