Modulatory Effect of Lippia alba Essential Oil on the Activity of Clinically Used Antimicrobial Agents on Salmonella typhi and Shigella dysenteriae Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Lippia alba Essential Oil
2.2. Bacterial Strains and Growth Conditions
2.3. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4. Determination of the Modulatory Effect of LaEO on the Activity of Clinically Used Antimicrobial Agents
2.5. Determination of the Effect of Time of Exposure to LaEO I and LaEO-ATM Association on Microbial Viability (Time Kill)
2.6. Determination of Biofilm Formation Inhibition by LaEO and LaEO-ATM Association
2.7. Determination of Preformed Biofilm Eradication by LaEO and the LaEO-ATM Association
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of the Essential Oil Extracted from the Lippia Alba Chemotype I Leaves
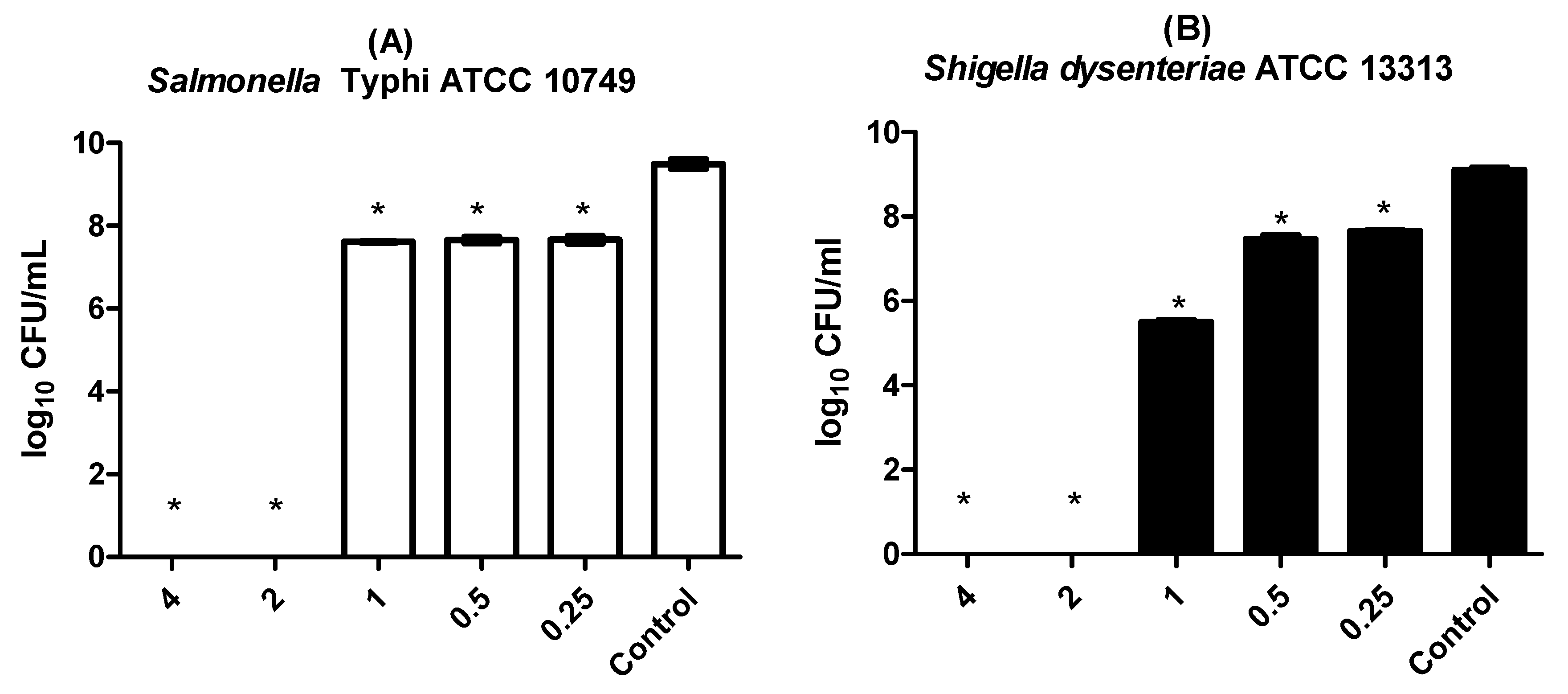
3.2. Antimicrobial Effect of LaEO I on Planktonic Cells
3.3. Modulatory Effect of LaEO on the Activity of Clinically used Antimicrobial Agents
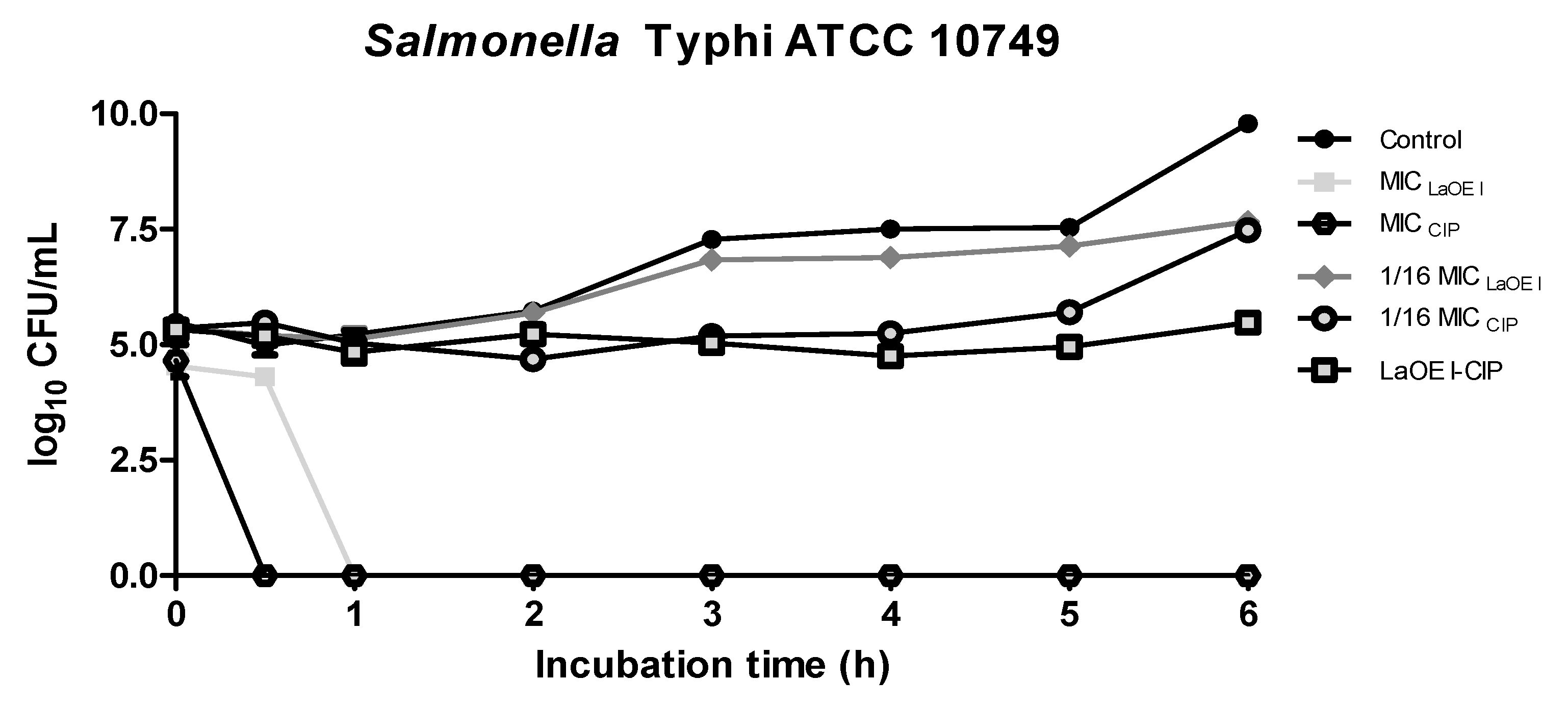
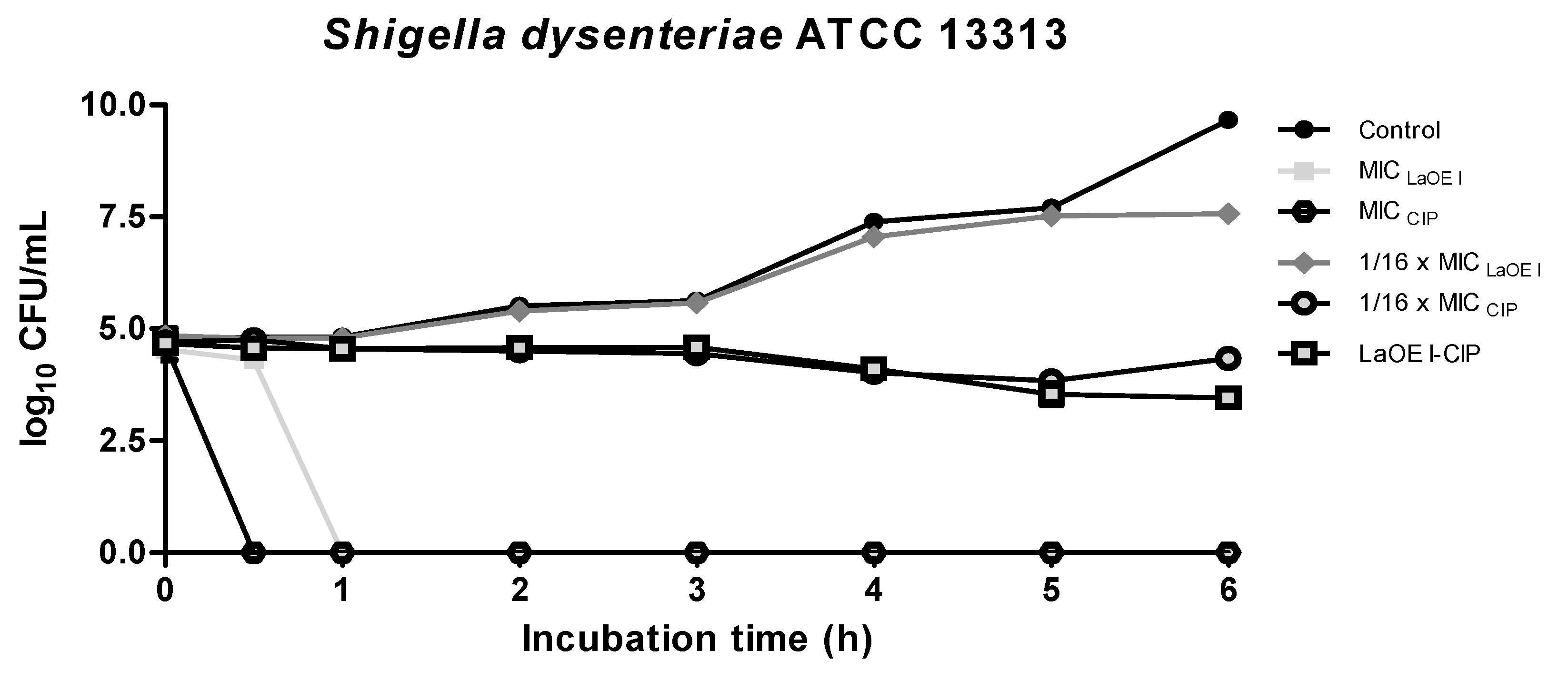
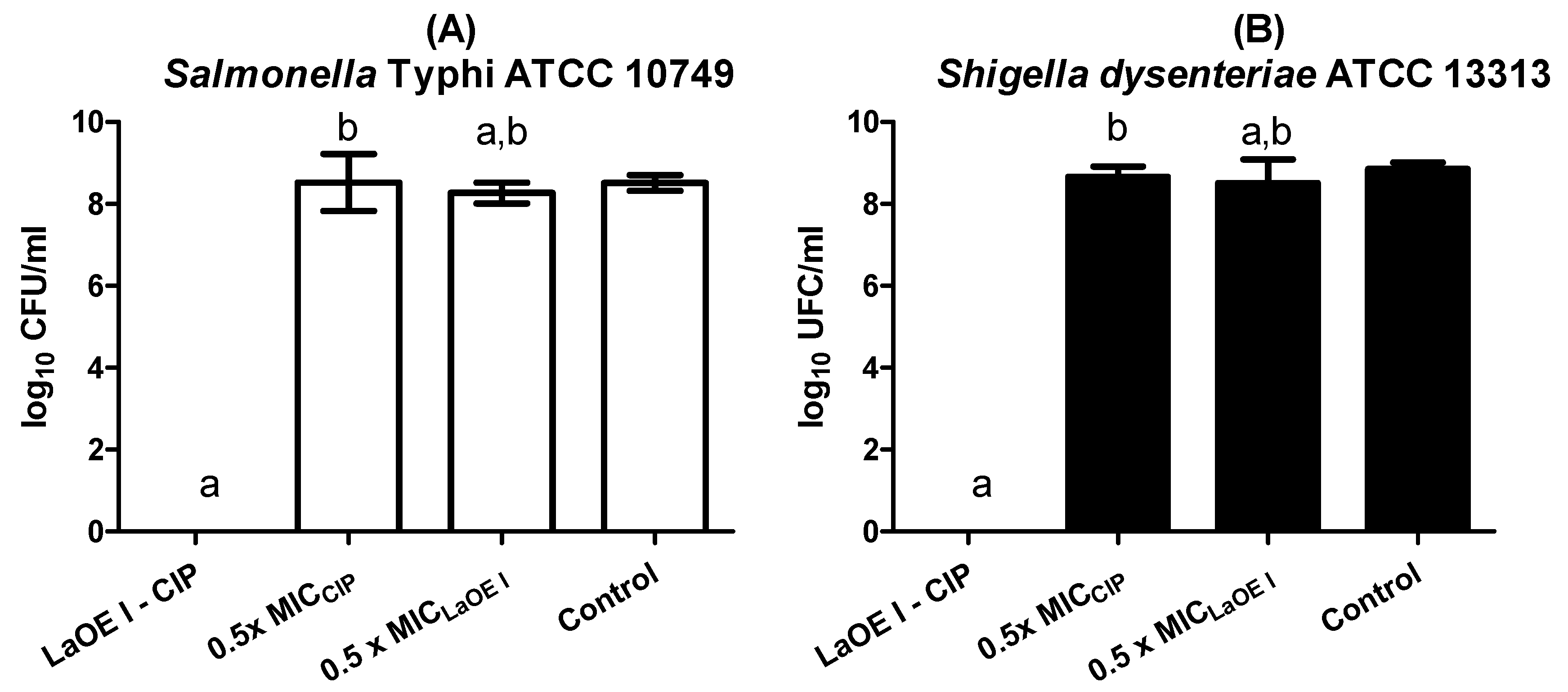
3.4. Effect of Time of Exposure to LaEO I and LaEO I-CIP Association on Microbial Viability (Time kill)
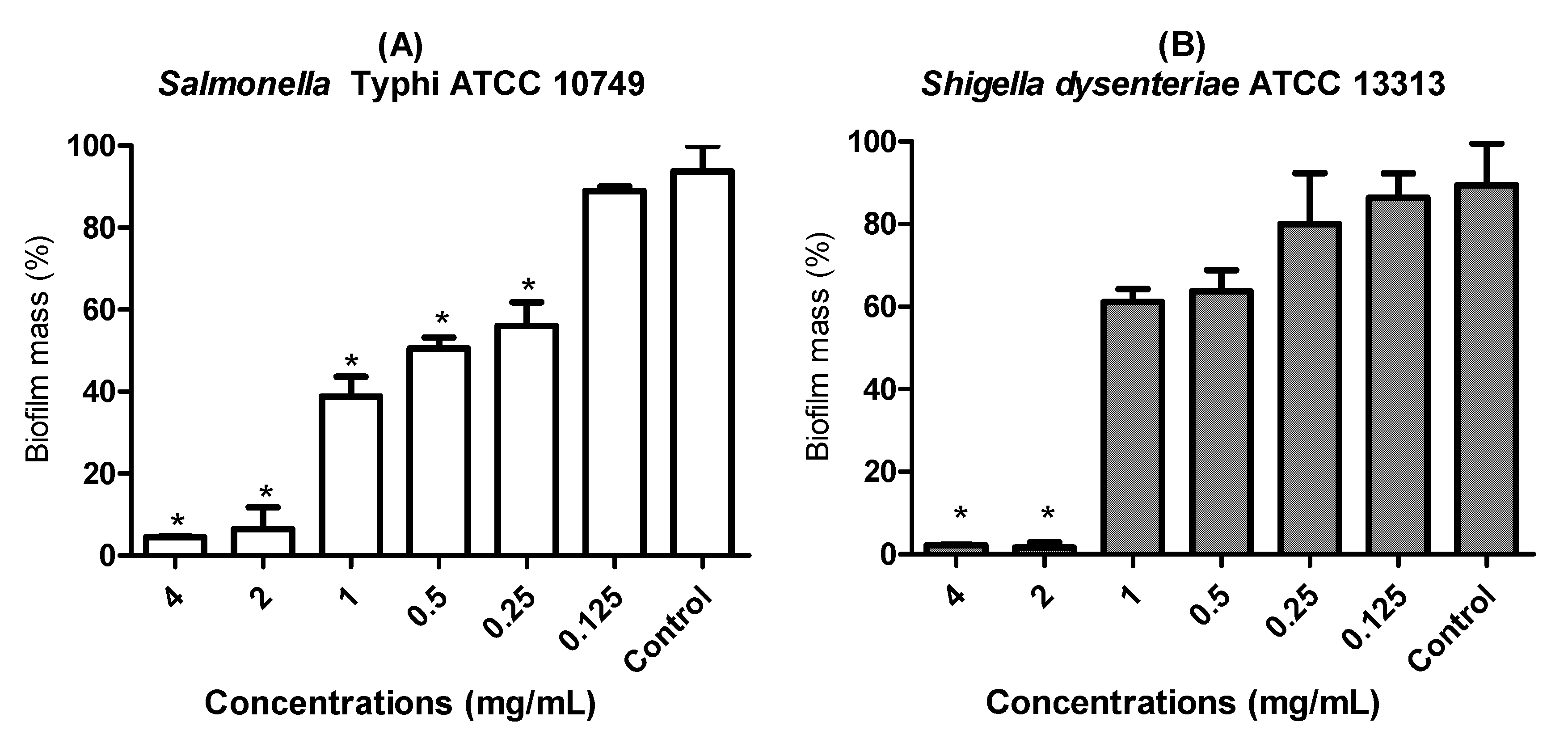
3.5. Anti-Biofilm Effect of LaEO I-CIP Association on Biofilm Formation Inhibition
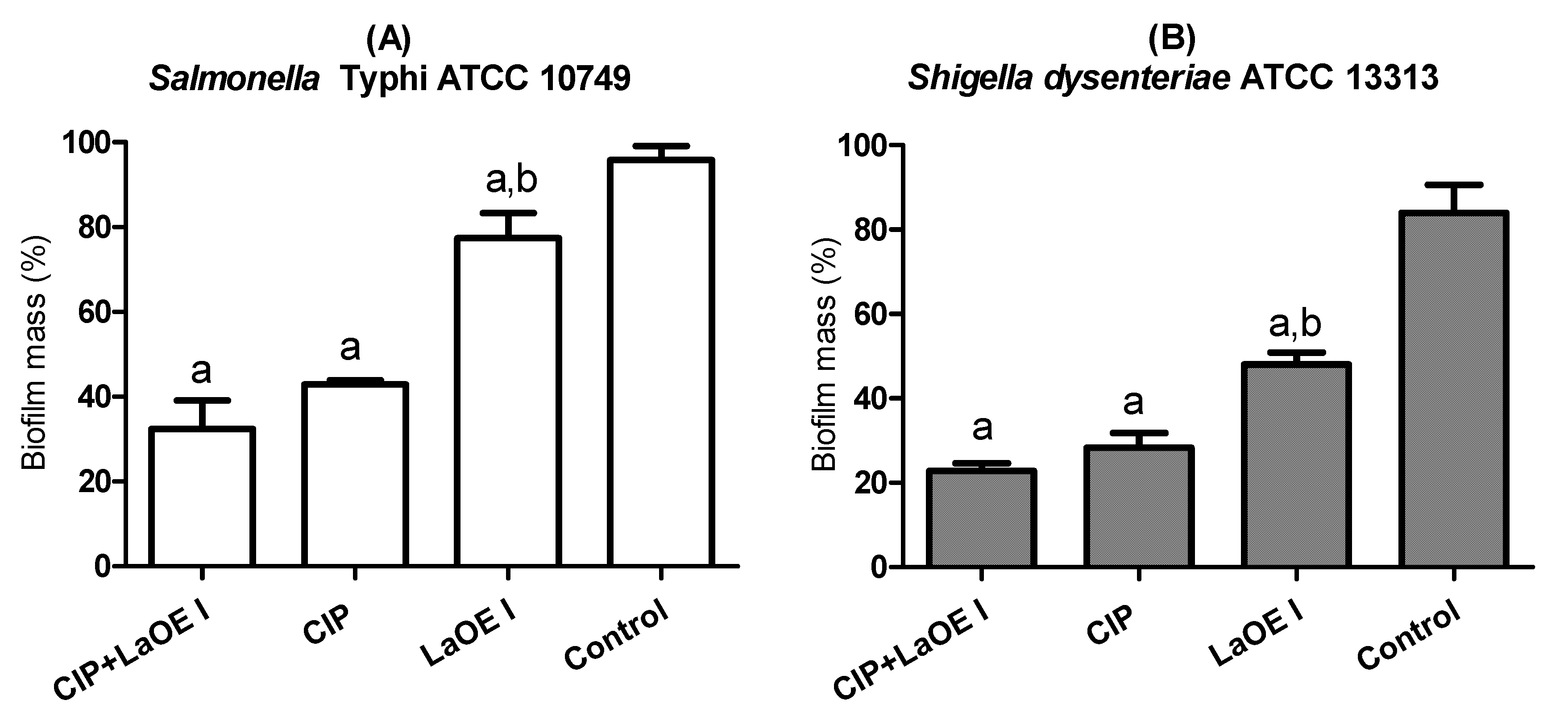
3.6. Anti-Biofilm Effect of the LaEO I-CIP Association on Biofilm Eradication
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. WHO’s First Ever Global Estimates of Foodborne Diseases Find Children under 5 Account for almost One Third of Deaths—2015. Available online: http://www.who.int/mediacentre/news/releases/2015/foodborne-diseaseestimates/en/ (accessed on 28 January 2017).
- Fhogartaigh, C.N.; Edgeworth, J.D. Bacterial gastroenteritis. Medicine 2009, 37, 586–593. [Google Scholar]
- BRASIL. Ministério da Saúde. Surtos de Doenças Transmitidas por Alimentos no Brasil. 2016. Available online: http://portalarquivos.saude.gov.br/images/pdf/2016/junho/08/Apresenta----o-Surtos-DTA-2016.pdf (accessed on 15 December 2016).
- Capalonga, R.; Ramos, R.C.; Both, J.M.; Soeiro, M.L.; Longaray, S.M.; Haas, S.; Tondo, E.C. Salmonella serotypes, resistance patterns, and food vehicles of salmonellosis in southern Brazil between 2007 and 2012. J. Infect. Dev. Ctries. 2014, 8, 811–817. [Google Scholar] [CrossRef] [PubMed]
- The, H.C.; Thanh, D.P.; Holt, K.E.; Thomson, N.R.; Baker, S. The genomic signatures of Shigella evolution, adaptation and geographical spread. Nat. Rev. Microbiol. 2016, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.W.; Gibson, D.L.; Kay, W.W.; Gunn, J.S. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 2008, 76, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, U.F.; Igwenagu, E.; Mu’azu, A.; Aliyu, S.; Umar, M.I. Intrigues of biofilm: A perspective in veterinary medicine. Vet. World 2016, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- García-Fernández, A.; Gallina, S.; Owczarek, S.; Dionisi, A.M.; Benedetti, I.; Decastelli, L.; Luzzi, I. Emergence of ciprofloxacin-resistant Salmonella enterica serovar Typhi in Italy. PLoS ONE 2015, 10, e0132065. [Google Scholar] [CrossRef]
- Gu, B.; Cao, Y.; Pan, S.; Zhuang, L.; Yu, R.; Peng, Z.; Tong, M. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe–America and Asia–Africa from 1998 to 2009. Int. J. Antimicrob. Agents 2012, 40, 9–17. [Google Scholar] [CrossRef]
- Braga, M.E.; Ehlert, P.A.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from Lippia alba: Global yields, kinetic data, and extract chemical composition. J. Supercrit. Fluid 2005, 34, 149–156. [Google Scholar] [CrossRef]
- Julião, L.S.; Tavares, E.S.; Lage, C.L.S.; Leitão, S.G. Cromatografia em camada fina de extratos de três quimiotipos de Lippia alba (Mill) NE Br.(erva-cidreira). Rev. Bras. Farmacogn 2003, 13, 36–38. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Macêdo, C.A.; Ribeiro, T.F.; Silva, A.A.; Da Silva, R.E.; de Morais, L.P.; Barbosa, R. Effect of the Lippia alba (Mill.) NE Brown essential oil and its main constituents, citral and limonene, on the tracheal smooth muscle of rats. Biotechnol. Rep. 2018, 17, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.G.; Costa-Júnior, L.M.; Blank, A.F.; da Silva Lima, A.; Menezes, T.S.A.; de Alexandria Santos, D.; de Fátima Arrigoni-Blank, M. Acaricidal activity of essential oils from Lippia alba genotypes and its major components carvone, limonene, and citral against Rhipicephalus microplus. Vet. Parasitol. 2015, 210, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.A.S. de. Influência dos fatores abióticos na composição química dos óleos essenciais. Hortic. Bras. 2009, 27, 4050–4063. [Google Scholar]
- Matos, F.J.A. As ervas cidreiras do Nordeste do Brasil. Estudo de três quimiotipos de Lippia alba (Mill) N.E. Brown (Verbenaceae). Parte I Farmacognosia. Rev. Bras. Farmacogn 1996, 72, 65–67. [Google Scholar]
- Machado, T.F.; Nogueira, N.A.P.; Pereira, R.D.C.A.; Sousa, C.T.D.; Batista, V.V. The antimicrobial efficacy of Lippia alba essential oil and its interaction with food ingredients. Braz. J. Microbiol. 2014, 45, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; Carvalho, M.G.D.; Júnior, F.E.A.C. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World J. 2017, 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.T.; Marques, R.A.; Silva, M.G.; Scherer, D.; Haubner, R.; Ulrich, C.M.; Owen, R.W. Composition of essential oils and ethanol extracts of the leaves of Lippia species: Identification, quantitation and antioxidant capacity. Rec. Nat. Prod. 2016, 10, 485. [Google Scholar]
- Adams, R.P. Review of Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2007, 18, 803–806. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard—Tenth Edition; CLSI document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Romeiro, R. Técnica de Microgota Para Contagem de Células Bacterianas Viáveis em uma Suspensão; UFV Viçosa: Viçosa, Brazil, 2007. [Google Scholar]
- Baron, E.J.; Peterson, L.R.; Finegold, S.M. Bailey & Scott’s Diagnostic Microbiology, 9th ed.; Mosby: St. Louis, MO, USA, 1994. [Google Scholar]
- Cleeland, R.; Squires, E. Evaluation of new antimicrobials in vitro and in experimental animal infections. Antibiot. Lab. Med. 1991, 3, 739–787. [Google Scholar]
- Shin, S.; Lim, S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J. Appl. Microbiol. 2004, 97, 1289–1296. [Google Scholar] [CrossRef]
- EUCAST. Determination of minimum inhibitory concentrations (MICs) of antibacterial agentes by broth diluition. Clin. Microbiol. J. 2003, 9, 1–7. [Google Scholar]
- Dodou, H.V.; de Morais Batista, A.H.; Sales, G.W.P.; de Medeiros, S.C.; Rodrigues, M.L.; Nogueira, P.C.N.; Nogueira, N.A.P. Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. J. Appl. Microbiol. 2017, 123, 853–860. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Anthony, J.A. In vitro antibacterial and time-kill assessment of crude methanolic stem bark extract of Acacia mearnsii De Wild against bacteria in shigellosis. Molecules 2012, 17, 2103–2118. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Mah, T.-F. Establishing the minimal bactericidal concentration of an antimicrobial agent for planktonic cells (MBC-P) and biofilm cells (MBC-B). J. Vis. Exp. 2014, 83, 50854. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Rivera, A.; Ortega-Cuadros, M.; Galvis-Pareja, D.; Jiménez-Rios, H.; Merini, L.J.; Martínez-Pabón, M.C. Effect of Lippia alba and Cymbopogon citratus essential oils on biofilms of Streptococcus mutans and cytotoxicity in CHO cells. J. Ethnopharmacol. 2016, 194, 749–754. [Google Scholar]
- Tavares, I.B.; Momenté, V.G.; do Nascimento, I.R. Lippia alba: Estudos químicos, etnofarmacológicos e agronômicos. Pesqui. Apl. Agrotecnol. 2011, 4, 204–220. [Google Scholar] [CrossRef]
- Szabo, M.R.; Radu, D.; Gavrilas, S.; Chambre, D.; Iditoiu, C. Antioxidant and antimicrobial properties of selected spice extracts. Int. J. Food Prop. 2010, 13, 535–545. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Pontone, M.; Toma, L.; Ensoli, F. Biofilm producing Salmonella typhi: Chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 2017, 18, 1887. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Molecular basis of Staphylococcus epidermidis infections. Semin. Immunopathol. 2012, 34, 2. [Google Scholar] [CrossRef]
- Aguila-Arcos, S.; Ding, S.; Aloria, K.; Arizmendi, J.M.; Fearnley, I.M.; Walker, J.E.; Alkorta, I. A commensal strain of Staphylococcus epidermidis overexpresses membrane proteins associated with pathogenesis when grown in biofilms. J. Membr. Biol. 2015, 248, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Sahal, G.; Bilkay, I.S. Multi drug resistance in strong biofilm forming clinical isolates of Staphylococcus epidermidis. Braz. J. Microbiol. 2014, 45, 539–544. [Google Scholar] [CrossRef]
- Zaidi, M.B.; Estrada-García, T. Shigella: A highly virulent and elusive pathogen. Curr. Trop. Med. Rep. 2014, 1, 81–87. [Google Scholar] [CrossRef]
| Number of Peak | Retention Time (min) | Formula | Component | % Area |
|---|---|---|---|---|
| 1 | 15.154 | C8H14O | Sulcatone | 0.95 |
| 2 | 15.416 | C10H16 | Myrcene | 12.56 |
| 3 | 17.480 | C10H14 | p-cymene | 4.06 |
| 4 | 19.527 | C10H16 | γ-Terpinene | 0.97 |
| 5 | 21.883 | C10H18O | Linalool | 1.45 |
| 6 | 26.890 | C10H16O | Camphor | 0.74 |
| 7 | 30.459 | C10H16O | Neral (cis-citral) | 25.90 |
| 8 | 30.762 | C10H14O | Carvone | 2.06 |
| 9 | 31.138 | C10H18O | Nerol | 0.74 |
| 10 | 32.160 | C10H16O | Geranial (trans-citral) | 34.16 |
| 11 | 38.387 | C15H24 | α-copaene | 3.13 |
| 12 | 40.927 | C15H24 | trans-Caryophyllene | 4.03 |
| 13 | 44.867 | C15H24 | Germacrene | 1.08 |
| 14 | 46.559 | C15H24 | γ-muurolene | 0.41 |
| 15 | 47.176 | C15H26O | Elemol | 1.88 |
| 16 | 47.316 | C15H26O | Nerolidol | 0.70 |
| 17 | 47.924 | C15H24O | Caryophyllene oxide | 3.47 |
| 18 | 48.062 | C15H26O | Guaiol | 0.58 |
| 19 | 48.857 | C15H26O | β-Eudesmol | 0.75 |
| 20 | 50.992 | C15H26O | Hedycaryol | 0.38 |
| Monoterpene | 17.59 | |||
| Oxygenated monoterpene | 39.15 | |||
| Sesquiterpene | 34.55 | |||
| Oxygenated sesquiterpene | 7.76 | |||
| Ketone | 0.95 | |||
| Strain | LaEO I 1 (mg/mL) | CIP 2 (μg/mL) | |
|---|---|---|---|
| MIC 3 | MBC 4 | MIC | |
| Salmonella typhi ATCC 10749 | 1 | 1 | 0.25 |
| Shigella dysenteriae ATCC 13313 | 1 | 1 | 0.25 |
| Salmonella typhi ATCC 10749 | Shigella dysenteriae ATCC 13313 | |||||||
|---|---|---|---|---|---|---|---|---|
| LaEO I-ATM | FICLaEO I 1 | FICATM 2 | FICI 3 | ME 4 | FICLaEO I 1 | FICATM 2 | FICI 3 | ME 4 |
| LaEO I-CIP 5 | 0.0625 | 0.0624 | 0.1249 | S | 0.0625 | 0.0624 | 0.1249 | S |
| LaEO I-AMI 6 | 0 | 0 | 0 | ND | 0 | 0 | 0 | ND |
| LaEO I-CAZ 7 | 0 | 0 | 0 | ND | 0 | 0 | 0 | ND |
| LaEO I-CPM 8 | 0.0625 | 0.125 | 0.1875 | S | 0.0625 | 0.0625 | 0.1249 | S |
| LaEO I-CRO 9 | 0.0625 | 0.25 | 0.3125 | S | 0.0625 | 0.0625 | 0.1249 | S |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, A.; Dodou, H.; Rodrigues, M.; Pereira, P.; Sales, G.; Medeiros, S.; Nogueira, N. Modulatory Effect of Lippia alba Essential Oil on the Activity of Clinically Used Antimicrobial Agents on Salmonella typhi and Shigella dysenteriae Biofilm. Sci. Pharm. 2018, 86, 52. https://doi.org/10.3390/scipharm86040052
Batista A, Dodou H, Rodrigues M, Pereira P, Sales G, Medeiros S, Nogueira N. Modulatory Effect of Lippia alba Essential Oil on the Activity of Clinically Used Antimicrobial Agents on Salmonella typhi and Shigella dysenteriae Biofilm. Scientia Pharmaceutica. 2018; 86(4):52. https://doi.org/10.3390/scipharm86040052
Chicago/Turabian StyleBatista, Andressa, Hilania Dodou, Matheus Rodrigues, Pedro Pereira, Gleilton Sales, Suelen Medeiros, and Nádia Nogueira. 2018. "Modulatory Effect of Lippia alba Essential Oil on the Activity of Clinically Used Antimicrobial Agents on Salmonella typhi and Shigella dysenteriae Biofilm" Scientia Pharmaceutica 86, no. 4: 52. https://doi.org/10.3390/scipharm86040052
APA StyleBatista, A., Dodou, H., Rodrigues, M., Pereira, P., Sales, G., Medeiros, S., & Nogueira, N. (2018). Modulatory Effect of Lippia alba Essential Oil on the Activity of Clinically Used Antimicrobial Agents on Salmonella typhi and Shigella dysenteriae Biofilm. Scientia Pharmaceutica, 86(4), 52. https://doi.org/10.3390/scipharm86040052






