Abstract
The aim of this study was to investigate the effect of adenosine in non-occluded or occluded femoral arteries (FA) that were isolated from healthy or diabetic Wistar rats. Determining the role of endothelium, and a transmembrane flow of potassium ions in adenosine actions were also of interest. Diabetes was experimentally induced by alloxan, while the vascular occlusion was performed for 45 min on randomly selected FA. Vascular tone changes were continuously recorded. Selected markers of endothelial dysfunction were measured in animal serum. Thus, adenosine produced a concentration-dependent relaxation of rat FA, which was endothelium-dependent, too, except in a group of diabetic animals. Moreover, serum asymmetric dimethylarginine (ADMA) levels were higher in diabetic animals, thus reflecting endothelial dysfunction (ED). Still, an occlusion of FA enhanced the relaxation effect of adenosine in endothelium-intact rings from diabetic animals. Oppositely, in the presence of high potassium concentration in the buffer, adenosine-induced relaxation was significantly reduced in all of the investigated groups/subgroups. These results suggest that in diabetic animals, an occlusion of FA most probably reversed adenosine-induced relaxation from endothelium-independent into an endothelium-dependent relaxation, thus indicating the possible protective mechanism against ischemic episodes of FA in the presence of diabetes.
1. Introduction
Adenosine is a ubiquitous extracellular signaling molecule [1]. This purine nucleoside is obtained during the complete dephosphorylation of adenine nucleotides, which is catalyzed by the enzyme 5′-nucleotidases located on the cell membrane or inside the cells [2]. Adenosine is widespread in almost all of the tissues in the human body, with a significant role in the regulation of different physiological processes, and because of that, it is considered to have a significant therapeutic potential [3]. Although it can be found almost everywhere in the human organism, adenosine concentrations are relatively low in physiological conditions. Inversely, in quite a few pathological conditions, such as hypoxia or anoxia, the concentration of adenosine could be significantly increased [4]. Today, we know that this increase in adenosine concentration is considered a protective mechanism. For example, it is known that if hypoxia occurs, the carotid bodies will release adenosine in order to protect the central nervous system [5].
The vascular actions of adenosine are commonly initiated through activation of specific G protein-coupled adenosine receptors, namely A1, A2A, A2B, and A3, with the second messenger systems primarily involving cyclic AMP and cyclic GMP [6]. Adenosine predominantly induces the vasodilation of arterial blood vessels. This process is usually independent of the presence of functional endothelial integrity [2]. Nevertheless, adenosine-induced vascular dilations can be partly or entirely endothelium-dependent, too [7,8]. Additionally, the main signaling pathway in adenosine action, at least at the level of smooth muscle cells, is known to include the potassium ion flow [2,9]. In consideration of the femoral artery, it was reported that in the femoral arteries of dogs and rabbits, adenosine induced endothelium-independent vasodilation via the activation of A2A receptors located on smooth muscle cells, whereas initial reports suggested that the A2 receptor-induced relaxation of rat femoral artery was partially endothelium-dependent [10].
The role of adenosine in the cardiovascular system is constantly being examined and re-evaluated. Still, the underlying adenosine mechanism of action, including the one at the level of the femoral artery of different species, is under investigation. In addition, limited data are available considering the possible changes in the transduction mechanisms of an adenosine-induced effect on the femoral artery during different pathological conditions, such as diabetes, and/or vascular occlusion. These pathological conditions are now known to be important in the process of peripheral artery disease development, which commonly affects femoral arteries. Also, diabetes is able to change the function of both endothelial [11] and smooth muscle layers [12] in the arterial wall.
Considering previously mentioned facts, this study was undertaken in order to investigate the effect of adenosine on isolated rat femoral artery, with the complete experimental procedure performed in two experimental groups (healthy versus diabetic rats), each divided into two subgroups (non-occluded versus occluded FA). Hence, it was of interest to evaluate whether pathological conditions, including alloxan-induced diabetes and/or vascular occlusion, affected the transduction mechanisms of adenosine-induced action. Finally, the role of endothelium and the potassium ion flow in this process were determined.
2. Materials and Methods
2.1. Animals
The experiments were performed in accordance with European Union (EU) (86/609/EEC) guidelines, together with the CPCSEA guidelines, and guidelines from the Good Laboratory Practice [13]. The described methodology was approved by the Ethics Committee for Experimental Animals Welfare Protection (Medical Faculty, University of Belgrade, 2010-1500/05).
Three-month-old male rats of Wistar strain were used in our experiments. The animals weighted between 150–350 g at the beginning of the experiments (considering the vascular ring analysis and the blood collection). All of the animals were kept in polycarbonate cages and had ad libitum access to a standard rodent diet and water. The environmental conditions were kept constant, with a 12/12-h light/day cycle, and adequate temperature (24 ± 1 °C) and humidity (55 ± 10%).
2.2. Experimental Design
Our experiments involved two main groups—the healthy group (without alloxan-induced diabetes), and the diabetic group (with alloxan-induced diabetes). Each group was further subdivided into three subgroups intended to be submitted to the vascular occlusion: non-operated, operated, and sham-operated animals.
In a group of sham-operated animals, adenosine (1–100 µM) produced a comparable concentration-dependent relaxation of the femoral artery (irrespective of endothelial presence) if compared to non-operated (healthy) animals. Considering this, and in accordance with “the 3Rs” principle of avoiding the further unnecessary use of experimental animals [13,14,15], from this point, the control group consisted of only non-operated animals.
2.3. Experimental Diabetes
In randomly selected animals, experimental diabetes was induced by a single intraperitoneal (i.p.) injection of 175 mg/kg of alloxan, previously dissolved in normal saline (0.9% NaCl, pH 7.4). Twenty-four hours before the injection, the food was withdrawn, and it was restored after the injection. In order to determine the success of diabetes development, we measured animals’ body weight, the blood glucose level, and the plasma RANTES-ccl5 level. In order to measure the blood glucose level, the blood samples from the rat tail vein were collected before fasting; then, they were collected after alloxan injection, and every seven days post-injection (animals were fed ad libitum in this period). At the end of the four-week period (after alloxan administration), animals with glucose levels higher than 11 mM were considered as diabetic [16,17].
The animals were two months old when alloxan was injected. This particular age was chosen because the follow-up of experimental animals with alloxan-induced diabetes lasted for four weeks (one month). In that way, the animals from the diabetic group could be the same age (three months old), as animals in other experimental groups at the beginning of the pharmacological analysis.
2.4. Vascular Occlusion
Randomly selected healthy and diabetic animals were anesthetized with the single intraperitoneal injection of urethane, at the dose of 125 mg/100 g. A tracheal cannula was regularly placed to facilitate the spontaneous breathing. The body temperature was regulated by the warming pad. Before the surgical procedure, the depth of anesthesia was determined. If an animal did not react to pinching the tail and space between the toes with tweezers, or to poking these areas with the needle, this was considered to be a good sign of deep anesthesia. If the pedal withdrawal reflex persisted, it was considered that the animals were in a superficial anesthesia. In that case, an additional time was given to the animal to enter a deep anesthesia, or in some cases, a slight increase in the anesthetic dose was performed.
Animals were allowed to stabilize for 90 min prior to the femoral artery occlusion. The animals were placed in a supine position to facilitate the incision that would be made in the inguinal area along the natural angle of the hind leg [18]. The surrounding connective tissue was bluntly dissected to visualize the femoral artery (FA) [18]. The FA was then occluded (by a cord) for a period of 45 min. The occluded femoral arteries were isolated for the following measurements of vascular tone changes. During the process of occlusion, an animal paw was monitored to observe possible changes in color and temperature, as signs of adequately performed occlusion. The similar procedure was performed in a group of sham-operated animals. The only difference was that the FA was not occluded in this group.
2.5. The Preparation of Vascular Rings and Measurement of Vascular Tone Changes
Animals in deep anesthesia were decapitated, while non-anesthetized animals were firstly stunned, and then decapitated. Afterward, both left and right FA was isolated, dissected from the surrounding tissue, cut into rings (four mm long), and placed into the Krebs–Ringer bicarbonate solution (composition in mM: NaCl 118.3; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.0; Ca-EDTA 0.026; glucose 11.1). In some randomly selected rings, endothelium was removed by gently rubbing the intimal surface with a stainless-steel wire. The rings were mounted between two stainless-steel triangles and further positioned into an organ bath field with Krebs–Ringer bicarbonate solution aerated with 95% O2 and 5% CO2 at 37 °C, and a pH level of 7.4. The upper triangle was attached to a displacement unit, allowing a fine adjustment of tension. The displacement unit was further connected to a force-displacement transducer (Hugo Sachs Elektronik F30 Type 372, Freiburg, Germany). An isometric tension was continuously recorded on the Rikadenki R-62 multi-pen electronic recorder (Rikadenki Kogyo Co., Ltd., Tokyo, Japan).
Once the initial setup procedure was done, the vascular rings were allowed to adjust for 45 min. Following this period, each vascular ring was gradually stretched to an optimal resting tension of 1.5 g during the period of 30 min. At the beginning of each experiment, the endothelial functional integrity was pharmacologically tested. In triplicate, the FA rings were pre-contracted with a submaximal concentration (EC50–EC70) of phenylephrine. Then, acetylcholine (one μmol/L) was added after the maximal response was obtained with phenylephrine. Between these controls, there was a resting period of 20 min. The presence of intact endothelium was considered to be positive if an acetylcholine relaxation of an initial phenylephrine pre-contraction was over 80%. In addition to an endothelial functional integrity, the morphological integrity of the endothelial layer was confirmed as well at the end of randomly selected experiments by staining these rings with hematoxylin and eosin. The concentration-response curves for adenosine were obtained in a cumulative fashion on phenylephrine pre-contracted arteries. Since preliminary experiments in the vascular rings of FA demonstrated a significant difference between the first and the second adenosine concentration-dependent relaxation (after 45 min and 60 min), we used one of the vascular rings as a time control, while the rest of the rings obtained from the same preparation were used to investigate the influence of endothelial denudation and/or hyperpolarizing buffer (KCl = 100 mM).
2.6. Blood Sample Collection
The blood was obtained from rat tail veins. A modified 21 G × 1″ butterfly needle system was used for blood collection. The needle was placed into the lateral tail vein, and the blood was collected into the collecting tube (less than 10% of a total rat blood volume).
The collecting tubes were left for two hours at room temperature in order to clot and extract the serum. After this two hours, the collected blood was centrifuged for 15 min at 1000× g, which finally separated the serum from the rest of the blood. For plasma separation, collecting tubes with EDTA were used. Samples were stored at −80 °C for further use.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
The serum level of asymmetric dimethylarginine (ADMA) and vascular cell adhesion protein-1/cluster of differentiation 106 (VCAM-1/CD106), as well as the plasma level of chemokine C-C motif ligand 5 (RANTES-ccl5) were quantified by using the ELISA method and the respective kit produced by the Cusabio Biotech Co., Ltd. (Wuhan, China). The kit was used according to the provided instructions. The microplate reader model DV 990 BV-6 was used for the measurements (GDV, Gio Da Vita, Rome, Italy).
2.8. Drugs and Solutions
The following chemicals were used: adenosine, alloxan, phenylephrine hydrochloride, urethane (Sigma-Aldrich, St Louis, MO, USA); acetylcholine iodide (Serva, Heidelberg, Germany). All of the agents, except alloxan, were dissolved in distilled water and diluted to the desired concentration with the buffer. Alloxan was dissolved in 0.9% NaCl. During the experimental procedure, all of the agents were added directly to the organ bath with a volume of 150 μL. The given concentrations were calculated as final concentrations in the bath solution.
2.9. Statistical Analysis
The vascular relaxation that was produced by each concentration of adenosine was expressed as the percentage of the previously induced maximal control contraction with phenylephrine. The results were expressed as the mean ± S.E.M., with N referring to the number of experiments. The maximal response and the sensitivity to the used agonist were determined, for each concentration-response curve, by conducting a non-linear regression analysis. Thus, the maximum effect was used as a measure of responsiveness (Emax—% of the phenylephrine-induced contraction), while the concentration of agonist was used as a measurement of the sensitivity to agonist (which produced 50% of the Emax, presented as pEC50; pEC50 = −log EC50). The area under the curve (AUC) was used to additionally compare the effect of adenosine on the FA in different groups/subgroups, with and without the hyperpolarizing solution. For obtaining the AUC, the values were calculated for each individual curve.
The statistical analysis was performed by using the Graph Pad Prism (Graph Pad Software Inc., San Diego, CA, USA). The results were expressed as the mean ± S.E.M (vascular tone change), or mean ± SD (body weight, blood glucose level, RANTES-ccl5, ADMA, and VCAM-1/CD106). The inside-group difference was determined via the unpaired Student’s two-tailed t-test. The between-group difference was determined through the one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test.
3. Results
3.1. Effect of Short-Term Diabetes on the Histology, Body Weight, Blood Glucose Level, and RANTES-ccl5 Level
The histological evaluation of morphological differences in vascular rings randomly obtained from healthy and diabetic animals failed to show any diversity, which suggests the morphological preservation of the vascular endothelium and smooth muscle cells after four weeks of diabetes. The control images of vascular rings with endothelium isolated from healthy and diabetic rats were presented in Figure 1. Conversely, significant differences in the body weight (277.70 ± 12.58 versus 254.30 ± 52.15, p = 0.021; Figure 2A), the blood glucose level (6.43 ± 0.86 versus 29.93 ± 2.99, p < 0.0001; Figure 2B), and the RANTES-ccl5 plasma level (0.01 ± 0.04 versus 0.70 ± 0.56, p = 0.015; Figure 2C) were recorded in between groups of healthy animals and those with alloxan-induced diabetes.
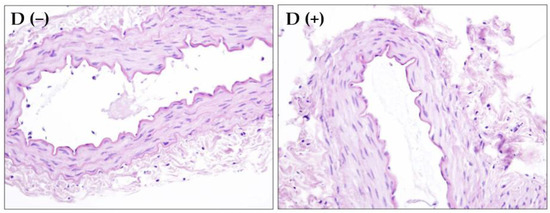
Figure 1.
Rat femoral artery vascular rings with endothelium isolated from healthy (D −) and diabetic (D +) animals after hematoxylin and eosin staining (magnification 400×).
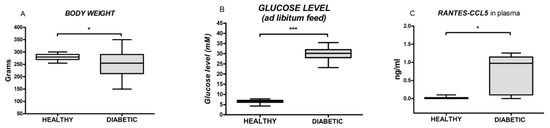
Figure 2.
Box and whiskers plots showing body weight, the blood glucose level and the plasma level of RANTES-CCL5 in healthy and diabetic animals. The upper and the lower hinge of the boxes represent the interquartile range, the midline represents the median value, and the whiskers represent the maximum and minimum values. * p < 0.05, *** p < 0.001.
3.2. The Role of Endothelium on Adenosine-Induced Relaxation in the Rat FA Obtained from Different Groups/Subgroups
Adenosine (1–100 µM) produced the concentration-dependent relaxation of the FA in all of the investigated groups/subgroups (Figure 3, Table 1). The recorded relaxation effect was endothelium-dependent in almost all of the groups and subgroups. The only exception was the group of non-operated diabetic animals, in which endothelial denudation failed to produce any significant alteration of adenosine action (Figure 3B). These results indicate that endothelium plays an important role in adenosine-induced relaxation in the FA, and that diabetes affects smooth muscle cells, which leads toward an enhanced response to adenosine.
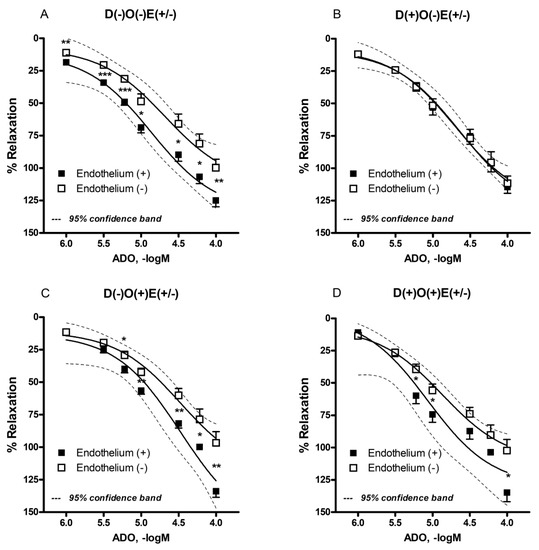
Figure 3.
Adenosine-induced relaxation in isolated rat femoral artery with and without endothelium. The femoral artery was obtained from healthy (A,C) and diabetic animals (B,D). Additionally, the femoral artery was occluded in randomly selected healthy (C) and diabetic animals (D). Each point is presented as the mean ± S.E.M (n = 5–26). Adenosine-induced vascular relaxation is expressed as the percentage of pre-contraction induced by phenylephrine. * p < 0.05, ** p < 0.01, *** p < 0.001. E = endothelium; D = diabetes; O = occlusion.

Table 1.
Adenosine-induced relaxation in isolated rat femoral artery obtained from different animal groups/subgroups in the presence or absence of KCl (100 mM).
In contrast to the maximal relaxation response, pEC50 values were not different in between the investigated groups (Table 1). On the other hand, the AUC values were lower after endothelial denudation in healthy, operated, and non-operated animals (Table 1). This result confirms that in a healthy group of animals, the endothelial layer has a prominent role regarding adenosine vascular effects.
The serum concentration of ADMA, a marker of endothelial dysfunction (ED), was higher in diabetic animals if compared to healthy animals (198.30 ± 11.25 vs. 158.30 ± 27.84, p = 0.044; Figure 4A). This means that the notable change occurred in endothelial function during diabetes, and that the difference in adenosine-induced relaxation in the diabetic group was in part a consequence of progressing endothelial dysfunction (ED). A measurement of VCAM-1/CD106 in animal serum failed to present any difference between the investigated groups (20.92 ± 1.52 versus 20.34 ± 0.79, p = 0.45; Figure 4B).
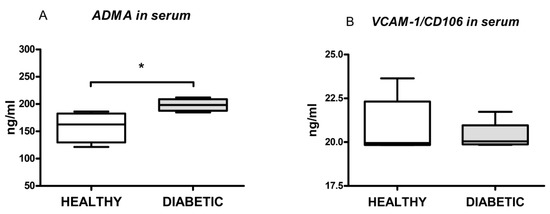
Figure 4.
Box and whiskers plots showing serum levels of asymmetric dimethylarginine (ADMA) and vascular cell adhesion protein-1/cluster of differentiation 106 (VCAM-1/CD160) in healthy and diabetic animals. The upper and the lower hinge of the boxes represent the interquartile range, the midline represents the median value, and the whiskers represent maximum and minimum values. * p < 0.05.
3.3. Effect of the Hyperpolarizing Solution on Adenosine-Induced Relaxation in the Rat FA Obtained from Different Groups/Subgroups
In the presence of hyperpolarizing solution, the maximal relaxant response of the FA (in all of the investigated groups/subgroups) to adenosine was almost abolished, if compared to the adenosine action in the regular buffer (Figure 5, Table 1; p < 0.001). The adenosine-induced relaxation was comparably reduced in all of the investigated groups/subgroups (Figure 5, Table 1, p > 0.05), thus indicating that the transmembrane flow of potassium ions has an important role in the vascular effects of adenosine on the FA. Similarly to the recorded maximal relaxant response, pEC50 and AUC values were significantly reduced in the presence of a hyperpolarizing solution (Table 1). The maximal relaxant responses, as well as pEC50 and AUC values, were reduced irrespective of endothelial presence. This means that, at the level of the FA, potassium flow plays a significant role in adenosine-induced relaxation.
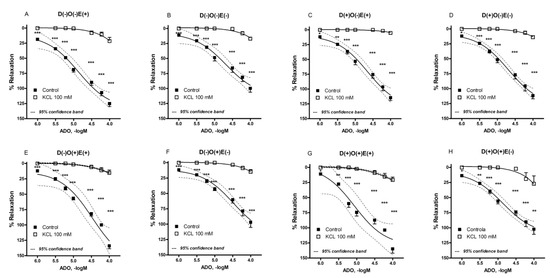
Figure 5.
Adenosine-induced relaxation in isolated rat femoral artery in the presence of hyperpolarizing solution (KCl = 100 mM). The femoral artery was obtained from non-diabetic (A,B,E,F) and diabetic animals (C,D,G,H). Additionally, femoral artery was occluded in randomly selected non-diabetic (E,F) and diabetic animals (G,H). Each point is presented as the mean ± S.E.M (n = four to eight). Adenosine-induced vascular relaxation is expressed as the percentage of pre-contraction induced by phenylephrine. * p < 0.05, ** p < 0.01, *** p < 0.001. E = endothelium; D = diabetes; O = occlusion.
4. Discussion
In our study, we investigated the relaxant response of the FA induced by adenosine. We also examined the role of endothelium in this process, as well as the transmembrane flow of potassium ions. The whole protocol was performed in two experimental groups (healthy versus diabetic rats), each divided into two subgroups (non-occluded versus occluded FA), with the goal of evaluating the role of different pathological conditions in adenosine-induced vascular actions. Since the femoral artery is known to be prone to different pathological disorders, involving endothelial dysfunction, atherosclerosis, transient or permanent ischemia, or diabetes-related morphological and functional changes, our study also aimed to provide an additional clinical insight into the etiology of the peripheral artery disease of lower limbs.
In the first part of this study, adenosine produced the concentration-dependent relaxation of the rat FA in all of the groups/subgroups. This response of FA was not surprising, since it is well known that adenosine mainly produces vasorelaxant effects [1,19,20]. The adenosine-induced relaxation was endothelium-dependent in all of the groups/subgroups, except in a group of animals with alloxan-induced diabetes. In this group, the majority of denuded blood vessels had an almost identical response compared to vascular rings with preserved endothelium. Additionally, the relaxant response of vascular rings obtained from diabetic animals, both with and without endothelium, was similar to the recorded maximal relaxant effect observed in intact vascular rings from healthy animals. This means that during diabetes, vascular smooth muscle cells probably developed a specific protective mechanism, which allowed them to react quite potently to adenosine stimuli. Interestingly, in consideration of the previous facts, it could be expected that the relaxation response of the FA would be even stronger in the presence of endothelium. Nevertheless, we did not record this phenomenon, which was most probably due to the concomitant development of endothelial dysfunction (ED) related to diabetes.
Diabetes is considered to be a significant risk factor for the development of ED [11,21,22,23]. ADMA, a well-confirmed marker of ED, is also an endogenous inhibitor endothelial nitric oxide synthase that reduces nitric oxide production [24,25,26]. In our study, the serum ADMA levels were higher in the group of diabetic animals, suggesting the possible development of ED. On the other hand, measurements of VCAM-1/CD106 failed to show any difference between healthy and diabetic animals. This marker is also an important indicator of ED [27]. The explanation for an increase of the ADMA level, while the VCAM-1/CD106 level was unchanged, most likely lies in diabetes lasting shortly (only four weeks), where ADMA is considered to represent an early marker of ED [25]. These results suggest that ED was probably developed during diabetes, which significantly diminished the endothelial part of adenosine-induced relaxation.
The occlusion of femoral arteries in healthy animals, with the subsequent isolation of intact or denuded rings, did not produce any significant difference in any adenosine-produced effect, which was comparable to the results obtained in the group of non-operated healthy animals. Although vascular injury was expected to occur during the FA occlusion, this procedure did not affect the relaxant mechanisms triggered by adenosine. Interestingly, an occlusion of the FA in diabetic animals enhanced the relaxant effect of adenosine in vascular rings with preserved endothelium. In vascular rings without endothelium, an occlusion procedure did not have a similar effect, and the maximal relaxant response was comparable to the maximal response in vascular rings obtained from diabetic animals without occlusion. The most probable explanation for these results could be regarding the development of protective mechanisms in the endothelial cells of diabetic animals during the vascular occlusion, which has most likely briefly prevailed endothelial dysfunction induced by diabetes. To the best of our knowledge, there is no other similar data in the literature, so the next step should be the revealing the nature of the underlying transduction mechanisms in this process.
In the second part of this study, we were interested in determining the influence of diabetes and/or vascular occlusion on the transmembrane potassium ion flow in the adenosine-induced relaxation of the investigated blood vessel. The opening of potassium ion channels, as a result of adenosine action, was previously described in various blood vessels [2]. This pattern was also found in our study. Thus, after administration of the solution with high potassium concentration (100 mM), the significant reduction of adenosine-induced relaxation was found in all of the investigated groups/subgroups, irrespective of the endothelial presence. In accordance, all of the measured parameters (Emax, pEC50, and AUC values) were reduced in the presence of hyperpolarizing solution. Although the reduction was found in all of the experimental groups/subgroups, a near complete abolishment of adenosine effects was found in the vascular rings with preserved endothelium from diabetic animals. Still, we did not detect any statistical difference in between the results obtained from vascular rings from different groups/subgroups of animals in the presence of hyperpolarizing solution.
This preliminary report has several limitations that need to be addressed. Namely, one of the primary goals was to elucidate the effect of short-term diabetes on adenosine action, additionally aiming to detect early changes in the morphological and functional characteristics of the examined blood vessel. However, the next step would certainly require an experimental protocol covering the prolonged period of diabetes action on the blood vessel, for example, over eight or 12 weeks. Secondly, since we aimed to examine adenosine-induced action in the context of endothelial dysfunction, as well as diabetes-induced and vascular occlusion-evoked changes in the responsiveness of the rat femoral artery, a comprehensive characterization of adenosine receptors was not carried out this time. Finally, although the initially designed methodological approach provided solid pieces of evidence in accordance with the defined aims of our study, some additional immunohistochemistry or immunofluorescence analysis would surely increase the factual merit of the provided data.
To conclude, we have demonstrated that adenosine induces the concentration-dependent relaxation of the rat FA. The adenosine-induced relaxation was endothelium-dependent in all of the investigated groups/subgroups, except in a group of diabetic animals. In diabetic animals, the development of ED led to a loss of an endothelial component of adenosine-induced relaxation. The occlusion of the FA in diabetic animals overcame the changes developed during ED, which reversed adenosine-induced relaxation into an endothelium-dependent one again. Transmembrane potassium ion flow has a crucial role in adenosine-induced relaxation, irrespective of the presence of investigated pathological conditions, or the presence of an intact endothelium.
Author Contributions
Conceived and designed the experiments, M.R. and M.S.; collected data, M.S. and R.J.; performed the experiments, M.S.; analyzed the data and wrote the manuscript, M.S. and M.R.; supervised the study and made critical revisions, M.R. and M.P.; all authors read, reviewed and approved the final manuscript.
Funding
This research was funded by the Ministry of Education and Science—Republic of Serbia (Grant number—175023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine: Physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Stojanović, M.; Janković, R.; Topalović, M.; Stojiljković, M. Effects of diabetes and vascular occlusion on adenosine-induced relaxant response of rat common carotid artery. Pharmacol. Rep. 2013, 65, 632–641. [Google Scholar] [CrossRef]
- Samsel, M.; Dzierzbicka, K. Therapeutic potential of adenosine analogues and conjugates. Pharmacol. Rep. 2011, 63, 601–617. [Google Scholar] [CrossRef]
- Peart, J.N.; Headrick, J.P. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol. Ther. 2007, 114, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Conde, S.V.; Monteiro, E.C. Hypoxia induces adenosine release from the rat carotid body. J. Neurochem. 2004, 89, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Collis, M.G. The vasodilator role of adenosine. Pharmacol. Ther. 1989, 41, 143–162. [Google Scholar] [CrossRef]
- Grbović, L.; Radenković, M.; Prostran, M.; Pešić, S. Characterization of adenosine action in isolated rat renal artery. Possible role of adenosine A(2A) receptors. Gen. Pharmacol. 2000, 35, 29–36. [Google Scholar] [CrossRef]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef]
- Radenković, M.; Grbović, L.; Pešić, S.; Stojić, D. Isolated rat inferior mesenteric artery response to adenosine: Possible participation of Na+/K+-ATPase and potassium channels. Pharmacol. Rep. 2005, 57, 824–832. [Google Scholar]
- Burnstock, G.; Ralevic, V. Purinergic signaling and blood vessels in health and disease. Pharmacol. Rev. 2013, 66, 102–192. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Walther, G.; Pérez-Martin, A.; Vicente-Salar, N.; Roche, E.; Vinet, A. Vascular smooth muscle function in type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetologia 2013, 56, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Gursel, O.; Tapan, S.; Sertoglu, E.; Taşçılar, E.; Eker, I.; Ileri, T.; Uysal, Z.; Kurekci, A.E. Elevated plasma asymmetric dimethylarginine levels in children with beta-thalassemia major may be an early marker for endothelial dysfunction. Hematology 2018, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Santos-Ruiz, M.; Ruiz de Adana, J.C.; Pindado, M.L.; Sánchez-Ferrer, A.; Hernández, A.; Rodríguez-Maas, L. Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans. J. Physiol. 2016, 594, 3045–3060. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, U.; Singh, S.P.; Yanamandra, S.; Mulajkar, D.; Grewal, R.S.; Singh, S.; Ashraf, M.Z.; Reddy, P.; Nair, V. Endothelial markers in high altitude induced systemic hypertension (HASH) at moderate high altitude. Med. J. Armed. Forces India 2017, 73, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M. The OECD principles of good laboratory practice and the current bioethics. In Bioethics and Pharmacology: Ethics in Preclinical and Clinical Drug Development; Todorović, Z., Prostran, M., Turza, K., Eds.; Transworld Research Network: Trivandrum, India, 2012; pp. 43–50. ISBN 978-81-7895-579-7. Available online: http://www.ressign.com/UserArticleDetails.aspx?arid=11925 (accessed on 22 October 2018).
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Perry, P. The ethics of animal research: A UK perspective. ILAR J. 2007, 48, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, M.; Prostran, M.; Janković, R.; Radenković, M. Clarification of serotonin-induced effects in peripheral artery disease observed through the femoral artery response in models of diabetes and vascular occlusion: The role of calcium ions. Clin. Exp. Pharmacol. Physiol. 2017, 44, 749–759. [Google Scholar] [CrossRef]
- Radenković, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef]
- Jespersen, B.; Knupp, L.; Northcott, C.A. Femoral arterial and venous catheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. J. Vis. Exp. 2012, 24, 3496. [Google Scholar] [CrossRef]
- El-Gowelli, H.M.; El-Gowilly, S.M.; Elsalakawy, L.K.; El-Mas, M.M. Nitric oxide synthase/K+ channel cascade triggers the adenosine A(2B) receptor-sensitive renal vasodilation in female rats. Eur. J. Pharmacol. 2013, 702, 116–125. [Google Scholar] [CrossRef]
- Casey, D.P.; Mohamed, E.A.; Joyner, M.J. Role of nitric oxide and adenosine in the onset of vasodilation during dynamic forearm exercise. Eur. J. Appl. Physiol. 2013, 113, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Eringa, E.C.; Serne, E.H.; Meijer, R.I.; Schalkwijk, C.G.; Houben, A.J.; Stehouwer, C.D.; Smulders, Y.M.; van Hinsbergh, V.W. Endothelial dysfunction in (pre)diabetes: Characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. [Google Scholar] [CrossRef]
- Prieto, D.; Contreras, C.; Sánchez, A. Endothelial dysfunction, obesity and insulin resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Huang, C.; Liu, M.; Chen, N.; Chen, W.; Yang, C.; Zhao, Y.; Li, X.; Duan, J.; Liu, S.; Yang, S. Survivin regulated by autophagy mediates hyperglycemia-induced vascular endothelial cell dysfunction. Exp. Cell. Res. 2018, 364, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc. Diabetol. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).