Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Plantspecimens
2.2. Extraction and Fractionation
2.3. Preliminary Phytochemical Analysis
2.3.1. Qualitative Determination
2.3.2. Quantitative Determination
2.4. Anticariogenic and Antiperiodontal Activity
2.5. Microorganisms
2.6. Inoculum Preparation for Cariogenic Bacteria
2.7. Inoculum Preparation for Periodontal Bacteria
2.8. Agar Diffusion Methods—Disk and Well
2.9. Minimum Inhibitory Concentration—Minimum Bactericidal Concentration
2.10. Statistical Analysis
3. Results and Discussion
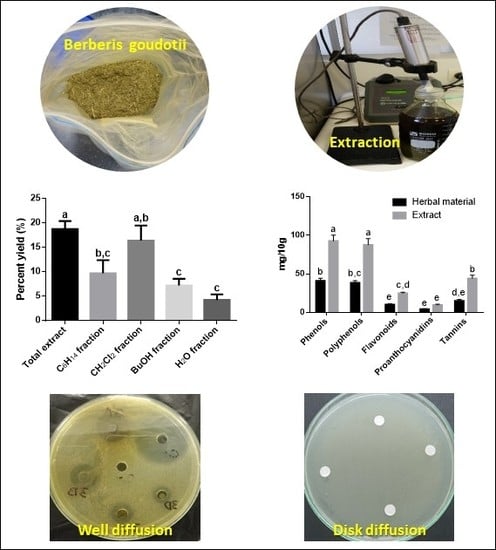
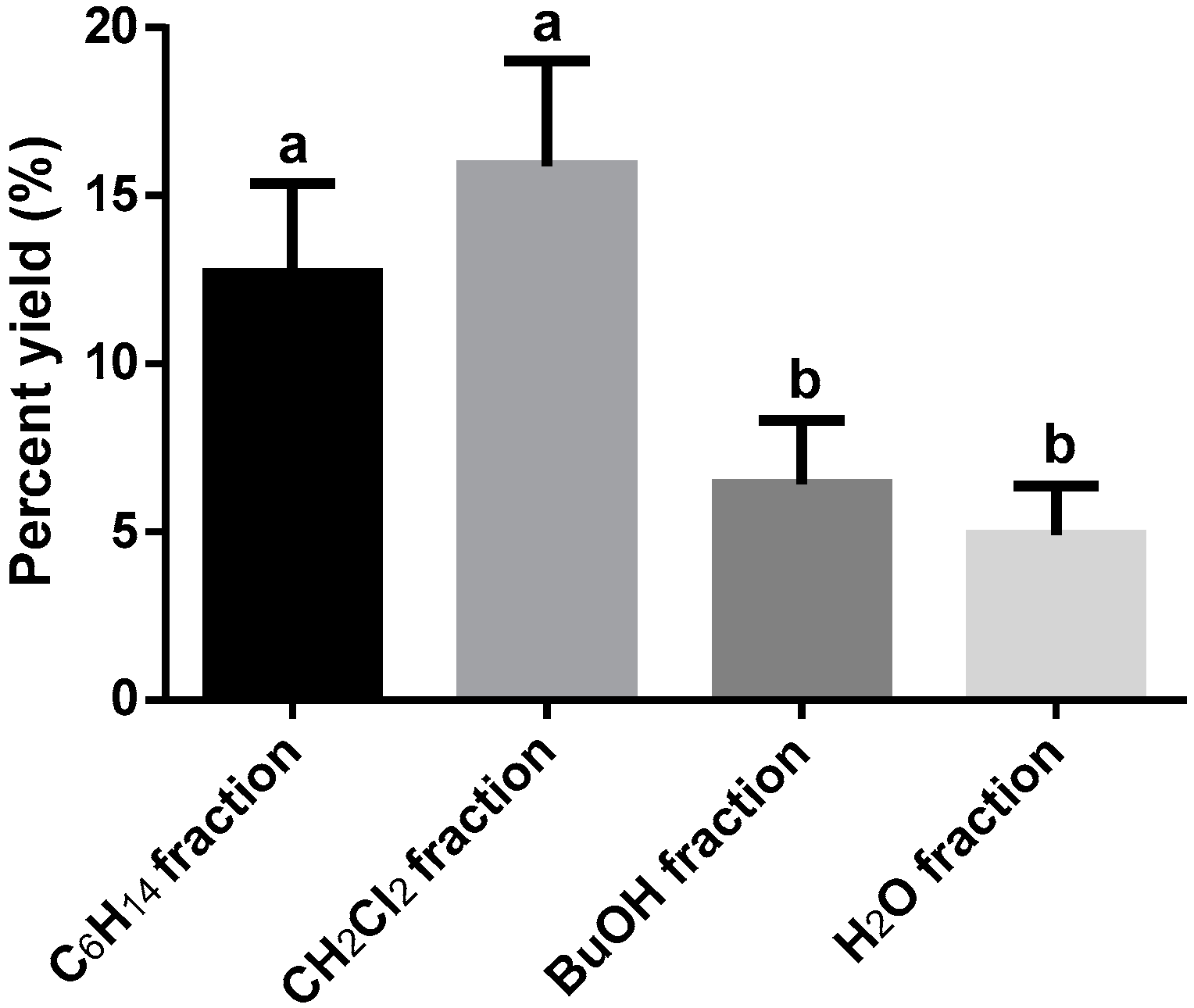
3.1. Extraction and Fractionation
3.2. Preliminary Phytochemical Analysis
3.3. Anticariogenic and Antiperiodontal Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locker, D. Concepts of oral health, disease and the quality of life. In Measuring Oral Health and Quality of Life; Slade, G.D., Ed.; Department of Dental Ecology, School of Dentistry, University of North Carolina: Chapel Hill, NC, USA, 1997; p. 172. [Google Scholar]
- Petersen, P.E. The world oral health report 2003: Continuous improvement of oral health in the 21st century—The approach of the who global oral health programme. Commun. Dent. Oral Epidemiol. 2003, 31 (Suppl. 1), 3–23. [Google Scholar] [CrossRef]
- Sheiham, A. Oral health, general health and quality of life. Bull. World Health Org. 2005, 83, 644. [Google Scholar] [PubMed]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, B. Streptococci of dental plaques. Caries Res. 1968, 2, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Grahnen, H.; Jonsson, G. Lactobacilli and Streptococci in the mouth of children. Caries Res. 1975, 9, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.J.; Adams, B.O.; Fitzgerald, D.B.; Knox, K.W. Cariogenicity of human plaque lactobacilli in gnotobiotic rats. J. Dent. Res. 1981, 60, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Keyes, P.H. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch. Oral Boil. 1960, 1, 304–320. [Google Scholar] [CrossRef]
- Gamboa, F.; Lamby, C.P.; Gómez, O.L.; Chaves, M.; Plazas, L.A.; Arévalo, A.; García, D.A.; Sarralde, A.L. Aspectos ecológicos orales, conocimiento microbiológico y molecular de microorganismos de importancia en caries dental y periodontitis crónica. In Experiencias y Resultados de Investigación en Odontología, 1st ed.; Roa-Molina, N.E., Ed.; Editorial Pontificia Universidad Javeriana: Bogotá, Colombia, 2018; Volume 1, p. 344. [Google Scholar]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe tooth loss: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 20s–28s. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontal. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontal. 1992, 63, 322–331. [Google Scholar] [CrossRef]
- Marinho, V.C.C.; Higgins, J.; Logan, S.; Sheiham, A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Chong, L.Y. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Tellez, M.; Pitts, N.B.; Ekstrand, K.R.; Ricketts, D.; Longbottom, C.; Eggertsson, H.; Deery, C.; Fisher, J.; Young, D.A.; et al. Caries management pathways preserve dental tissues and promote oral health. Commun. Dent. Oral Epidemiol. 2013, 41, e12–e40. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Ekstrand, K.R. International caries detection and assessment system (icdas) and its international caries classification and management system (iccms)—Methods for staging of the caries process and enabling dentists to manage caries. Commun. Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.P.; Haffajee, A.D.; Socransky, S.S. Microbiological goals of periodontal therapy. Periodontology 2000 2006, 42, 180–218. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Signoretto, C.; Corrocher, G.; Pardo, A.; Pighi, J.; Rovera, A.; Caccuri, F.; Nocini, P.F. A topical desiccant agent in association with ultrasonic debridement in the initial treatment of chronic periodontitis: A clinical and microbiological study. New Microbial. 2015, 38, 393–407. [Google Scholar]
- Bracke, J.; Basara, M.; Savord, E.; Dunaway, A.; Watkins, M. Pilot evaluation of a simple adjunctive method for improved removal of oral biofilm during conventional scaling and root planing therapy. J. Boil. Regul. Homeost. Agents 2015, 29, 6–9. [Google Scholar]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Invest. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Laugisch, O.; Ramseier, C.A.; Salvi, G.E.; Hagi, T.T.; Burgin, W.; Eick, S.; Sculean, A. Effects of two different post-surgical protocols including either 0.05% chlorhexidine herbal extract or 0.1% chlorhexidine on post-surgical plaque control, early wound healing and patient acceptance following standard periodontal surgery and implant placement. Clin. Oral Invest. 2016, 20, 2175–2183. [Google Scholar]
- Matarese, G.; Ramaglia, L.; Cicciu, M.; Cordasco, G.; Isola, G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-year randomized controlled clinical trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A review of pre-clinical and clinical studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [PubMed]
- Henley-Smith, C.J.; Steffens, F.E.; Botha, F.S.; Lall, N. Predicting the influence of multiple components on microbial inhibition using a logistic response model—A novel approach. BMC Complement. Altern. Med. 2014, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Milovanova-Palmer, J.; Pendry, B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J. Herb. Med. 2018, 12, 33–48. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of Bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
- Hwang, J.K.; Chung, J.Y.; Baek, N.I.; Park, J.H. Isopanduratin a from Kaempferia pandurata as an active antibacterial agent against cariogenic streptococcus mutans. Int. J. Antimicrob. Agents 2004, 23, 377–381. [Google Scholar] [CrossRef]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in vitro investigation of indigenous south african medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef]
- Gamboa, F.; Muñoz, C.-C.; Numpaque, G.; Sequeda-Castañeda, L.G.; Gutierrez, S.J.; Tellez, N. Antimicrobial activity of Piper marginatum Jacq and Ilex guayusa Loes on microorganisms associated with periodontal disease. Int. J. Microbiol. 2018, 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Paniagua Zambrana, N.Y.; Romero, C.; Hart, R.E. Astonishing diversity—The medicinal plant markets of bogotá, colombia. J. Ethnobiol. Ethnomed. 2018, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Bernal, H.Y.; García-Martínez, H.; Quevedo-Sánchez, G.F. Pautas Para el Conocimiento, Conservación y uso Sostenible de las Plantas Medicinales Nativas en Colombia; Ministerio de Ambiente, Vivienda y Desarrollo Territorial e Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2011; p. 232.
- García-Barriga, H. Berberis rigidifolia. In Flora Medicinal de Colombia. Botánica Médica; Imprenta Nacional: Bogotá, Colombia, 1974; Volume I, pp. 314–317. [Google Scholar]
- Marín, C.; Parra, S. Bitácora de Flora: Guía Visual de Plantas de Páramos en Colombia; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2015.
- Sequeda-Castañeda, L.G.; Célis, C.; Gutiérrez, S.; Luengas-Caicedo, P.E. Berberis rigidifolia Kunth. (Berberidaceae) Colombian endemic plant. PhOL 2016, 2016, 134–138. [Google Scholar]
- Díaz, M.A.; Moreno-Castro, J.D.; Sequeda-Castañeda, L.G. Berberis Goudotii—Pictures; Editorial PUJ: Bogotá, Colombia, 2014. [Google Scholar]
- Method 2.9.35 —Powder fineness. Available online: https://www.drugfuture.com/Pharmacopoeia/EP7/DATA/20934E.PDF (accessed on 4 February 2017).
- Parimelazhagan, T. Pharmacological Assays of Plant-Based Natural Products; Springer International Publishing: Basel, Switzerland, 2016; Volume 71. [Google Scholar]
- Sharapin, N. Fundamentos de Tecnología de Productos Fitoterapéuticos; Convenio Andrés Bello: Santafé de Bogotá, Colombia, 2000. [Google Scholar]
- WHO. Quality Control Methods for Herbal Materials; World Health Organization: Geneva, Switzerland, 2011; p. 187. [Google Scholar]
- Carvajal-Rojas, L.; Hata-Uribe, Y.; Sierra-Martínez, N.; Rueda-Niño, D. Análisis fitoquímico preliminar de hojas, tallos y semillas de cupatá (Strychnos schultesiana krukoff). Colomb. For. 2009, 12, 161–170. [Google Scholar] [CrossRef]
- Domínguez, X.A. Métodos de Investigación Fitoquímica; Limusa: Ciudad de México, México, 1988. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer-Verlag Berlin Heidelberg: Munich, Germany, 1996. [Google Scholar]
- Lock, O.R. Invetigacion Fitoquimica. Métodos en el Estudio de Productos Naturales; Fondo Editorial de la Pontifiicia Universidad Católica del Perú: Lima, Perú, 2016. [Google Scholar]
- ANVISA. Farmacopeia brasileira—Taninos totais. In Volume 2—Monografias; Agência Nacional de Vigilância Sanitária—Fundação Oswaldo Cruz: Brasília, Brasil, 2010; p. 899. [Google Scholar]
- Luengas-Caicedo, P.E.; Braga, F.C.; Brandão, G.C.; Oliveira, A.B. Seasonal and intraspecific variation of flavonoids and proanthocyanidins in Cecropia glaziovi Sneth. leaves from native and cultivated specimens. Z. Naturforsch. C 2007, 62, 701–709. [Google Scholar] [CrossRef]
- Isaza, J.H.; Veloza, L.A.; Guevara, C.A.; Ávila, Y.P.; Díaz, O. A spectrometric approach to the phenoltotals in species of melastomataceae familiy. Actual. Biol. 2005, 27, 75–79. [Google Scholar]
- Palomino, L.R.; García, C.M.; Gil, J.H.; Rojano, B.A.; Durango, D.L. Determination of phenolic content and evaluation of antioxidant activity of propolis from antioquia (colombia). Vitae 2009, 16, 388–395. [Google Scholar]
- Luengas-Caicedo, P.E. Contribuição para a Padronização de Extratos de Folhas de Cecropia Glaziovii Snethl: Estudos de Variação Sazonal e Intra-Específica de Flavonóides e Proantocianidinas, de Metodologias de Extração e de Atividade Vasorelaxante; Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2005. [Google Scholar]
- Hiermann, A.; Karting, T.; Azzam, S. Ein beitrag zur quantitativen bestimmung der procyanidine in crataegus. Sci. Pharm. 1986, 54, 331–337. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Cavalieri, S.J.; Rankin, I.D.; Harbeck, R.J.; Sautter, R.L.; McCarter, Y.S.; Sharp, S.E.; Ortez, J.H.; Spiegel, C.A. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Washington, DC, USA, 2009; p. 240. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard—Eighth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- CLSI. Performance Standard for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007.
- Ramirez, L.S.; Díaz, H.E. Actividad antibacteriana de extractos y fracciones del ruibardo (Rumex conglomeratus). Sci. Tech. 2007, 23. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the Minimal Inhibitory Concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Bonilla, D.M.; Mendoza, Y.; Moncada, C.E.; Murcia, O.; Rojas, Á.P.; Calle, J.; Pinzón, R.; Neiro, L. Efecto del aceite esencial de Rosmarinus officinalis sobre Porphyromonas gingivalis cultivada in vitro. Rev. Colomb. Cienc. Quím. Farm. 2016, 45, 275. [Google Scholar] [CrossRef]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999.
- Franco-Ospina, L.A.; Matiz-Melo, G.E.; Pájaro-Bolívar, I.B.; Gómez-Estrada, H.A. In vitro antibacterial activity of extracts and fractions of Physalis peruviana L. and Caesalpinia pulcherrima (L.) Swartz. Bol. Latinoam. Caribe. Plant. Med. Aromat. 2013, 12, 230–237. [Google Scholar]
- Lesaffre, E.; Feine, J.; Leroux, B.; Declerck, D. Statistical and Methodological Aspects of Oral Health Research; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Infostat. Available online: http://www.infostat.com.ar/ (accessed on 9 August 2018).
- Ekstrom, C.; Sørensen, H. Introduction to Statistical Data Analysis for the Life Sciences; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jones, D. Pharmaceutical Statistics; Pharmaceutical Press: London, UK, 2002. [Google Scholar]
- Belwal, T.; Giri, L.; Bhatt, I.D.; Rawal, R.S.; Pande, V. An improved method for extraction of nutraceutically important polyphenolics from Berberis jaeschkeana c.K. Schneid. Fruits. Food Chem. 2017, 230, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Zovko Koncic, M.; Kremer, D.; Karlovic, K.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. 2010, 48, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Azuola, R.; Vargas, P. Extracción de Sustancias Asistida por Ultrasonido (EUA). Tecnol. Marcha 2007, 20, 30–40. [Google Scholar]
- Londoño-Londoño, J.A. Aprovechamiento de residuos de la agroindustria de cítricos: Extracción y caracterización de flavonoides. In Cítricos: Cultivo, Poscosecha e Industrialización; Corporación Universitaria Lasallista: Caldas, Antioquia, 2011; pp. 395–416. [Google Scholar]
- Vardanega, R.; Santos, D.T.; Meireles, M.A.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88–95. [Google Scholar]
- Chan, C.H.; See, T.Y.; Yusoff, R.; Ngoh, G.C.; Kow, K.W. Extraction of bioactives from Orthosiphon stamineus using microwave and ultrasound-assisted techniques: Process optimization and scale up. Food Chem. 2017, 221, 1382–1387. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Lucchessi, M.E.; Abdelly, C.; Magné, C. Ultrasound-assisted extraction: Effect of extraction time and solvent power on the levels of polyphenols and antioxidant activity of Mesembryanthemum edule L. Aizoaceae shoots. Trop. J. Pharm. Res. 2012, 11. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalan, E.B.; Micol, V.; Garcia-Perez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mo, K.; Fei, S.; Zu, Y.; Yang, L. Efficient approach for the extraction of proanthocyanidins from Cinnamomum Longepaniculatum leaves using ultrasonic irradiation and an evaluation of their inhibition activity on digestive enzymes and antioxidant activity in vitro. J. Sep. Sci. 2017, 40, 3100–3113. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Qadir, S.A.; Kwon, M.C.; Han, J.G.; Ha, J.H.; Chung, H.S.; Ahn, J.; Lee, H.Y. Effect of different extraction protocols on anticancer and antioxidant activities of Berberis koreana bark extracts. J. Biosci. Bioeng. 2009, 107, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.S.H. Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Bonesi, M.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Berberis aetnensis and b. Libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer’s disease and antioxidant activity. J. Pharm. Pharmacol. 2013, 65, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Alamzeb, M.; Khan, M.R.; Mamoon Ur, R.; Ali, S.; Khan, A.A. Isolation, structure elucidation and enzyme inhibition studies of a new hydroxy ester and other compounds from berberis jaeschkeana schneid stem. Nat. Prod. Res. 2015, 29, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, D.; Paredes, M.; Ramos, D.; Gonzalez, K.; Díaz, R.; Núñez, D. Aqueous and methanol extracts of Berberis darwinii h. (Berberidaceae) inhibit innate celular responses in human monocytes in vitro treated. Bol. Latinoam. Caribe. Plant. Med. Aromat. 2014, 13, 81–91. [Google Scholar]
- Quevedo, R.; Antolinez, S.; Moreno, B.; Fajardo, V. Tabienina a: Un nuevo alcaloide aporfinabencilisoquinolina oxidado. Sci. Tech. 2007, 13, 167–169. [Google Scholar]
- Quevedo, R.; Núñez, L.; Moreno, B. A rare head–head binding pattern in bisbenzylisoquinoline alkaloids. Nat. Prod. Res. 2011, 25, 934–938. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Kaushik, N. Phytochemical and pharmacological studies in genus Berberis. Phytochemistry Reviews 2013, 11, 523–542. [Google Scholar] [CrossRef]
- Khan, I.; Najeebullah, S.; Ali, M.; Shinwari, Z. Phytopharmacological and ethnomedicinal uses of the genus Berberis (Berberidaceae): A review. Trop. J. Pharm. Res. 2016, 15, 2047–2057. [Google Scholar] [CrossRef]
- Manosalva, L.; Mutis, A.; Díaz, J.; Urzúa, A.; Fajardo, V.; Quiroz, a. Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS. Bol. Latinoam. Caribe. Plant. Med. Aromat. 2014, 13, 323–334. [Google Scholar]
- Martínez, J.L.; Russo, A.; Vinet, R.; Jaimes, L.; Russo, L.; Laurido, C. Berberis chilensis gillies ex Hook: Alkaloids and pharmacologiacl activities. Pharmacologyonline 2018, 1, 58–67. [Google Scholar]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M. Phytochemistry and pharmacology of Berberis species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar] [PubMed]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-Gharaghoshi, H.; Rafieian-Kopaei, M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017, 20, 569–587. [Google Scholar]
- Sabir, S.; Tahir, K.; Rashid, N.; Naz, S.; Masood, B.; Shah, M.A. Phytochemical and antioxidant studies of Berberis lycium. Pak. J. Pharm. Sci. 2013, 26, 1165–1172. [Google Scholar]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla g. Forst. berries in relation to light intensity and fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-Assisted Extraction (MAE) conditions using polynomial design for improving antioxidant phytochemicals in Berberis asiatica Roxb. Ex DC. Leaves. Ind. Crops Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Furrianca, M.C.; Alvear, M.; Zambrano, T.; Fajardo, V.; Salazar, L.A. Phytochemical constituents of the root of Berberis microphylla. Asian J. Pharm. Clin. Res. 2017, 10, 225–227. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, S. Perspective on plant products as antimicrobials agents: A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Paredes, F.; Roca, J.J. Acción de los antibióticos. Offarm 2004, 23, 116–124. [Google Scholar]
- Molina-López, J. Terapéutica. Drogas Antibacterianas. Available online: http://www.facmed.unam.mx/deptos/microbiologia/bacteriologia/terapeutica.html (accessed on 15 November 2018).
- Olsson-Liljequist, B.; Nord, C.E. Methods for susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 1994, 18, S293–S296. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman. Studies in natural products chemistry. In Methods for Evaluating Antimicrobial Activity of Plant Extracts; Elsevier Science: Amsterdam, The Netherlands, 2016; Volume 47. [Google Scholar]
- CYTED. Manual de técnicas de investigación. Proyecto X-1: Búsqueda de principios bioactivos en plantas de la región. In Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo; Comité científico: Madrid, España, 1995. [Google Scholar]
- Ramirez, L.S.; Marin-Castaño, D. Metodologias para evaluar in vitro la actividad antibacteriana de compuestos de origen vegetal. Sci. Tech. 2009, XV, 263–268. [Google Scholar]
- Rios, J.L.; Recio, M.C.; Villar, A. Screening methods for natural products with antimicrobial activity: A review of the literature. J. Ethnopharmacol. 1988, 23, 127–149. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry; OUP Oxford: Oxford, UK, 2010. [Google Scholar]
- Valgas, C.; Souza, S.M.d.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Ortíz-Ardila, A.E.; Correa-Cuadros, J.P.; Celis-Zambrano, C.A.; Rodríguez-Bocanegra, M.X.; Robles-Camargo, J.; Sequeda-Castañeda, L.G. Antioxidant and antimicrobial capacity Cecropia mutisiana Mildbr. (cecropiaceae) leave extracts. Emir. J. Food Agric. 2017, 29, 25–35. [Google Scholar] [CrossRef]
- Das, K.; Tiwari, R.K.S.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agents: Current methods and future trends. J. Med. Plant. Res. 2010, 4, 104–111. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Othman, M.; Loh, H.S.; Wiart, C.; Khoo, T.J.; Lim, K.H.; Ting, K.N. Optimal methods for evaluating antimicrobial activities from plant extracts. J. Microbiol. Methods 2011, 84, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.A.M.D.; Pinto, F.G.d.S.; Fruet, T.K.; Jorge, T.C.M.; Moura, A.C.d. Comparação de métodos para avaliação da atividade antimicrobiana e determinação da concentração inibitória mínima (cim) de extratos vegetais aquosos e etanólicos. Arq. Inst. Biol. 2014, 81, 218–225. [Google Scholar] [CrossRef]
- Bernatova, S.; Samek, O.; Pilat, Z.; Sery, M.; Jezek, J.; Jakl, P.; Siler, M.; Krzyzanek, V.; Zemanek, P.; Hola, V.; et al. Following the mechanisms of bacteriostatic versus bactericidal action using Raman spectroscopy. Molecules 2013, 18, 13188–13199. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Padgham, C.; Hedges, A. Clinical pharmacology & therapeutics. In Proceedings of the Plenary Lectures Symposia and Therapeutic Sessions of the First World Conference on Clinical Pharmacology & Therapeutics, London, UK, 3–9 August 1980. [Google Scholar]
- Liu, Z. Preparation of botanical samples for biomedical research. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Haloui, T.; Farah, A.; Balouiri, M.; Chraibi, M.; Fadil, M.; Benbrahim, K.F.; Alaoui, A.B. Bacteriostatic and bactericidal profile of leaves and twigs essential oils of moroccan Pistacia lentiscus L. J. Appl. Pharm. Sci. 2015, 5, 50–53. [Google Scholar] [CrossRef]
- Vieira, D.R.P.; Amaral, F.M.M.; Maciel, M.C.G.; Nascimento, F.F.R.F.; Libério, A.S. Plantas e constituintes químicos empregados em odontologia: Revisão de estudos etnofarmacológicos e de avaliação da atividade antimicrobiana in vitro em patógenos orais. Rev. Bras. Plant. Med. 2014, 16, 135–167. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 15. [Google Scholar] [CrossRef]

| Metabolite Class (Test) | Positive, If | Result | Comments |
| Alkaloids (Dragendorff′s test) | Precipitated orange | + | Little precipitation |
| Coumarins (Fluorescence test) | Blue or green fluorescence | − | − |
| Cardiotonic glycosides (Kedde′s test) | Blue or violet color | − | − |
| Steroids and triterpenes (Liebermann-Burchard test) | Blue or green color Purple color | + | Purple, possibly triterpenes |
| Flavonoids (Shinoda′s test) | Red, orange, or violet color | + | Red, possibly flavones |
| Phenols (Ferric chloride test) | Green, blue, or black color | + | Blue, possibly derived from pyrogallol |
| Naphtho- and anthraquinones (Bornträger-Kraus′s test) | Intense red color | − | − |
| Saponins (Foam and hemolysis test) | Formation of stable foam. Hemolysis | − | − |
| Sesquiterpene lactones (Ferric hydroximate test) | Violet or coffee color | − | − |
| Tannis (Gelatine-salt test) | White precipitate | + | Precipited |
| Phenolic Metabolite Quantification | |||
| Metabolite Class | Aerial Parts (P) | Extract (E) | P/E Relation |
| Phenols (mg GA/10 g) | 41.1 ± 3.3 a,y | 92.5 ± 7.7 b,z | 2.3 |
| Polyphenols (mg PG/10 g) | 38.4 ± 2.9 a,y | 87.7 ± 8.1 b,z | 2.3 |
| Flavonoids (mg QE/10 g) | 10.1 ± 1.2 a,u | 24.8 ± 1.5 b,x | 2.5 |
| Proanthocyanidins (mg CI/10 g) | 4.3 ± 0.5 a,t | 9.5 ± 1.2 b,u | 2.2 |
| Tannins (mg PG/10 g) | 15.1 ± 1.7 a,v | 44.1 ± 4.3 b,y | 2.9 |
| Microorganism | Total Extract (µg/µL) | Hexane Fraction (µg/µL) | Butanol Fraction (µg/µL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 | 25 | 1 | 50 | 25 | 1 | 50 | 25 | 1 | |
| L. acidophilus | - | - | - | - | - | - | - | - | - |
| L. casei | - | - | - | - | - | - | - | - | - |
| S. mutans | 51.2 ± 6.3 a,b | 36.2 ± 5.7 b,c,d,e,f | - | - | - | - | - | - | - |
| S. sobrinus | 38.8 ± 5.7 a,b,c,d,e | 23.5 ± 3.2 e,f,g | - | - | - | - | - | - | - |
| F. nucleatum | 40.6 ± 6.4 a,b,c,d | 28.3 ± 4.5 d,e,f,g | 19.7 ± 3.8 g | 28.8 ± 3.0 t,u,v | 20.8 ± 1.5 v,x,y | 12.9 ± 2.5 y,z | 33.8 ± 2.0 t | 30.0 ± 3.8 t,u | 21.5 ± 3.6 u,v,x,y |
| P. gingivalis | 46.5 ± 4.5 a,b,c | 25.1 ± 3.1 d,e,f,g | 17.9 ± 1.9 g | 22.3 ± 1.2 u,v,x | 16.1 ± 2.4 x,y,z | 7.9 ± 1.6 z | 28.1 ± 2.2 t,u,v | 22.9 ± 2.4 u,v,x | 16.0 ± 2.6 x,y,z |
| P. intermedia | 53.3 ± 6.2 a | 32.3 ± 2.4 c,d,e,f,g | 22.1 ± 4.1 f,g | 35.2 ± 3.5 s,t | 24.6 ± 1.5 u,v,x | 17.2 ± 0.9 x,y | 43.3 ± 2.9 s | 28.1 ± 1.6 t,u,v | 19.1 ± 2.7 x,y |
| Microorganism | Total Extract (µg/µL) | Hexane Fraction (µg/µL) | Butanol Fraction (µg/µL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |||||||
| Agar 1 | Broth 2 | Agar 1 | Broth 2 | Agar 1 | Broth 2 | Agar 1 | Broth 2 | Agar 1 | Broth 2 | Agar 1 | Broth 2 | |
| Lactobacillus acidophilus | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Lactobacillus casei | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Streptococcus mutans | 4 | 1 | 4 a | 1 a | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Streptococcus sobrinus | 4 | 1 | 4 a | 1 a | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Fusobacterium nucleatum | 1 | 0.12 | 1 b | 0.12 b | 1 | 1 | 1 a | 1 a | 1 | 0.12 | 1 b | 0.12 b |
| Porphyromonas gingivalis | 1 | 0.12 | 1 b | 0.12 b | 1 | 1 | 1 a | 1 a | 1 | 0.12 | 1 b | 0.12 b |
| Prevotella intermedia | 1 | 0.12 | 1 b | 0.12 b | 1 | 1 | 1 a | 1 a | 1 | 0.12 | 1 b | 0.12 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeda-Castañeda, L.G.; Muñoz-Realpe, C.C.; Celis-Zambrano, C.A.; Gutiérrez-Prieto, S.J.; Luengas-Caicedo, P.E.; Gamboa, F. Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities. Sci. Pharm. 2019, 87, 2. https://doi.org/10.3390/scipharm87010002
Sequeda-Castañeda LG, Muñoz-Realpe CC, Celis-Zambrano CA, Gutiérrez-Prieto SJ, Luengas-Caicedo PE, Gamboa F. Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities. Scientia Pharmaceutica. 2019; 87(1):2. https://doi.org/10.3390/scipharm87010002
Chicago/Turabian StyleSequeda-Castañeda, Luis G., Camila C. Muñoz-Realpe, Crispín A. Celis-Zambrano, Sandra J. Gutiérrez-Prieto, Pilar E. Luengas-Caicedo, and Fredy Gamboa. 2019. "Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities" Scientia Pharmaceutica 87, no. 1: 2. https://doi.org/10.3390/scipharm87010002
APA StyleSequeda-Castañeda, L. G., Muñoz-Realpe, C. C., Celis-Zambrano, C. A., Gutiérrez-Prieto, S. J., Luengas-Caicedo, P. E., & Gamboa, F. (2019). Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities. Scientia Pharmaceutica, 87(1), 2. https://doi.org/10.3390/scipharm87010002