Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Gelling Agent for Hydrogel Base
2.3. Evaluation of Hydrogel Base
2.4. Preparation Solid Dispersion
2.5. Preparation and Evaluation of Hydrogel Usnic Acid (UA) and Usnic Acid in Solid Dispersion (UA-SD)
2.6. Spreadability and Syneresis Test
2.7. Usnic Acid Assay in Hydrogel
2.8. Cycling and Stability Test
2.9. In Vitro Antibacterial Activity
3. Results
3.1. Optimization of Hydrogel Base
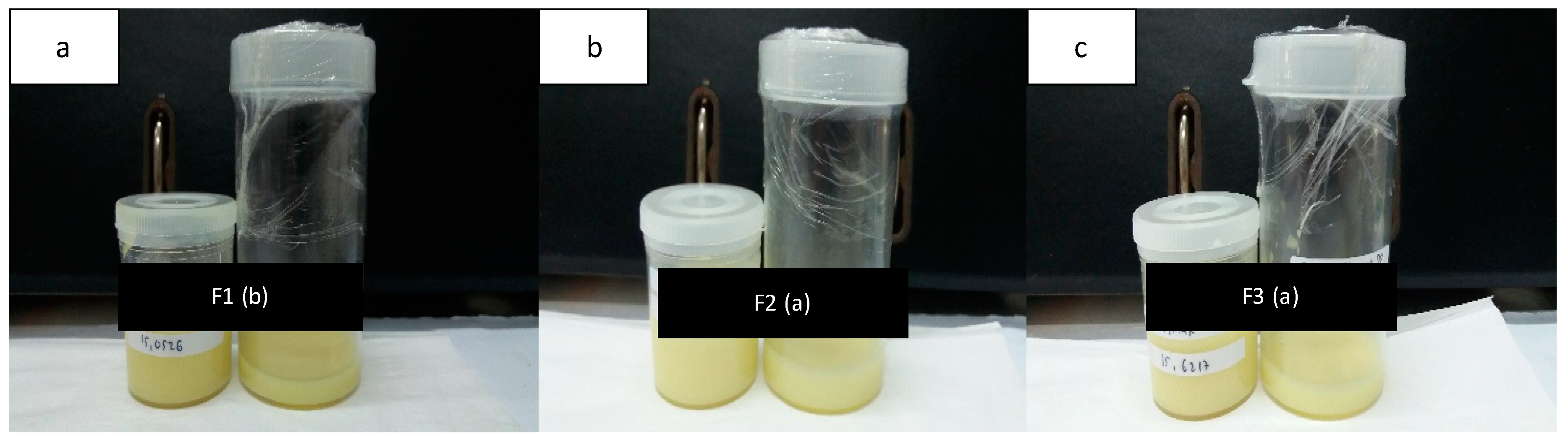
3.2. Hydrogel of Usnic Acid and Usnic Acid—Solid Dispersion
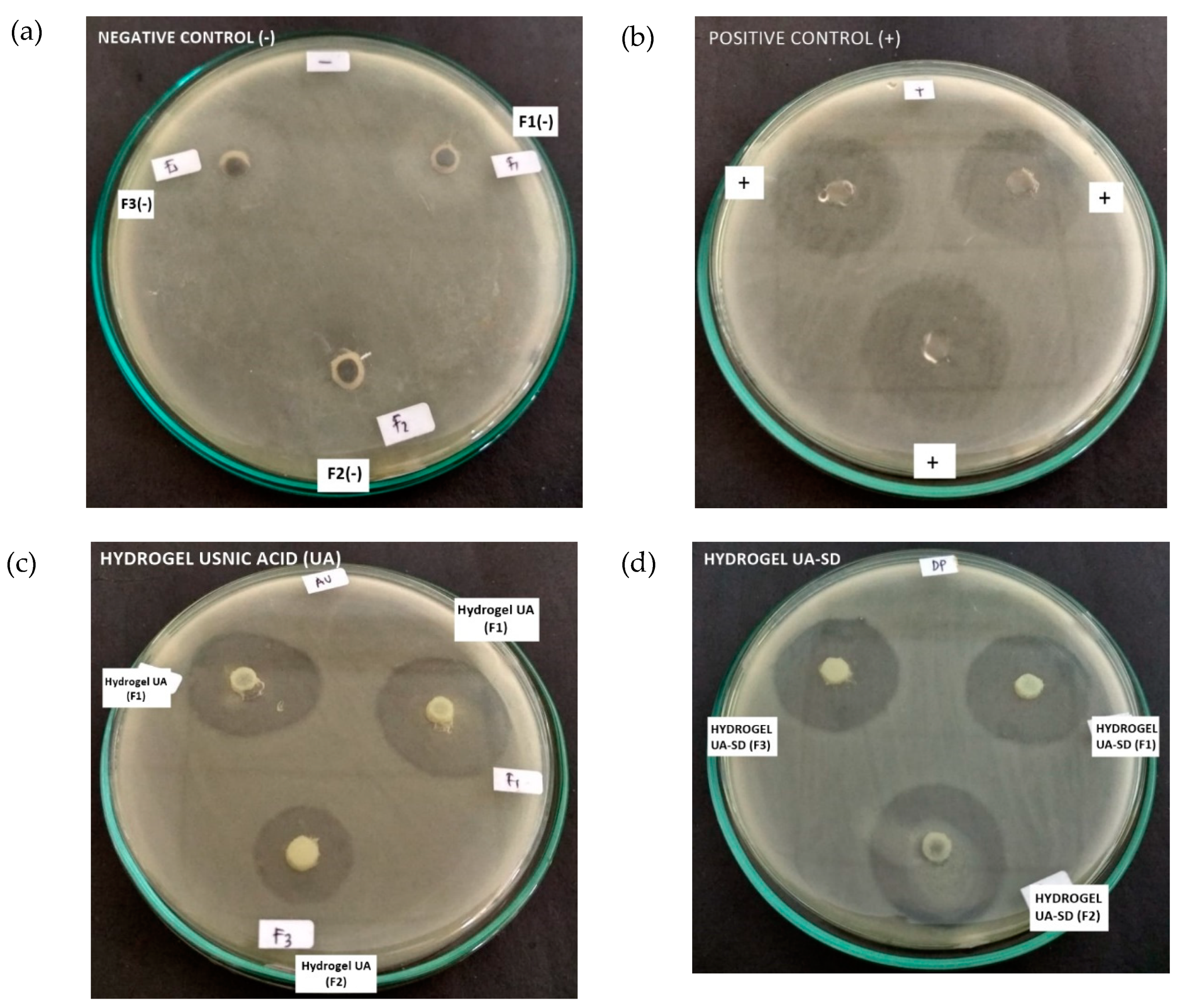
3.3. In Vitro Antibacterial Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madamombe, I.T.; Afolayan, A.J. Evaluation of Antimicrobial Activity of Extracts from South African Usnea barbata. Pharm. Biol. 2003, 43, 199–202. [Google Scholar] [CrossRef]
- Perry, N.B.; Benn, M.H.; Brennan, N.J.; Burgess, E.J.; Ellis, G.; Galloway, D.J.; Lorimer, S.D.; Tangney, R.S. Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist 1999, 6, 627–636. [Google Scholar] [CrossRef]
- Campanella, L.; Delfini, M.; Ercole, P.; Iacoangeli, A.; Risuleo, G. Molecular characterization and action of usnic acid: A drug that inhibits proliferation of mouse polyomavirus in vitro and whose main target is RNA transcription. Biochimie 2002, 84, 329–334. [Google Scholar] [CrossRef]
- Mayer, M.; O’Neill, M.A.; Murray, K.E.; Santos-Magalhães, N.S.; Carneiro-Leão, A.M.A.; Thompson, A.M.; Appleyard, V.C.L. Usnic acid: A non-genotoxic compound with anti-cancer properties. Anticancer. Drugs 2005, 16, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Antioxidant and antibacterial activities of lichen Usnea ghattensis in vitro. Biotechnol. Lett. 2005, 27, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Umeyama, K.; Yamazaki, M.; Kinoshita, Y.; Yamamoto, Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995, 61, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, C.S.; Viswanathan, S.; Kannappa Reddy, M.; Parvathavarthini, S.; Kundu, A.B.; Sukumar, E. Anti-inflammatory activity of (+)-usnic acid. Fitoterapia 2000, 71, 564–566. [Google Scholar] [CrossRef]
- Cocchietto, M.; Skert, N.; Nimis, P.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttr, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Martinez-Chapa, S.O.; Parra-Saldívar, R. Medicinal Plants Versus Skin Disorders. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–221. [Google Scholar]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Stanković, M.; Nikolić, L.; Nikolić, G.; Ilić-Stojanović, S.; Popsavin, M.; Zlatković, S.; Kundaković, T. Inclusion complexes with cyclodextrin and usnic acid. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 173–182. [Google Scholar] [CrossRef]
- Ribeiro-Costa, R.M.; Alves, A.J.; Santos, N.P.; Nascimento, S.C.; Gonçalves, E.C.P.; Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. In vitro and in vivo properties of usnic acid encapsulated into PLGA-microspheres. J. Microencapsul. 2004, 21, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zaini, E.; Nisak, R.K.; Utami, R.D.; Fitriani, L.; Ismed, F. Effect of milling on physicochemical properties of usnic acid isolated from usnea sp. Orient. J. Chem. 2017, 33, 3031–3036. [Google Scholar] [CrossRef]
- Fitriani, L.; Afriyanti, I.; Afriyani, A.; Ismed, F.; Zaini, E. Solid Dispersion of Usnic acid–HPMC 2910 Prepared by Spray drying and Freeze drying Techniques. Orient. J. Chem. 2018, 34, 2083–2088. [Google Scholar] [CrossRef]
- Fitriani, L.; Rismawati, E.; Umar, S.; Zaini, E. Solid Dispersion of Usnic Acid-PVP K30 and Evaluation of Antioxidant Activity. Rasayan J. Chem. 2018, 11, 1643–1648. [Google Scholar]
- Eady, E.A.; Fitzgerald, D.J. Antibacterial or Anti-Acne Formulations Containing Usnic Acid or an Usnate and a Metal Salt. U.S. Patent Application No. 13/996,427, 28 November 2013. [Google Scholar]
- Carbomer (HV-505, HV-505HC, HV-504, HV-501, HV-505E, HV-504E, HV-501E, HV-505ED). Available online: https://www.sumitomoseika.co.jp/documents/pc_dts_aqupec.pdf (accessed on 4 December 2018).
- Bessadóttir, M. The Effects of the Lichen Metabolites Usnic Acid and Protolichesterinic Acid on Energy and Lipid Metabolism in Cancer Cells. Ph.D. Thesis, University of Iceland, Reykjavík, Iceland, December 2014. [Google Scholar]
- Małolepsza-Jarmołowska, K. Pharmaceutical assessment of thermosensitive hydrophilic gels with polyvinyl pyrrolidone K-30 containing lactic acid complexed with chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2013, 18, 123–131. [Google Scholar]
- Małolepsza-Jarmołowska, K. The Effect of Selected Polymers on the Properties of Thermosensitive Hydrophilic Gels Containing Lactic Acid Complexed with Chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2010, 15, 135–142. [Google Scholar]
- Nasri, H.; Bahmani, M.; Shahinfard, N.; Nafchi, A.M.; Saberianpour, S.; Kopaei, M.R. Medicinal plants for the treatment of acne vulgaris: A review of recent evidences. Jundishapur J. Microbiol. 2015, 8, e25580. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Taresco, V.; Crisante, F.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Water soluble usnic acid-polyacrylamide complexes with enhanced antimicrobial activity against Staphylococcus epidermidis. Int. J. Mol. Sci. 2013, 14, 7356–7369. [Google Scholar] [CrossRef] [PubMed]
| Materials | Formula 1 | Formula 2 | Formula 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 3c | |
| Aqupec HV−505 (%) | 0.15 | 0.175 | 0.20 | − | − | − | − | − | − |
| Sodium alginate (%) | − | − | − | 1.5 | 1.75 | 2 | − | − | − |
| HPMC K 100 M (%) | − | − | − | − | − | − | 1.5 | 1.75 | 2 |
| Glycerin (%) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| TEA (%) | 0.4 | 0.4 | 0.4 | − | − | − | − | − | − |
| Distilled water ad (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| No. | Hydrogel Base | Homogeneity | pH | Volume of Wash out | Viscosity |
|---|---|---|---|---|---|
| 1 | F1a | Homogenous | 6.33 | 16 mL | 259.07 cps |
| 2 | F1b | Homogenous | 6.20 | 17 mL | 2595.39 cps |
| 3 | F1c | Homogenous | 6.53 | 18 mL | 3703.75 cps |
| 4 | F2a | Homogenous | 5.40 | 32 mL | 3815.19 cps |
| 5 | F2b | Homogenous | 5.37 | 37 mL | 5115.17 cps |
| 6 | F2c | Homogenous | 6.00 | 40 mL | 14,203.22 cps |
| 7 | F3a | Homogenous | 5.80 | 40 mL | 5544.87 cps |
| 8 | F3b | Homogenous | 5.40 | 50 mL | 9811.03 cps |
| 9 | F3c | Homogenous | 5.40 | 60 mL | 15,811.03 cps |
| Formula | Homogeneity | pH | Viscosity (cps) | Usnic Acid Assay (%) |
|---|---|---|---|---|
| F1b UA | No | 5.63 ± 0.09 | 2328.67 | 97.56 ± 0.19 |
| F1b UA-SD | Homogeneous | 5.67 ± 0.05 | 2270.74 | 99.23 ± 0.29 |
| F2a UA | No | 5.70 ± 0.08 | 2038.95 | 96.94 ± 0.51 |
| F2a UA-SD | Homogeneous | 5.50 ± 0.08 | 2017.03 | 98.19 ± 0.10 |
| F3a UA | No | 5.67 ± 0.05 | 3612.63 | 96.90 ± 0.29 |
| F3a UA-SD | Homogeneous | 5.50 ± 0.14 | 3635.04 | 97.77 ± 0.29 |
| Formula | Spreadability (cm) | Syneresis (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Load 0 g | Load 1 g | Load 3 g | Load 5 g | Load 7 g | 24 h | 48 h | 72 h | |
| F1b UA | 1.90 | 2.30 | 2.75 | 3.25 | 3.90 | 11.20 | 11.13 | 11.11 |
| F1b UA-SD | 2.85 | 3.45 | 3.95 | 4.45 | 5.10 | 11.18 | 11.15 | 11.14 |
| F2a UA | 1.85 | 2.35 | 2.70 | 2.85 | 3.00 | 11.05 | 11.37 | 11.31 |
| F2a UA-SD | 2.05 | 2.45 | 2.70 | 2.95 | 3.10 | 11.03 | 11.03 | 10.93 |
| F3a UA | 2.20 | 2.70 | 3.00 | 3.20 | 3.40 | 11.02 | 11.95 | 10.95 |
| F3a UA-SD | 2.40 | 2.85 | 3.15 | 3.55 | 3.65 | 11.04 | 10.94 | 11.01 |
| Formula | pH | Homogeneity | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| F1b UA | 5.63 ± 0.09 | 5.67 ± 0.00 | No | No |
| F1b UA-SD | 5.67 ± 0.05 | 5.67 ± 0.03 | Homogeneous | Homogeneous |
| F2a UA | 5.70 ± 0.08 | 5.63 ± 0.05 | No | No |
| F2a UA-SD | 5.50 ± 0.08 | 5.47 ± 0.05 | Homogeneous | Homogeneous |
| F3a UA | 5.67 ± 0.05 | 5.63 ± 0.05 | No | No |
| F3a UA-SD | 5.50 ± 0.14 | 5.20 ± 0.02 | Homogeneous | Homogeneous |
| Formula | pH Homogeneity | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | Week 8 | |
| F1b | 6.30 Homogeneous | 6.20 Homogeneous | 6.20 Homogeneous | 6.20 Homogeneous |
| F1b UA | 5.63 No | 5.81 No | 5.83 No | 5.83 No |
| F1b UA-SD | 5.67 Homogeneous | 5.63 Homogeneous | 5.63 Homogeneous | 5.63 Homogeneous |
| F2a | 6.60 Homogeneous | 6.30 Homogeneous | 6.00 Homogeneous | 6.00 Homogeneous |
| F2a UA | 5.50 No | 5.50 No | 5.50 No | 5.47 No |
| F2a UA-SD | 5.70 Homogeneous | 5.70 Homogeneous | 5.70 Homogeneous | 5.70 Homogeneous |
| F3a | 5.83 Homogeneous | 5.73 Homogeneous | 5.73 Homogeneous | 5.73 Homogeneous |
| F3a UA | 5.50 No | 5.43 No | 5.43 No | 5.43 No |
| F3a UA-SD | 5.57 Homogeneous | 5.57 Homogeneous | 5.57 Homogeneous | 5.57 Homogeneous |
| Formula | Diameter Inhibition (mm) ± SD |
|---|---|
| F1b | 0 ± 0.00 |
| F1b UA | 30 ± 0.05 |
| F1b UA-SD | 32 ± 0.05 |
| F2a | 0 ± 0.00 |
| F2a UA | 29 ± 0.05 |
| F2a UA-SD | 30 ± 0.05 |
| F3a | 0 ± 0.00 |
| F3a UA | 20 ± 0.05 |
| F3a UA-SD | 26 ± 0.05 |
| Positive control (+) | 30 ± 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriani, L.; Afifah; Ismed, F.; Bakhtiar, A. Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne. Sci. Pharm. 2019, 87, 1. https://doi.org/10.3390/scipharm87010001
Fitriani L, Afifah, Ismed F, Bakhtiar A. Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne. Scientia Pharmaceutica. 2019; 87(1):1. https://doi.org/10.3390/scipharm87010001
Chicago/Turabian StyleFitriani, Lili, Afifah, Friardi Ismed, and Amri Bakhtiar. 2019. "Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne" Scientia Pharmaceutica 87, no. 1: 1. https://doi.org/10.3390/scipharm87010001
APA StyleFitriani, L., Afifah, Ismed, F., & Bakhtiar, A. (2019). Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne. Scientia Pharmaceutica, 87(1), 1. https://doi.org/10.3390/scipharm87010001




