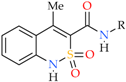
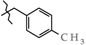
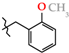
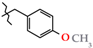
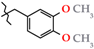
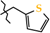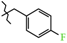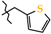Abstract
The analysis of our previous studies on the search for synthetic analgesics among N-R-amides of bicyclic hetaryl-3-carboxylic acids has been performed; on its basis N-hetaryl(aryl)-alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides have been selected as new study objects. The “one pot synthesis” of these compounds, which is simple to perform and at the same time highly effective, has been offered. The method consists in the initial reaction of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid and N,N′-carbonyldiimidazole in anhydrous N,N-dimethylformamide with the subsequent amidation of imidazolide formed with hetarylalkyl- or benzylamines in the same solvent. The peculiarities of 1H- and 13C-NMR spectra of the substances obtained, as well as their electrospray ionization liquid chromato-mass spectra are discussed. According to the results of the pharmacological tests carried out on the model of carrageenan inflammation it has been found that all without exception N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides demonstrate the statistically significant analgesic and anti-inflammatory properties. Among the substances presented in this article analgesics and antiphlogistics, which increase the pain threshold and suppress the inflammatory response more effectively than Lornoxicam and Diclofenac in the same doses, have been identified. The molecular and crystal structures of a large group of the substances synthesized have been studied by X-ray diffraction analysis. Comparison of these data with the results of biological tests has revealed the fact of excellent correlation between the molecular conformations of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides recorded in the crystal and the potency of their analgesic effect. N-Thiophen-2-ylmethyl- and N-4-methoxybenzyl-amides of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid has shown a high analgesic and anti-inflammatory effect, therefore, they deserve more careful research.
1. Introduction
For the first time, the analgesic properties of 4-hydroxy-2-quinolones were accidentally detected in pharmacological tests of N-[(dialkylamino)alkyl]-1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxamides hydrochlorides with the general formula I (Figure 1). These compounds were synthesized on the basis of the preliminary virtual screening and were initially tested as potential opioid receptors antagonists [1]. But very soon the direction of our research began to shift towards the search for analgesics since amides I much more often showed the ability to decrease the pain threshold than to block opioid receptors. First in the circle of the study objects alkyl-, arylalkyl-, and hetarylalkylamides of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid [2,3] were involved, then 1-N-ethyl derivatives II (R = Et) [4] and, finally, their analogs II unsubstituted in position 1 (R = H) [5,6]. As a result, only one compound was selected from the whole group—N-(pyridin-3-ylmethyl)-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxamide (III), which effectively relieved pain of the central and peripheral origin. Being an almost non-toxic substance, pyridin-3-ylmethylamide III revealed its analgesic properties mainly through the activation of nicotinic acetylcholine receptors, caused the ulcerogenic action in a dose significantly superior to the therapeutic one, and it allowed to recommend it to the preclinical tests as a promising pain-killer [4].
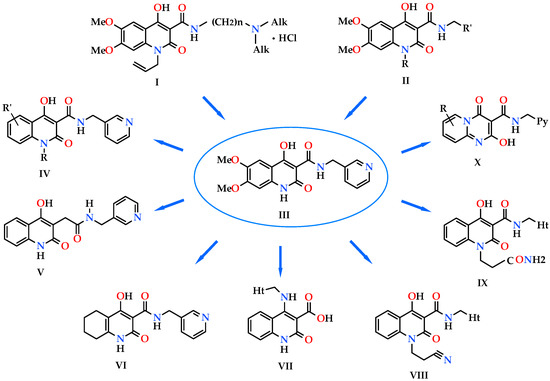
Figure 1.
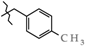
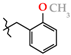
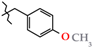
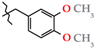
Analgesics created on the basis of hetarylalkylamines and bicyclic hetaryl-3-carboxylic acids [1,2,3,4,5,6,7,8,9,10,11].
Meanwhile, having decided on the lead compound we did not stop searching for new analgesics. Moreover, this search now has a purposeful nature, and actually it has become a study of close structural analogs of pyridin-3-ylmethylamide III, in which molecule various chemical changes were introduced, but by preserving the aminoalkylhetaryl fragment.
Thus, in particular, it has been found that the substituents in the benzene moeity of the quinolone nucleus, as well as at the cyclic nitrogen atom (amides IV), have very little effect on the analgesic properties [4]. But the removal of the terminal pyridin-3-ylmethylaminocarbonyl group from the bicyclic base by only one methylene link, i.e., transition from quinoline-carboxylic to quinoline-acetic acids (amide V), is accompanied by an approximate two times decrease in activity [4].
The high analgesic activity of N-(pyridin-3-ylmethyl)-4-hydroxy-2-oxo-1,2,5,6,7,8-hexahydro-quinoline-3-carboxamide (VI) detected during the primary pharmacological screening initially caused an increased interest in it as a potentially new lead compound. However, very soon this substance was found to have an increased propensity to form numerous polymorphic forms, and in most cases they were low active forms [7]. Unfortunately, factors causing changes in the phase composition are still unclear. Therefore, further study of amide VI as an analgesic is considered inappropriate, at least, until the conditions, which would allow obtaining exceptionally high active crystalline modifications of the substance in pharmacological respect, are found, and they will be able to guarantee their stability during storage.
Transfer of the aminoalkylhetaryl fragment to position 4 of the quinolone nucleus is very interesting as for pharmacology [8], but at the same time it creates pharmaceutical problems—acids VII appeared to be insufficiently stable compounds and decarboxylated relatively easy (in solution even at room temperature [9]).
Quinolonepropanenitriles VIII, products of their hydration (quinolonepropaneamides IX) and hydrolysis (the corresponding quinolonepropanoic acids) are deprived of this drawback. Interest to substances of this group is generated by their expressed analgesic properties [10], as well as by their practically unlimited possibilities for further chemical transformations.
In general, N-(pyridinylmethyl)-2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxamides (X) are somewhat inferior in activity to quinoline analogs although highly active analgesics have been identified among them [11].
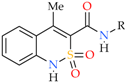
Summing up this short review two important, in our opinion, factors should be noted: the presence of the hetarylalkylamide fragment in the molecule contributes to the manifestation of analgesic properties, and rather great changes are permissible in the structure of the bicyclic hetaryl-3-carboxylic acid. Hence, our next step in the search for new analgesics—N-hetarylalkyl-substituted amides of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid seems quite logical and natural, and this message is devoted to these amides. The simultaneous involvement of the structurally close N-benzyl-substituted analogs of these compounds in the circle of objects under study is also of interest, i.e., the actual replacement of a heterocycle in the amide fragment by a phenyl or substituted phenyl nucleus. The expediency and even the need for such an addition become more obvious and reasonable if we take into account the high efficiency and productivity of the methodology of bioisosteric replacements [6,12,13,14,15,16,17], which is well-proven when creating new biologically active substances.
2. Materials and Methods
2.1. Chemistry
1H- and 13C-NMR (proton and carbon nuclear magnetic resonance) spectra were obtained on a Varian Mercury-400 (Varian Inc., Palo Alto, CA, USA) instrument (400 and 100 MHz, respectively) in hexadeuterodimethyl sulfoxide (DMSO-d6) with tetramethylsilane as internal standard. The chemical shift values were recorded on a δ scale and the coupling constants (J) in hertz. The following abbreviations were used in reporting spectra: s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, m = multiplet. The electrospray ionization liquid chromato-mass spectra (ESI-LC/MS) were recorded on a modular Agilent Technologies 1260 Infinity system with 6530 Accurate-Mass Q-TOF LC/MS (G6530B#200 ESI) mass-spectrometric detector (Agilent Technologies, Inc., Santa Clara, CA, USA). The chromatography conditions were: Agilent Extend-C18 column of 2.1 × 50 mm; the sorbent particle size 1.8 μm; the mobile phase flow rate 0.25 mL/min; the column temperature 30 °C; the injection volume 1.0 μL; the mobile phase composition 0.1% formic acid in methanol. The elemental analysis was performed on a Euro Vector EA-3000 (Eurovector SPA, Redavalle, Italy) microanalyzer. The melting points were determined in a capillary using a electrothermal IA9100X1 (Bibby Scientific Limited, Stone, UK) digital melting point apparatus. In the synthesis of N-hetaryl(aryl)alkyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k described in this article the commercial N,N′-carbonyldiimidazole (CDI) and the anhydrous N,N-dimethylformamide (DMF) for peptide synthesis of Aldrich company (St. Louis, MO, USA) were used. The synthesis of the starting methyl 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxyate (1) and anhydrous 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid (3) was carried out by the methods described in [17,18] respectively.
2.2. General Procedure for the Synthesis of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k
N,N′-Carbonyldiimidazole (1.78 g, 0.011 mol) was added to a solution of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid 3 (2.39 g, 0.01 mol) in anhydrous DMF (5 mL) and protected from atmospheric moisture using a CaCl2 tube. It was kept for approximately 2 h at 90 °C until CO2 evolution had ceased. To remove CO2 residues, dry argon was passed in the reaction mixture through a thin capillary for 5 min, after that 0.01 mol of the corresponding hetarylalkylamine or benzylamine was added and kept for 4 h at the temperature of 90 °C. The reaction mixture was cooled, diluted by adding cold water, and adjusted to pH ~4 by adding dilute (1:1) hydrochloric acid (acetic acid was used in the isolation of N-pyridinylmethyl- and N-imidazolylpropyl-amides 2a–c,i). The precipitate formed was filtered, washed with cold water, dried, and recrystallized from ethanol. Hetarylalkylamides 2a–i and benzylamides 3a–k were colorless or white with yellowish crystals.
N-(Pyridin-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2a). The yield was: 2.99 g (91%); colorless crystals; melting point (mp) 177–179 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.63 (br. s, 1H, SO2NH), 9.10 (t, 1H, J = 5.6, CONH), 8.49 (d, 1H, J = 5.2, H-6′), 7.76 (t, 1H, J = 7.2, H-4′), 7.71 (d, 1H, J = 8.4, H-5), 7.45 (t, 1H, J = 7.6, H-7), 7.41 (d, 1H, J = 8.0, H-3′), 7.25 (t, 1H, J = 6.0, H-5′), 7.19 (t, 1H, J = 7.6, H-6), 7.11 (d, 1H, J = 8.0, H-8), 4.51 (d, 2H, J = 5.6, NCH2), 2.33 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.8 (C=O), 158.4, 149.4, 140.0, 138.0, 137.3, 131.8, 131.5, 127.7, 123.3, 122.7, 121.5, 121.1, 118.6 (3-C), 45.2 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 330 (100) [M + H]+, 222 (22), 109 (10) [pyridin-2-ylmethylamine + H]+. This was analytically calculated (Anal. Calcd.) for C16H15N3O3S: C, 58.35; H, 4.59; N, 12.76; S, 9.73%. We found: C, 58.44; H, 4.68; N, 12.85; S 9.64%.
N-(Pyridin-3-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2b). The yield was: 3.06 g (93%); colorless crystals; mp 243–245 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.67 (br. s, 1H, SO2NH), 9.13 (t, 1H, J = 6.0, CONH), 8.55 (s, 1H, H-2′), 8.45 (d, 1H, J = 4.4, H-6′), 7.73 (d, 1H, J = 7.6, H-4′), 7.69 (d, 1H, J = 8.4, H-5), 7.45 (t, 1H, J = 7.6, H-7), 7.35 (t, 1H, J = 6.4, H-5′), 7.18 (t, 1H, J = 7.6, H-6), 7.11 (d, 1H, J = 8.2, H-8), 4.44 (d, 2H, J = 6.0, NCH2), 2.24 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.7 (C=O), 149.2, 148.7, 139.8, 138.0, 135.7, 134.8, 131.8, 131.5, 127.7, 123.9, 123.3, 121.1, 118.6 (3-C), 40.9 (NHCH2), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 330 (100) [M + H]+, 222 (24), 109 (11) [pyridin-3-ylmethylamine + H]+. The Anal. Calcd. was for C16H15N3O3S: C, 58.35; H, 4.59; N, 12.76; S, 9.73%. We found: C, 58.43; H, 4.51; N, 12.82; S 9.80%.
N-(Pyridin-4-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide monohydrate (2c). The yield was: 3.12 g (90%); colorless crystals; mp 252–254 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.61 (br. s, 1H, SO2NH), 9.15 (t, 1H, J = 6.0, CONH), 8.50 (d, 2H, J = 5.6, H-2′,6′), 7.70 (d, 1H, J = 8.0, H-5), 7.45 (t, 1H, J = 7.6, H-7), 7.34 (d, 2H, J = 5.6, H-3′,5′), 7.19 (t, 1H, J = 7.6, H-6), 7.12 (d, 1H, J = 8.0, H-8), 4.45 (d, 2H, J = 6.0, NCH2), 2.29 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.9 (C=O), 149.9 (C-2′,6′), 148.4, 139.9, 138.0, 131.8, 131.4, 127.7, 123.3, 122.7 (C-3′,5′), 121.1, 118.6 (3-C), 42.3 (NHCH2), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 330 (100) [M + H]+, 222 (28), 109 (14) [pyridin-4-ylmethylamine + H]+. The Anal. Calcd. was for C16H15N3O3S ∙ H2O: C, 55.32; H, 4.93; N, 12.10; S, 9.23%. We found: C, 55.30; H, 4.85; N, 12.01; S 9.30%.
N-(Furan-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2d). The yield was: 3.02 g (95%); white with yellowish crystals; mp 190–192 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.58 (br. s, 1H, SO2NH), 9.00 (t, 1H, J = 6.0, CONH), 7.68 (dd, 1H, J = 8.0 and 1.2, H-5), 7.55 (d, 1H, J = 1.6, H-5′), 7.44 (tt, 1H, J = 7.6 and 1.2, H-7), 7.18 (tt, 1H, J = 8.0 and 1.2, H-6), 7.10 (dd, 1H, J = 8.0 and 1.2, H-8), 6.38 (dd, 1H, J = 3.2 and 1.6, H-4′), 6.28 (d, 1H, J = 3.2, H-3′), 4.39 (d, 2H, J = 6.0, NCH2), 2.24 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.5 (C=O), 152.1, 142.7, 139.8, 138.0, 131.7, 131.4, 127.7, 123.3, 121.1, 118.5 (3-C), 111.0, 107.5, 36.6 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 319 (43) [M + H]+, 222 (24), 98 (9) [furan-2-ylmethylamine + H]+. The Anal. Calcd. was for C15H14N2O4S: C, 56.59; H, 4.43; N, 8.80; S, 10.07%. We found: C, 56.66; H, 4.50; N, 8.73; S 10.15%.
N-(5-Methylfuran-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2e). The yield was: 3.19 g (96%); white with yellowish crystals; mp 179–181 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.56 (br. s, 1H, SO2NH), 8.94 (t, 1H, J = 6.0, CONH), 7.68 (d, 1H, J = 8.4, H-5), 7.44 (tt, 1H, J = 7.6 and 1.0, H-7), 7.18 (tt, 1H, J = 7.6 and 1.0, H-6), 7.10 (d, 1H, J = 8.0, H-8), 6.14 (d, 1H, J = 3.1, H-3′), 5.97 (dt, 1H, J = 3.1 and 1.2, H-4′), 4.33 (d, 2H, J = 5.8, NCH2), 2.26 (s, 3H, CH3), 2.21 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 151.1, 150.2, 139.8, 138.0, 131.7, 131.5, 127.7, 123.3, 121.1, 118.5 (3-C), 108.2, 106.9, 36.7 (NHCH2), 17.7 (4-CH3), 13.8 (5′-CH3). ESI-LC/MS (m/z, %): 333 (44) [M + H]+, 222 (39), 112 (7) [5-methylfuran-2-ylmethylamine + H]+. The Anal. Calcd. was for C16H16N2O4S: C, 57.82; H, 4.85; N, 8.43; S, 9.65%. We found: C, 57.75; H, 4.91; N, 8.36; S 9.58%.
N-(Tetrahydrofuran-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2f). The yield was: 2.84 g (88%); colorless crystals; mp 154–156 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.55 (br. s, 1H, SO2NH), 8.51 (t, 1H, J = 6.0, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.4, H-7), 7.18 (t, 1H, J = 7.6, H-6), 7.09 (d, 1H, J = 8.0, H-8), 3.93 (quin, 1H, J = 6.0, 2′-CHO), 3.75 (q, 1H, J = 6.8, 5′-CHO), 3.61 (q, 1H, J = 6.8, 5′-CHO), 3.32-3.22 (m, 2H, NCH2), 2.28 (s, 3H, 4-CH3), 1.93–1.85 (m, 1H, 3′-CH), 1.83-1.73 (m, 2H, 4′-CH2), 1.63-1.56 (m, 1H, 3′-CH). 13C-NMR (100 MHz, DMSO-d6): δ 160.6 (C=O), 139.5, 138.0, 131.8, 131.6, 127.7, 123.2, 121.1, 118.5 (3-C), 77.3 (2′-C), 67.7 (5′-C), 43.6 (NHCH2), 29.1 (3′-C), 25.6 (4′-C), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 323 (100) [M + H]+, 222 (33), 102 (9) [tetrahydrofuran-2-ylmethylamine + H]+. The Anal. Calcd. was for C15H18N2O4S: C, 55.89; H, 5.63; N, 8.69; S, 9.95%. We found: C, 55.97; H, 5.58; N, 8.76; S 10.03%.
N-(Thiophen-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2g). The yield was: 3.01 g (90%); white with yellowish crystals; mp 195–197 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.59 (br. s, 1H, SO2NH), 9.13 (t, 1H, J = 5.8, CONH), 7.69 (dd, 1H, J = 8.0 and 1.2, H-5), 7.44 (td, 1H, J = 7.2 and 1.6, H-7), 7.38 (dd, 1H, J = 5.0 and 1.2, H-5′), 7.18 (t, 1H, J = 7.6, H-6), 7.10 (d, 1H, J = 8.4, H-8), 7.02 (d, 1H, J = 3.6, H-3′), 6.95 (dd, 1H, J = 5.2 and 3.6, H-4′), 4.56 (d, 2H, J = 6.0, NCH2), 2.24 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 141.9, 139.9, 138.0, 131.8, 131.4, 127.7, 127.2, 126.3, 125.8, 123.3, 121.1, 118.5 (3-C), 38.2 (NHCH2), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 335 (36) [M + H]+, 222 (30), 114 (5) [tiophen-2-ylmethylamine + H]+. The Anal. Calcd. was for C15H14N2O3S2: C, 53.87; H, 4.22; N, 8.38; S, 19.18%. We found: C, 53.95; H, 4.17; N, 8.30; S 19.11%.
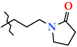
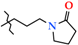
N-[3-(2-Oxopyrrolidin-1-yl)-propyl]-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2h). The yield was: 3.34 g (92%); colorless crystals; mp 107–109 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.58 (br. s, 1H, SO2NH), 8.49 (t, 1H, J = 5.4, CONH), 7.70 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.4, H-7), 7.18 (t, 1H, J = 7.4, H-6), 7.10 (d, 1H, J = 8.0, H-8), 3.32 (t, 2H, J = 7.2, NHCH2CH2CH2N), 3.24-3.15 (m, 4H, NHCH2CH2CH2N + 5′-CH2), 2.26 (s, 3H, 4-CH3), 2.19 (t, 2H, J = 8.0, 3′-CH2), 1.90 (quin, 2H, J = 8.0, NHCH2CH2CH2N), 1.66 (quin, 2H, J = 7.2, 4′-CH2). 13C-NMR (100 MHz, DMSO-d6): δ 174.5 (2′-C=O), 160.4 (C=O), 139.5, 137.9, 131.9, 131.7, 127.6, 123.3, 121.1, 118.5 (3-C), 46.9 (5′-C), 40.4 (NHCH2CH2CH2N), 37.5 (NHCH2), 30.9 (3′-C), 27.2 (NHCH2CH2CH2N), 18.1 (4′-C), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 364 (100) [M + H]+, 222 (9), 143 (4) [3-(2-oxopyrrolidin-1-yl)-propylamine + H]+. The Anal. Calcd. was for C17H21N3O4S: C, 56.18; H, 5.82; N, 11.56; S, 8.82%. We found: C, 56.13; H, 5.75; N, 11.48; S 8.73%.
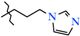
N-(3-Imidazol-1-ylpropyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2i). The yield was: 2.87 g (83%); white with yellowish crystals; mp 144–146 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.44 (br. s, 1H, SO2NH), 8.56 (t, 1H, J = 6.1, CONH), 7.83 (s, 1H, H-2′), 7.64 (dd, 1H, J = 8.0 and 1.0, H-5), 7.38 (td, 1H, J = 7.7 and 1.0, H-7), 7.25 (d, 1H, J = 1.0, H-4′), 7.09 (td, 1H, J = 7.6 and 1.1, H-6), 7.03 (d, 1H, J = 8.1, H-8), 6.99 (d, 1H, J = 1.0, H-5′), 4.04 (t, 2H, J = 6.6, NHCH2CH2CH2N), 3.14 (q, 2H, J = 6.1, NHCH2CH2CH2N), 2.25 (s, 3H, 4-CH3), 1.91 (quin, 2H, J = 6.7, NHCH2CH2CH2N). 13C-NMR (100 MHz, DMSO-d6): δ 161.4 (C=O), 140.0, 139.1, 137.5, 131.3, 130.7, 127.4, 127.2, 121.9, 120.6, 120.4, 119.2 (3-C), 44.3 (NHCH2CH2CH2N), 36.6 (NHCH2), 30.9 (NHCH2CH2CH2N), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 347 (100) [M + H]+, 222 (16), 126 (3) [3-imidazol-1-ylpropylamine + H]+. The Anal. Calcd. was for C16H18N4O3S: C, 55.48; H, 5.24; N, 16.17; S, 9.26%. We found: C, 55.56; H, 5.30; N, 16.12; S 9.33%.
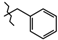
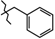
N-Benzyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3a). The yield was: 3.12 g (95%); colorless crystals; mp 225–227 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.58 (br. s, 1H, SO2NH), 9.03 (t, 1H, J = 5.7, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.45 (t, 1H, J = 7.7, H-7), 7.36-7.27 (m, 4H, H-2′,3′,5′,6′), 7.23 (t, 1H, J = 6.6, H-4′), 7.18 (t, 1H, J = 7.8, H-6), 7.11 (d, 1H, J = 8.0, H-8), 4.41 (d, 2H, J = 6.0, NCH2), 2.25 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.6 (C=O), 139.6, 139.2, 137.9, 131.7, 128.8 (2C), 127.8 (2C), 127.6, 127.4, 123.3, 123.2, 121.1, 118.5 (3-C), 43.2 (NHCH2), 17.9 (4-CH3). ESI-LC/MS (m/z, %): 329 (79) [M + H]+, 222 (28), 91 (3) [C6H5-CH2]+. The Anal. Calcd. was for C17H16N2O3S: C, 62.18; H, 4.91; N, 8.53; S 9.76%. We found: C, 62.26; H, 4.99; N, 8.44; S 9.85%.
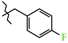
N-(4-Fluorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3b). The yield was: 3.22 g (93%); colorless crystals; mp 247–249 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.60 (br. s, 1H, SO2NH), 9.05 (t, 1H, J = 5.7, CONH), 7.68 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.7, H-7), 7.36 (t, 2H, J = 7.0, H-3′,5′), 7.18 (t, 1H, J = 7.7, H-6), 7.15-7.08 (m, 3H, H-8,3′,5′), 4.39 (d, 2H, J = 5.9, NCH2), 2.23 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 162.7/160.2 (d, JC–F = 242.4, C-4′), 160.5 (C=O), 139.7, 137.9, 135.4, 131.7, 131.6, 129.8/129.7 (d, 3JC–F = 8.1, C-2′,6′), 127.7, 123.3, 121.1, 118.5 (3-C), 115.6/115.4 (d, 2JC–F = 21.3, C-3′,5′), 42.5 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 347(81) [M + H]+, 222 (11), 109 (20) [4-F-C6H4-CH2]+. The Anal. Calcd. was for C17H15FN2O3S: C, 58.95; H, 4.36; N, 8.09; S 9.26%. We found: C, 59.04; H, 4.42; N, 8.03; S 9.17%.
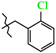
N-(2-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3c). The yield was: 3.29 g (91%); colorless crystals; mp 252–254 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.63 (br. s, 1H, SO2NH), 9.07 (t, 1H, J = 5.5, CONH), 7.71 (d, 1H, J = 8.0, H-5), 7.49 (dd, 1H, J = 7.2 and 1.5, H-3′), 7.44 (t, 1H, J = 7.6, H-7), 7.41 (d, 1H, J = 6.9, H-6′), 7.35-7.27 (m, 2H, H-4′,5′), 7.19 (t, 1H, J = 7.5, H-6), 7.12 (d, 1H, J = 8.0, H-8), 4.48 (d, 2H, J = 5.8, NCH2), 2.30 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.3 (C=O), 140.0, 137.9, 136.0, 132.5, 131.8, 131.5, 129.6, 129.4, 129.3, 127.7, 127.6, 123.3, 121.1, 118.5 (3-C), 41.2 (NHCH2), 18.0 (4-CH3). ESI-LC/MS (m/z, %): 363/365 (93/36) [M + H]+, 222 (24), 125/127 (12/5) [2-Cl-C6H4-CH2]+. The Anal. Calcd. was for C17H15ClN2O3S: C, 56.28; H, 4.17; N, 7.72; S 8.84%. We found: C, 56.37; H, 4.25; N, 7.64; S 8.92%.
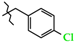
N-(4-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3d). The yield was: 3.48 g (96%); colorless crystals; mp 229–231 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.60 (br. s, 1H, SO2NH), 9.06 (t, 1H, J = 5.7, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.1, H-7), 7.38 (d, 2H, J = 9.8, H-3′,5′), 7.35 (d, 2H, J = 9.8, H-2′,6′), 7.19 (t, 1H, J = 7.6, H-6), 7.11 (d, 1H, J = 8.0, H-8), 4.40 (d, 2H, J = 5.9, NCH2), 2.24 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.6 (C=O), 139.7, 138.3, 137.9, 132.0, 131.7, 131.6, 129.7 (2C), 128.7 (2C), 127.7, 123.3, 121.1, 118.5 (3-C), 42.5 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 363/365 (87/38) [M + H]+, 222 (19), 125/127 (17/7) [4-Cl-C6H4-CH2]+. The Anal. Calcd. was for C17H15ClN2O3S: C, 56.28; H, 4.17; N, 7.72; S 8.84%. We found: C, 56.35; H, 4.26; N, 7.80; S 8.77%.
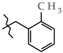
N-(2-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3e). The yield was: 3.08 g (90%); colorless crystals; mp 235–237 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.57 (br. s, 1H, SO2NH), 8.92 (t, 1H, J = 5.4, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.6, H-7), 7.32 (t, 1H, J = 4.4, H-4′), 7.18 (t, 1H, J = 7.6, H-6), 7.15-7.12 (m, 3H, H-3′,5′,6′), 7.10 (d, 1H, J = 8.1, H-8), 4.39 (d, 2H, J = 5.8, NCH2), 2.30 (s, 3H, 2′-CH3), 2.26 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 139.6, 138.0, 136.7, 136.3, 131.8, 131.6, 130.4, 128.3, 127.7, 127.5, 126.2, 123.3, 121.2, 118.5 (3-C), 41.4 (NHCH2), 19.2 (CH3), 17.9 (CH3). ESI-LC/MS (m/z, %): 347 (76) [M + H]+, 239 (<1), 222 (5), 105 (20) [2-Me-C6H4-CH2]+. The Anal. Calcd. was for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.08; H, 5.23; N, 8.21; S 9.42%.
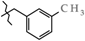
N-(3-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3f). The yield was: 3.21 g (94%); colorless crystals; mp 109–111 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.58 (br. s, 1H, SO2NH), 9.00 (t, 1H, J = 5.7, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.6, H-7), 7.22-7.17 (m, 2H, H-6,5′), 7.16 (s, 1H, H-2′), 7.12-7.08 (m, 2H, H-8,4′), 7.04 (d, 1H, J = 7.4, H-6′), 4.37 (d, 2H, J = 6.0, NCH2), 2.27 (s, 3H, 3′-CH3), 2.25 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.6 (C=O), 139.6, 139.4, 138.0, 137.8, 131.8, 131.7, 128.7, 128.5, 128.0, 127.7, 124.8, 123.3, 121.2, 118.5 (3-C), 43.1 (NHCH2), 21.5 (CH3), 17.9 (CH3). ESI-LC/MS (m/z, %): 347 (71) [M + H]+, 239 (<1), 222 (14), 105 (24) [3-Me-C6H4-CH2]+. The Anal. Calcd. was for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.06; H, 5.22; N, 8.13; S 9.29%.
N-(4-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3g). The yield was: 3.25 g (95%); colorless crystals; mp 218–220 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.57 (br. s, 1H, SO2NH), 8.98 (t, 1H, J = 5.5, CONH), 7.68 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.6, H-7), 7.21 (d, 2H, J = 7.9, H-2′,6′), 7.16 (t, 1H, J = 7.6, H-6), 7.12-7.07 (m, 3H, H-8,3′,5′), 4.36 (d, 2H, J = 5.7, NCH2), 2.31 (s, 3H, 4′-CH3), 2.26 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 139.5, 138.0, 136.4, 136.2, 131.8, 131.7, 129.3 (2C), 127.8 (2C), 127.6, 123.3, 121.1, 118.5 (3-C), 42.9 (NHCH2), 21.2 (CH3), 17.8 (CH3). ESI-LC/MS (m/z, %): 347 (75) [M + H]+, 239 (<1), 222 (4), 105 (22) [4-Me-C6H4-CH2]+. The Anal. Calcd. was for C18H18N2O3S: C, 63.14; H, 5.30; N, 8.18; S 9.36%. We found: C, 63.18; H, 5.37; N, 8.10; S 9.42%.
N-(2-Methoxybenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3h). The yield was: 3.22 g (90%); colorless crystals; mp 203–205 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.60 (br. s, 1H, SO2NH), 8.83 (t, 1H, J = 4.7, CONH), 7.69 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.5, H-7), 7.30 (d, 1H, J = 7.3, H-6′), 7.24 (t, 1H, J = 8.0, H-4′), 7.17 (t, 1H, J = 7.8, H-6), 7.10 (d, 1H, J = 8.0, H-8), 6.96 (d, 1H, J = 8.1, H-5′), 6.89 (t, 1H, J = 7.4, H-3′), 4.37 (d, 2H, J = 4.8, NCH2), 3.78 (s, 3H, 2′-OMe), 2.27 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.5 (C=O), 157.1, 139.7, 138.0, 131.8, 131.7, 128.7, 128.4, 127.7, 126.4, 123.3, 121.2, 120.6, 118.5 (3-C), 110.9, 56.8 (OCH3), 38.4 (NHCH2), 17.8 (2-CH3). ESI-LC/MS (m/z, %): 359 (77) [M + H]+, 222 (5), 121 (38) [2-MeO-C6H4-CH2]+. The Anal. Calcd. was for C18H18N2O4S: C, 60.32; H, 5.06; N, 7.82; S 8.95%. We found: C, 60.25; H, 5.14; N, 7.74; S 9.00%.
N-(4-Methoxybenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3i). The yield was: 3.33 g (93%); colorless crystals; mp 181–183 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.59 (br. s, 1H, SO2NH), 8.98 (t, 1H, J = 5.7, CONH), 7.68 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.5, H-7), 7.25 (d, 2H, J = 8.4, H-2′,6′), 7.18 (t, 1H, J = 7.6, H-6), 7.10 (d, 1H, J = 8.0, H-8), 6.87 (d, 2H, J = 8.4, H-3′,5′), 4.33 (d, 2H, J = 5.9, NCH2), 3.71 (s, 3H, 4′-OMe), 2.22 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 158.8, 139.5, 137.9, 131.8, 131.6, 131.2, 129.2 (2C), 127.6, 123.3, 121.1, 118.5 (3-C), 114.2 (2C), 55.5 (OCH3), 42.6 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 359 (36) [M + H]+, 239 (<1), 222 (13), 121 (39) [4-MeO-C6H4-CH2]+. The Anal. Calcd. was for C18H18N2O4S: C, 60.32; H, 5.06; N, 7.82; S 8.95%. We found: C, 60.27; H, 5.11; N, 7.88; S 9.04%.
N-(3,4-Dimethoxybenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3j). The yield was: 3.53 g (91%); colorless crystals; mp 186–188 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.60 (br. s, 1H, SO2NH), 8.95 (t, 1H, J = 5.7, CONH), 7.68 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.6, H-7), 7.18 (t, 1H, J = 7.6, H-6), 7.10 (d, 1H, J = 8.0, H-8), 6.96 (s, 1H, H-2′), 6.87 (d, 1H, J = 8.2, H-5′), 6.83 (d, 1H, J = 8.2, H-6′), 4.34 (d, 2H, J = 5.9, NCH2), 3.72 (s, 3H, 4′-OMe), 3.70 (s, 3H, 3′-OMe), 2.25 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 149.2, 148.2, 139.5, 137.9, 131.8, 131.7, 131.6, 127.7, 123.3, 121.1, 119.8, 118.5 (3-C), 112.1, 111.5, 56.0 (OCH3), 55.8 (OCH3), 42.8 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 389 (39) [M + H]+, 239 (2), 222 (17), 151 (42) [3,4-(MeO)2-C6H3-CH2]+. The Anal. Calcd. was for C19H20N2O5S: C, 58.75; H, 5.19; N, 7.21; S 8.25%. We found: C, 58.82; H, 5.27; N, 7.30; S 8.17%.
N-(1,3-Benzodioxol-5-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3k). The yield was: 3.57 g (96%); colorless crystals; mp 207–209 °C; 1H-NMR (400 MHz, DMSO-d6): δ 11.58 (br. s, 1H, SO2NH), 8.97 (t, 1H, J = 5.6, CONH), 7.68 (d, 1H, J = 8.0, H-5), 7.44 (t, 1H, J = 7.6, H-7), 7.18 (t, 1H, J = 7.5, H-6), 7.11 (d, 1H, J = 8.1, H-8), 6,88 (s, 1H, H-2′), 6.83 (d, 1H, J = 7.9, H-5′), 6.79 (d, 1H, J = 7.9, H-6′), 5.96 (s, 2H, O-CH2-O), 4.31 (d, 2H, J = 5.9, NCH2), 2.24 (s, 3H, 4-CH3). 13C-NMR (100 MHz, DMSO-d6): δ 160.4 (C=O), 142.7, 146.6, 139.6, 138.0, 133.1, 131.7, 131.5, 127.6, 123.2, 121.1, 121.0, 118.5 (3-C), 108.5, 108.4, 101.3 (OCH2O), 42.9 (NHCH2), 17.8 (4-CH3). ESI-LC/MS (m/z, %): 373 (39) [M + H]+, 222 (8), 135 (38) [(3-OCH2O-4)-C6H3-CH2]+. The Anal. Calcd. was for C18H16N2O5S: C, 58.06; H, 4.33; N, 7.52; S 8.61%. We found: C, 57.98; H, 4.29; N, 7.47; S 8.56%.
2.3. X-ray Structural Analysis of N-(Pyridin-4-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide monohydrate (2c)
The crystals of pyridin-4-ylmethylamide monohydrate 2c (C16H15N3O3S ∙ H2O) were monoclinic, colorless. At 273 K: a 9.0159(4), b 9.0159(4), c 14.1003(6) Å; β 101.805(3)°; V 1590.4(1) Å3, Z 4, space group P21/c, dcalc 1.451 g/cm3, µ(MoKα) 0.230 mm−1, F(000) 728. The unit cell parameters and intensities of 17,139 reflections (4,622 independent reflections, Rint = 0.056) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited, Oxford, UK) using MoKα radiation, a Charge Coupled Device (CCD) detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry, Göttingen, Germany) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The hydrogen atoms taking part in the formation of the intermolecular hydrogen bonds were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.175 for 4622 reflections (R1 0.054 for 3202 reflections with F > 4σ (F), S = 0.998). The final atomic coordinates, and the crystallographic data for the molecule of pyridin-4-ylmethylamide monohydrate 2c have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877570 [20].
2.4. X-ray Structural Analysis of N-(Furan-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2d)
The crystals of furan-2-ylmethylamide 2d (C15H14N2O4S) were monoclinic, white with yellowish. At 273 K: a 21.331(3), b 4.8822(6), c 14.649(2) Å; β 103.09(1)°; V 1486.0(3) Å3, Z 4, space group P21/c, dcalc 1.423 g/cm3, µ(MoKα) 0.238 mm−1, F(000) 664. The unit cell parameters and intensities of 15,304 reflections (4321 independent reflections, Rint = 0.172) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were found from the electron density difference maps and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.176 for 4266 reflections (R1 0.069 for 1242 reflections with F > 4σ (F), S = 0.770). The final atomic coordinates, and the crystallographic data for the molecule of furan-2-ylmethylamide 2d have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877571 [21].
2.5. X-ray Structural Analysis of N-(5-Methylfuran-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2e)
The crystals of 5-methylfuran-2-ylmethylamide 2e (C16H16N2O4S) were orthorhombic, white with yellowish. At 273 K: a 17.842(5), b 17.718(6), c 4.842(1) Å; V 1530.6(8) Å3, Z 4, space group Pna21, dcalc 1.442 g/cm3, µ(MoKα) 0.234 mm−1, F(000) 696. The unit cell parameters and intensities of 14,963 reflections (4274 independent reflections, Rint = 0.218) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were found from the electron density difference maps and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms taking part in the formation of the hydrogen bonds were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.124 for 4237 reflections (R1 0.068 for 1410 reflections with F > 4σ (F), S = 0.774). The final atomic coordinates, and the crystallographic data for the molecule of 5-methylfuran-2-ylmethylamide 2e have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877572 [22].
2.6. X-ray Structural Analysis of N-(Thiophen-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2g)
The crystals of thiophen-2-ylmethylamide 2g (C15H14N2O3S2) were monoclinic, white with yellowish. At 20 °C: a 12.0760(7), b 9.3641(5), c 14.9258(9) Å; β 111.304(7)°; V 1572.5(2) Å3, Z 4, space group P21/c, dcalc 1.413 g/cm3, µ(MoKα) 0.352 mm−1, F(000) 696. The unit cell parameters and intensities of 6539 reflections (2762 independent reflections, Rint = 0.040) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 50°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were found from the electron density difference maps and refined using the “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms). The hydrogen atoms participating in the formation of intermolecular hydrogen bonds were refined in an isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.129 for 2702 reflections (R1 0.047 for 1933 reflections with F > 4σ (F), S = 0.954). The final atomic coordinates, and the crystallographic data for the molecule of thiophen-2-ylmethylamide 2g have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1865723 [23].
2.7. X-ray Structural Analysis of N-Benzyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3a)
The crystals of benzylamide 3a (C17H16N2O3S) were monoclinic, colorless. At 273 K: a 22.251(1), b 4.8672(3), c 14.6486(9) Å; β 99.511(5)°; V 1564.6(2) Å3, Z 4, space group P21/c, dcalc 1.394 g/cm3, µ(MoKα) 0.223 mm−1, F(000) 688. The unit cell parameters and intensities of 13,948 reflections (4468 independent reflections, Rint = 0.057) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.139 for 4468 reflections (R1 0.053 for 2737 reflections with F > 4σ (F), S = 0.933). The final atomic coordinates, and the crystallographic data for the molecule of benzylamide 3a have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877573 [24].
2.8. X-ray Structural Analysis of N-(2-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3c)
The crystals of 2-chlorobenzylamide 3c (C17H15ClN2O3S) were monoclinic, colorless. At 273 K: a 22.978(2), b 4.8596(4), c 14.607(1) Å; β 97.299(8)°; V 1617.9(3) Å3, Z 4, space group P21/c, dcalc 1.490 g/cm3, µ(MoKα) 0.384 mm−1, F(000) 752. The unit cell parameters and intensities of 15,264 reflections (4711 independent reflections, Rint = 0.109) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The hydrogen atoms taking part in the formation of the intermolecular hydrogen bonds were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.156 for 4644 reflections (R1 0.063 for 2100 reflections with F > 4σ (F), S = 0.865). The final atomic coordinates, and the crystallographic data for the molecule of 2-chlorobenzylamide 3c have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877574 [25].
2.9. X-ray Structural Analysis of N-(4-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3d)
The crystals of 4-chlorobenzylamide 3d (C17H15ClN2O3S) were orthorhombic, colorless. At 273 K: a 14.670(3), b 4.8269(7), c 46.405(8) Å; V 3286(1) Å3, Z 4, space group Pca21, dcalc 1.467 g/cm3, µ(MoKα) 0.378 mm−1, F(000) 1504. The unit cell parameters and intensities of 23,754 reflections (4934 independent reflections, Rint = 0.185) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 50°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The hydrogen atoms taking part in the formation of the intermolecular hydrogen bonds were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.236 for 4895 reflections (R1 0.098 for 2848 reflections with F > 4σ (F), S = 1.009). The final atomic coordinates, and the crystallographic data for the molecule of 4-chlorobenzylamide 3d have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877575 [26].
2.10. X-ray Structural Analysis of N-(2-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3e)
The crystals of 2-methylbenzylamide 3e (C18H18N2O3S) were orthorhombic, colorless. At 273 K: a 14.575(3), b 4.863(1), c 23.864(5) Å; V 1691.6(6) Å3, Z 4, space group Pna21, dcalc 1.344 g/cm3, µ(MoKα) 0.210 mm−1, F(000) 720. The unit cell parameters and intensities of 16,633 reflections (4923 independent reflections, Rint = 0.164) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 50°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.222 for 2904 reflections (R1 0.087 for 1568 reflections with F > 4σ (F), S = 0.941). The final atomic coordinates, and the crystallographic data for the molecule of 2-methylbenzylamide 3e have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877576 [27].
2.11. X-ray Structural Analysis of N-(3-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3f)
The crystals of 3-methylbenzylamide 3f (C18H18N2O3S) were monoclinic, colorless. At 273 K: a 23.758(3), b 4.8532(4), c 14.864(1) Å; β 101.49(1)°; V 1679.5(3) Å3, Z 4, space group P21/c, dcalc 1.354 g/cm3, µ(MoKα) 0.211 mm−1, F(000) 720. The unit cell parameters and intensities of 15,723 reflections (4869 independent reflections, Rint = 0.102) were measured on an Xcalibur-3 diffractometer (Oxford Diffraction Limited) using MoKα radiation, a CCD detector, graphite monochromator, and ω-scanning to 2θmax 60°. The structure was solved by the direct method using the SHELXTL program package (Institute of Inorganic Chemistry) [19]. The positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq for the non-hydrogen atom bonded to a given hydrogen atom (n = 1.5 for methyl, and n = 1.2 for the other hydrogen atoms) of the carrier atom. The hydrogen atoms taking part in the formation of the intermolecular hydrogen bonds were refined within isotropic approximation. The structure was refined using F2 full-matrix least-squares analysis in the anisotropic approximation for non-hydrogen atoms to wR2 0.172 for 4778 reflections (R1 0.067 for 2238 reflections with F > 4σ (F), S = 0.890). The final atomic coordinates, and the crystallographic data for the molecule of 3-methylbenzylamide 3f have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC 1877577 [28].
2.12. Pharmacology: Analgesic and Anti-Inflammatory Tests
All biological experiments were carried out in full accord with the European Convention on the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and the Ukrainian Law No. 3447-IV “On protection of animals from severe treatment” [29] (project ID 3410U14, approved 15 October 2015). The pharmacological research was carried out with the permission and under the supervision of the Commission on Bioethics (N.I. Pirogov Vinnitsa National Medical University, Vinnitsa, Ukraine).
The anti-inflammatory action with the simultaneous assessment of the analgesic effect of all N-hetaryl(aryl)alkyl-substituted 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k synthesized was studied on the standard model of carrageenan edema [30,31]. The studies were conducted on white Wistar male rats weighing 200–250 g. The test substances, Lornoxicam (Wasserburger Arzneimittelwerk GmbH, Wasserburger, Germany) and Diclofenac (KRKA, Novo Mesto, Slovenia) were introduced intraperitoneally in the form of fine aqueous suspensions stabilized with Tween-80 in the screening dose of 20 mg/kg. The animals of the control group received an equivalent amount of water with Tween-80. Other details of pharmacological experiments, as well as statistical processing of the results were described in detail earlier [18].
3. Results and Discussion
3.1. Chemistry
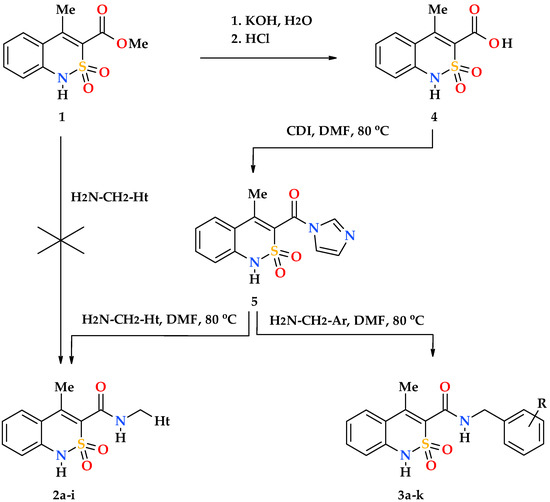
Unfortunately, the reactivity of methyl 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate (1) appeared to be extremely low. At least all our attempts to obtain target N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k by the reaction of this ester and hetarylalkylamines or benzylamines in boiling ethanol or under thermolysis at 120 °C without a solvent were unsuccessful. Taking this fact into account it was necessary to introduce an additional stage—hydrolysis of ester 1 to the corresponding acid 4 in the synthetic scheme (Scheme 1). In principle, it is possible to activate the carbon carbonyl atom of the carboxylic group of acid 4 to the level that allows obtaining its N-R-amides in different ways. In this study we used N,N′-carbonyldiimidazole, a reagent that is well-known in organic synthesis. The experiments conducted have shown that in DMF, which has a sufficiently high solubility, 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid imidazolide (5) is easily formed in relatively mild conditions. It is clear that the solvent used should not contain water, as well as the impurities of dimethylamine, ammonia and other amines that are often present in it. A commercially available anhydrous N,N-dimethylformamide for peptide synthesis meets all these criteria. In other cases, in order to avoid adverse reactions, the solvent must be purified and dried independently before starting the experiments (see, for example [32]).
Scheme 1.
Synthesis of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carbox-amides 2 and 3: 2a Ht = pyridin-2-yl; 2b Ht = pyridin-3-yl; 2c Ht = pyridin-4-yl; 2d Ht = furan-2-yl; 2e Ht = 5-methylfuran-2-yl; 2f Ht = tetrahydrofuran-2-yl; 2g Ht = thiophen-2-yl; 2h Ht = 3-(2-oxo-pyrrolidin-1-yl)-ethyl; 2i Ht = 3-imidazol-1-yl-ethyl; 3a R = H; 3b R = 4-F; 3c R = 2-Cl; 3d R = 4-Cl; 3e R = 2-Me; 3f R = 3-Me; 3g R = 4-Me; 3h R = 2-OMe; 3i R = 4-OMe; 3j R = 3,4-(OMe)2; 3k R = 3-O-CH2-O-4.
As a rule, imidazolides are intermediate compounds and are not isolated from the reaction mixture in a pure form. Our study is no exception, therefore, the whole chain of successive stages of conversion of acid 4 into imidazolide 5, and then in N-hetarylalkylamides 2a–i and benzylamides 3a–k was carried out by the principle of “one pot synthesis”, i.e., in one reactor. The method allows obtaining target compounds with high yields and purity (see Section 2.2) and can be recommended as a preparative one.
To confirm the structure of all N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k obtained the elemental analysis, 1H- and 13C-NMR spectroscopy, and electrospray ionization liquid chromato-mass spectrometry were used. The features of the spatial structure of this group of substances were studied on using X-ray diffraction analysis.
Interpretation of 1H NMR spectra of all hetarylalkylamides 2a–i and benzylamides 3a–k does not cause difficulties. The signals of all protons of the substances studied have the intensity and multiplicity corresponding to their chemical environment and are located in the spectrum regions that are typical for their nature.
In 13C NMR spectra, on the contrary, only signals of carbon atoms of methyl and methylene groups in the aliphatic part of the spectrum can be confidently identified, and signals of carbonyl and 3-C carbon atoms can be detected in the weak field (see Section 2.2). The aromatic region of 13C NMR spectra of hetarylalkylamides 2a–i and benzylamides 3a–k provides additional information only about the total number of carbon aromatic atoms. Usually, this is quite enough to confirm unambiguously the structure of the substance in combination with the data of other methods of analysis. The problem of the specific assignment of all signals of carbon aromatic atoms, if necessary, can be also solved, but it requires the additional use of special NMR methods (for example, two-dimensional experiments).
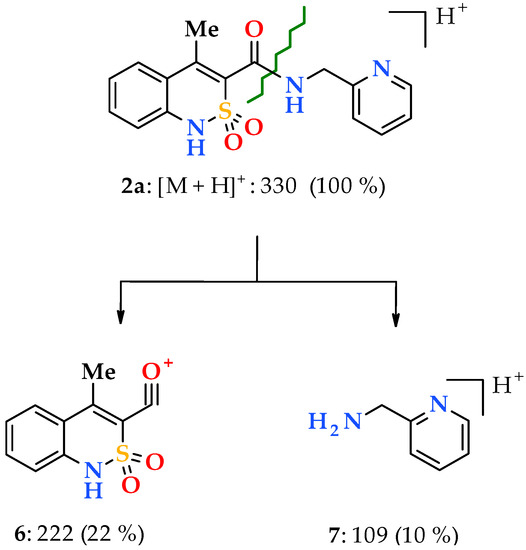
Unfortunately, the electrospray ionization liquid chromato-mass spectra despite all their advantages are not highly informative. Typically, under such conditions, molecular ions easily form complexes with cations (for example, [M + H]+ or [M + Na]+), various clusters ([2M + H]+) and adducts ([M + solvent + H]+), as well as their multicharged ions. However, knowing these features the interpretation of the recorded spectra is significantly simplified. Thus, in particular to confirm the structure of N-hetarylalkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i the most analytically important peaks are sufficient for consideration. These are usually the most intense peak in the spectrum corresponding to the protonated molecular ion [M + H]+, and much less intense peaks of its two fragments: acylium-cation 6 with m/z 222, which is common for all the substances studied, and the ion [hetarylalkylamine + H]+ 7, which is characteristic for each sample (Scheme 2).
Scheme 2.
The primary fragmentation of the protonated molecular ion of pyridin-2-ylmethylamide 2a.
Transition to N-benzyl-substituted 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3a–k significantly reduces the stability of protonated molecular ions [M + H]+, which peak intensity now does not reach the maximum possible 100% value in any of the spectra registered by us (see Section 2.2). However, more significant differences between N-hetarylalkylamides 2a–i and their N-benzyl substituted analogs 3a–k are observed in the primary fragmentation of their protonated molecular ions. If in the first case under the effect of electrospray ionization there is the terminal amide bond CO‒NHCH2Ht breaking (Scheme 2), now the main direction of the decomposition is the benzyl bond CONH‒CH2Ar breaking (the so-called β-breaking [33]) with formation of resonance stable benzyl cations 8 (Scheme 3). As a rule, benzyl cations of type 8 are quickly regrouped into energetically more favorable and stable structures of tropylium cations 9, but depending on the substituents in the aromatic nucleus they can also preserve the original benzyl structure [33].
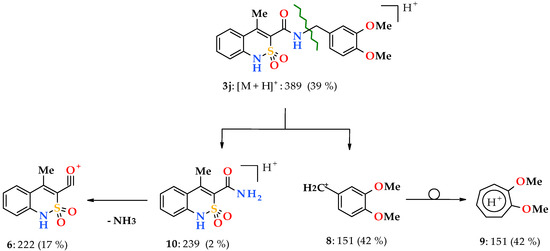
Scheme 3.
The primary fragmentation of the protonated molecular ion of N-(3,4-dimethoxybenzyl)amide 3j.
A protonated cation of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide 10 with m/z 239 as the most massive fragment formed during the primary fragmentation of N-benzylamides 3a–k protonated molecular ions is rarely recorded in the mass spectra, and in the form of peaks of an extremely low intensity, not more than 2%. The cause for this effect is obviously in the tendency of cation 10 to lose a molecule of NH3 easily and transform into an acylium-cation 6, which is more stable and therefore typical for both N-hetarylalkylamides 2a–i, and N-benzylamides 3a–k. The only difference is that in the latter case the acylium-cation 6 is a product of the secondary destruction of protonated molecular ions, but not the primary one.
The analysis of cluster and adduct peaks with m/z values significantly exceeding the molecular masses of the compounds studied is of interest only for the works devoted exclusively to mass spectrometry.
3.2. Evaluation of the Analgesic and Anti-Inflammatory Activity
Analysis of the results of pharmacological tests shows that our assumption concerning the feasibility of studying N-hetarylalkyl-substituted amides of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid was completely justified. Without exception, all samples showed analgesic effect, and some of them are almost as active as Lornoxicam in the same dose (Table 1). Curiously, in the group of N-pyridinylmethylamides the most powerful analgesic was again meta-isomer 2b. Furan-2-ylmethyl derivative 2d and its thiophene analog 2g are biologically very similar, whereas methylation of the furan nucleus or its hydrogenation (amides 2e and 2f, respectively) lead to a decline in analgesic properties.

Table 1.
The Analgesic Activity of Hetarylalkylamides 2a–i, Benzylamides 3a–k, and Reference Drugs.
These observations indicate that in the series of N-hetarylalkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i approximately the same structural and biological regularities recorded by us earlier for similar derivatives of 4-hydroxy-2-oxo-1,2-dihydroquinoline-[4,6] and 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-[11] 3-carboxylic acids remain. In other words, the above mentioned assertion that the analgesic effect of the groups of substances studied is more sensitive to the structure of the hetarylalkylamide fragment than to the bicyclic hetarylcarboxylic acid has received one more experimental confirmation.
The study of the anti-inflammatory activity of N-hetarylalkylamides 2a–i brought new interesting touches to the overall picture of our research. Pain and inflammation often accompany each other. Therefore, drugs that can effectively eliminate both of these reactions at the same time are very popular in modern medicine. N-Pyridinylmethylamides 2a–c has demonstrated the anti-inflammatory action of low to moderate level (Table 2), and therefore, unlikely to have any further prospects. The only the fact should be noted that meta-isomer 2b appeared to be the most active.

Table 2.
The Anti-Inflammatory Activity of Hetarylalkylamides 2a–i, Benzylamides 3a–k, and Reference Drugs.
N-(Tetrahydrofuran-2-ylmethyl)-amide 2f as an antiphlogistic agent (and indeed as an analgesic) is also of a little interesting. The same can be said about hetarylpropylamides 2h and 2i although they showed very good analgesic properties. Furan-2-ylmethylamides 2d and 2e by the strength of the anti-inflammatory activity are comparable to Lornoxicam and Diclofenac, but significantly inferior to them by the analgesic action. Of all the N-hetarylalkyl-substituted derivatives presented in this work only N-thiophen-2-ylmethylamide 2g has shown a high analgesic and anti-inflammatory activity simultaneously, therefore, it can be considered as a candidate for a more detailed study.
Replacement to N-benzyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 3a–k really appeared to be bioisosteric since the ability to suppress both pain and inflammation to some extent was found in the vast majority of the test samples. In this case, the basic structure of this series—unsubstituted N-benzylamide 3a—showed moderate biological effects. Halogenation of the aromatic ring—amides 3b–d—was accompanied by almost complete loss of activity, therefore, this modification should be considered extremely unsuccessful. Methylation of the benzyl fragment in ortho- and para-position almost did not affect the anti-inflammatory properties, but the meta-methyl substituent deactivated the molecule. At the same time, after the introduction of methyl groups the analgesic action increased approximately twice compared to the base N-benzylamide 3a. However, meta-isomer is an exception here—its analgesic activity remains at the initial level.
Methoxybenzylamides demonstrated the similar structural and biological regularities: a high and statistically significant activity of ortho- and para-monosubstituted derivatives 3h and 3i in the absence of the activity in the sample 3j with a meta-methoxy group. It is interesting that the replacement of methoxy groups by a dioxole cycle annelated with the benzene nucleus and formed on the basis of oxygen atoms in the same positions 3 and 4 (modification 3j → 3k) was very useful. Thus, the anti-inflammatory properties of N-(1,3-benzodioxol-5-ylmethyl)-amide 3k reach the maximum level among all compounds from the group of N-benzylamides 3a–k. The analgesic effect also increases, though rather insignificantly. In general, according to the results of our tests only two substances—N-thiophen-2-ylmethyl- and N-4-methoxybenzyl-amides of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid are of real interest for further expanded physico-chemical and pharmacological study as potential analgesics with a powerful anti-inflammatory effect.
3.3. The Molecular and Crystal Structure Study
The facts observed and repeatedly confirmed experimentally that, at least, the analgesic activity of N-R-amides of bicyclic hetaryl-3-carboxylic acids was mainly determined by their terminal amide fragment served as a prerequisite for the study of the molecular and crystal structure of several N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides presented in this article. There are several reasons for the interest in such a study. First of all, it is, of course, scientific curiosity and the desire to determine exactly how the general structure of the molecule (and therefore, the activity) changes during the transition from one N-hetaryl(aryl)alkyl-amide 2 or 3 to another. The next important point is the introduction of test samples in the form of an aqueous suspension, which provides their entry into the body of the experimental animal in the initial crystalline state. And finally, the last argument in favor of our research is that in pharmaceutical and medical chemistry it is well known that the molecular and crystal structure of organic compounds has a significant impact on their pharmacological properties, and therefore, often becomes the object of close attention of researchers [34,35,36,37,38,39].
As the objects of X-ray diffraction studies we deliberately chose several N-hetaryl(aryl)alkyl-amides 2 and 3, which showed the possibility of different levels of pharmacological properties and had mono-crystals that were suitable for such analysis. Thus, in the case of one of the most biologically active compounds—N-thiophen-2-ylmethylamide 2g—it was found that its thiazine heterocycle was in the conformation of the distorted sofa with puckering parameters [40] S = 0.61, Θ = 55.8°, Ψ = 14.0° (Figure 2). The deviations of the S(1) and C(8) atoms from the mean plane of the remaining atoms of this cycle were 0.81 Å and 0.19 Å, respectively. The N(1) atom had pyramidal configuration with very small extent of pyramidality (the sum of the bond angles centered at it was 357°). Some repulsion between the methyl group and the substituent at the C(8) atom (the shortened intramolecular contact H(15b)…C(9) is 2.51 Å compared to the van der Waals radii sum [41] 2.87 Å) caused the rotation of the substituent relatively the thiazine cycle (the C(7)−C(8)−C(9)−O(1) torsion angle was 67.5(2)°) and elongation of the C(7)−C(8) and C(8)−C(9) bonds (1.349(2) Å and 1.498(2) Å) compared to their mean values [42] 1.326 Å and 1.455 Å, respectively. In addition the repulsion between the methyl group and benzene ring was observed (the shortened intramolecular contacts were: H(15a)…C(5) 2.83 Å, H(5)…C(15) 2.60 Å). The thiophene cycle was located in orthogonal position to the carbamide fragment (the C(9)−N(2)−C(10)−C(11) torsion angle was −94.2(2)°) and was disordered over two positions (A and B) with equal populations due to rotation around the C(10)−C(11) bond (the N(2)−C(10)−C(11)−S(2) torsion angle was 77.8(2)° in conformer A and −94.2(2)° in conformer B).
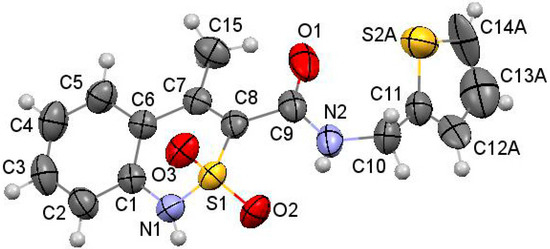
Figure 2.
The molecular structure of thiophen-2-ylmethylamide 2g with atoms represented by thermal vibration ellipsoids of 50% probability.
In the crystal phase molecules of thiophen-2-ylmethylamide 2g form the chains along the [010] crystallographic direction due to formation of the N(1)−H…O(1)’ (1 − x, −0.5 + y, 0.5 − z) H…O 1.93 Å, N−H…O 166° and N(2)−H…O(3)’ (1 − x, −0.5 + y, 0.5 − z) H…O 2.23 Å, N−H…O 167° hydrogen bonds. The molecules within a chain is located in “head-to-tail” way relatively each other (Figure 3).
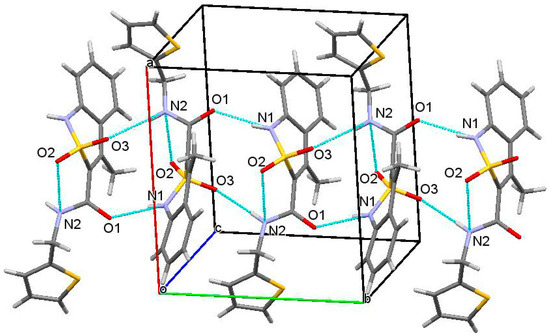
Figure 3.
Packing of molecules of thiophen-2-ylmethylamide 2g in the crystal phase. “Head-to-tail” chain along [0 1 0] crystallographic direction.
Externally crystals of N-thiophen-2-ylmethylamide 2g and all its analogs 2c, 2d, 2e, 3a, 3c, 3d, 3e and 3f also subjected to X-ray analysis are similar and look like thin sticks. This circumstance makes it possible to exclude such an important parameter for all biologically active substances as the crystal habit from the factors that could have a significant impact on their properties [43,44,45]. A similar conclusion can be made in relation to the crystal structure since with the exception of N-thiophen-2-ylmethylamide 2g and N-pyridin-4-ylmethylamide 2c isolated in the form of a monohydrate the molecules of all compounds studied form the same structural motif—hydrogen-bound chains, in which molecules are arranged by the “head-to-head” type (Figure 4). Consequently, the experimental differences observed in the biological properties of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides are stipulated not by the shape of their crystals and the crystal lattice, but mainly by the peculiarities of the molecular structure.
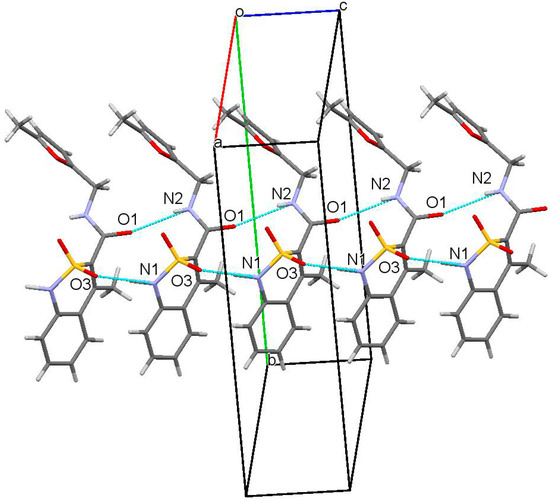
Figure 4.
Packing of molecules of 5-methylfuran--2-ylmethylamide 2e in the crystal phase. “Head-to-head” chain along [0 0 1] crystallographic direction.
Analysis of X-ray diffraction data shows that the spatial structure of the benzothiazine-3-carbamide fragment in all samples studied remains virtually unchanged. However, the location of N-hetaryl(aryl)methyl substituents in the crystals changes significantly. In particular, in the range of N-thiophen-2-ylmethylamide 2g → N-2-methylbenzylamide 3e → N-5-methylfuran-2-ylmethyl-amide 2e → N-3-methylbenzylamide 3f → N-benzylamide 3a → N-furan-2-ylmethylamide 2d → N-pyridin-4-ylmethylamide 2c → N-2-chlorobenzylamide 3c → N-4-chlorobenzylamide 3d their gradual turn in the direction of the plane of the benzothiazine bicycle is observed. It is interesting that in the same sequence and gradually the analgesic activity of these compounds decreases (Figure 5). Therefore, our study clearly shows that the strength of the analgesic effect (but not the anti-inflammatory one) of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides determines the spatial location of their terminal amide fragments. Thus, the high activity of N-thiophen-2-ylmethylamide 2g is presumably provided by conformer 2g-B, which feature is an almost right angle between the planes of the thiophene and benzothiazine cycles (Table 3). Conformer 2g-A (shown in Figure 5) is structurally very similar and differs only in approximately 180° turn of the thiophene nucleus around the 3-CONHCH2—Ht bond. It is difficult to answer unambiguously how this change in the structure affects the manifestations of the analgesic action. But, judging by the position of the oxygen atom in the bioisosteric furan cycle of highly active N-5-methylfuran-2-ylmethylamide 2e and low active N-furan-2-ylmethylamide 2d (Figure 5, Table 3) the preference should be given to conformer 2g-B. However, the presence of conformer 2g-A in the crystal is also not critical since, in general, N-thiophen-2-ylmethylamide 2g remains one of the most powerful analgesics (unlike N-4-chlorobenzyl derivative 3d, in which one of the conformers completely deactivates the sample).
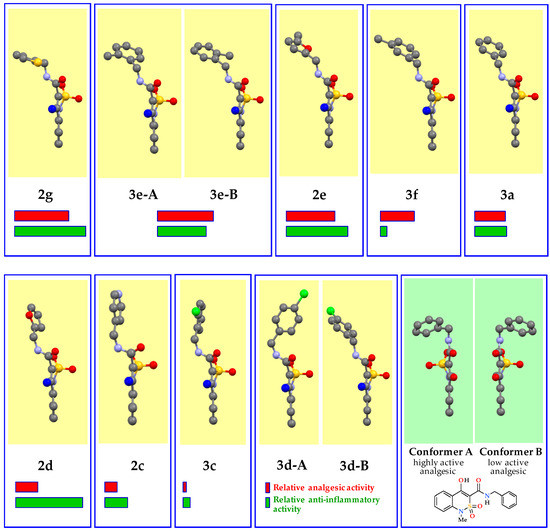
Figure 5.
Conformers of some N-hetaryl(aryl)methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carbox-amides (according to the data of X-ray diffraction analysis), their relative analgesic (red stripe) and anti-inflammatory (green stripe) activity. The standard representation of different atoms in different colors. The C-atom of the 4-methyl group of the benzothiazine cycle faces the viewer and for better perception is highlighted in blue.

Table 3.
The Most Important Torsion Angles that Determine the Spatial Position of N-Hetaryl(aryl)-methyl Substituents in Molecules of Some N-R-amides 2 and 3.
N-2-Methylbenzylamide 3e (more precisely its conformer 3e-B) and N-5-methylfuran-2-ylmethylamide 2e are structurally very close to conformer 2g-B and differ from it mainly only by turning the aryl(hetaryl)methyl fragment around the 3-CONH—CH2(Ar)Ht bond at an angle of approximately 90°. Hence, there is the approximately equally high level of analgesic properties of these compounds (Table 3, highlighted in beige). Obviously, it is this conformation that provides the closest interaction of molecules of the substance with pain receptors and, consequently, the maximum biological effect.
Further rotation around the abovementioned 3-CONH—CH2(Ar)Ht bond for another 20–40° is accompanied by a decline in the analgesic activity to a moderate level—N-benzylamide 3a and its conformational close analogs 3e-A, 3f (Table 3, highlighted in blue). Turning the next 20° gives low active N-furan-2-ylmethylamide 2d (Figure 5, Table 3), then there is a complete loss of analgesic properties. In the group of inactive compounds N-4-chlorobenzylamide 3d is particularly indicative. In general, it appears to be biologically completely inert although its crystals consist of a quarter of 3d-B conformer, which is structurally similar to a moderately active N-benzylamide 3a. Probably, a possible reason for this effect in the crystal is the presence of a pharmacologically inactive partner 3d-A, which is characterized by the strongest deviation of the aryl fragment in the direction of benzothiazine bicycle of all compounds studied (Figure 5).
All structural and biological regularities described relate exclusively to analgesic properties of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2 and 3. But with regard to their anti-inflammatory activity the data presented above clearly illustrate a completely different picture (Figure 5). There are no clear correlations here. Highly, moderately and low active analgesics may well turn out to be both powerful antiphlogistics and completely inactive substances regardless of the spatial structure (see, for example N-thiophen-2-ylmethylamide 2g, N-3-methyl-benzylamide 3f and N-furan-2-ylmethylamide 2d, respectively). Moreover, this result is not surprising and even quite natural since the ability to suppress the inflammatory reaction is implemented through the impact on completely different biological targets.
Most likely, the regularities found by us are valid only for N-R-amides of the benzyl type, and are unlikely to manifest themselves in the case of hetaryl-, aryl-, phenethyl- or alkylamides. However, even such a limited range of objects allows us to speak about preservation of those conformations of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2 and 3 in the solution, they were formed during formation of their crystals. Here, perhaps, it would be appropriate to mention one of the most controversial and yet unsolved problems of crystal chemistry, which has a direct relationship to the question under consideration: whether any differences between different polymorphic forms of the same substance remain after their transfer from the crystalline state to the solution [46,47]. The often biased and skeptical attitude towards this issue that prevails in the scientific community is well known. But the numerous experimental facts accumulated so far clearly indicate that polymorphic forms of organic substances in the solution can still retain a number of individual properties, including the original conformation [47]. It is clear that such conformational stability cannot last indefinitely. However, those few hours, during which the crystalline (or just dissolved) substance introduced into the body enters the biological fluids, and then into the target organ and has its specific effect, is obviously a rather real term.
Finally, another important point should be mentioned. All compounds considered in this section crystallize in centrosymmetric space groups, it indicates the existence of their symmetric equivalents in crystals with a mirror-opposite conformation due to the presence of a plane of symmetry. In other words, the crystals of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2 and 3 studied contain not one or two conformers, but two or four, respectively—those indicated in Figure 5 and Table 3 and their mirror reflections. Therefore, it is impossible to state unambiguously that the high analgesic activity, for example, of N-5-methylfuran-2-ylmethylamide 2e, is provided by the conformer depicted in Figure 5, but not by its symmetric equivalent. An accurate answer to these questions can be given only in some rare cases when it is possible to separate such conformers and subject them to comparative pharmacological tests. The first study of this type was N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide obtained in the form of two pseudo-enantiomeric polymorphic forms (see Figure 5), of which only one showed the high analgesic activity [48]. This example is interesting because it allowed experimentally to give a quantitative assessment of the effect on the biological properties of the molecule conformation. In contrast to the classical polymorphs, here significant differences in the activity of pseudo-enantiomeric conformer A and B can not be explained by different solubility since all the parameters of their molecular and crystal structure (bond lengths, valence angles, packaging, habit, etc.) are absolutely the same. Only torsion angles determining the conformational individuality differ, and even then not by absolute values, but only by signs (+or −).
4. Conclusions
This work describes the preparative method for obtaining new N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides, which are of interest as potential analgesic and anti-inflammatory agents. The structure of all compounds synthesized has been confirmed by the data of elemental analysis, 1H and 13C NMR spectroscopy, and electrospray ionization liquid chromato-mass spectrometry. The pharmacological screening has revealed highly active substances with the analgesic and anti-inflammatory actions that are not inferior and even superior to Lornoxicam and Diclofenac among N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Based on a detailed analysis of the molecular and crystal structure of a large group of substances and comparison of the X-ray diffraction data obtained with the results of biological tests the molecular conformation has been recognized to be the main criterion of the analgesic activity of the compounds under study. N-Thiophen-2-ylmethyl- and N-4-methoxybenzyl-amides of 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid are recommended for the in-depth pharmacological study as an agent of the complex analgesic and anti-inflammatory action.
Author Contributions
The synthesis of the compounds presented in this work and analysis of their characteristics were performed by I.V.U., G.M.H. and A.A.B. The liquid chromato-mass-spectrometric and X-ray structural studies were performed by L.A.G., G.S. and S.V.S., V.V.G. respectively. The pharmacological studies were conducted by N.I.V. and O.V.M. The manuscript was written by I.V.U., G.M.H. and O.V.M.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Candidate of Chemistry Evgene S. Gladkov (SSI “Institute for Single Crystal” National Academy of Sciences of Ukraine, Kharkiv, Ukraine) for his help in registration of NMR spectra of the compounds synthesized.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ukrainets, I.V.; Sidorenko, L.V.; Davidenko, A.A.; Yarosh, A.K. 4-Hydroxy-2-quinolones. 174. Hydro-chlorides of [(alkylamino)alkyl]amides of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid—A new class of the opioid receptors antagonists. Chem. Heterocycl. Compd. 2010, 46, 445–451. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A.; Shishkina, S.V. 4-Hydroxy-2-quinolones. 180. Synthesis, chemical reactions, and analgesic activity of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. Chem. Heterocycl. Compd. 2010, 46, 1084–1095. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Jaradat, N.A.; Bevz, O.V.; Turov, A.V. 4-Hydroxy-2-quinolones. 204. Synthesis, bromination, and analgetic properties of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid arylalkylamides. Chem. Heterocycl. Compd. 2012, 48, 1347–1356. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Gorokhova, O.V.; Jaradat, N.A.; Petrushova, L.A.; Mospanova, E.V.; Savchenkova, L.V.; Kuz’min, V.E.; Lyahovsky, A.V. 4-Hydroxyquinolin-2-ones and their close structural analogues as a new source of highly effective pain-killers. In Pain and Treatment; Racz, G.B., Noe, C.E., Eds.; InTech: Rijeka, Croatia, 2014; pp. 21–73. [Google Scholar]
- Ukrainets, I.V.; Bevz, O.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 202. Synthesis, chemical and biological properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. Chem. Heterocycl. Compd. 2012, 48, 320–326. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A. Using bioisosteric replacements to enhance the analgesic properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxamides. Pharm. Chem. J. 2016, 50, 365–368. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, O.V.; Bereznyakova, N.L.; Davidenko, O.O. Polymorphism and the analgesic activity of N-(3-pyridylmethyl)-4-hydroxy-2-oxo-1,2,5,6,7,8-hexahydroquinoline-3-carboxamide. J. Org. Pharm. Chem. 2015, 13, 41–46. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 195. Synthesis of novel, potential analgesics based on 4-(hetarylmethyl)amino-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2011, 47, 67–73. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Davidenko, A.A.; Mospanova, E.V.; Sidorenko, L.V.; Svechnikova, E.N. 4-Hydroxy-2-quinolones. 176. 4-R-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Synthesis, physicochemical and biological properties. Chem. Heterocycl. Compd. 2010, 46, 559–568. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Gorokhova, O.V.; Andreeva, K.V. Transformation of 3-(3-arylalkylcarbamoyl-4-hydroxy-2-oxo-1,2-dihydroquinolin-1-yl)propanenitriles into amides and acids. Russ. J. Org. Chem. 2013, 49, 867–871. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Bereznyakova, N.L.; Sim, G.; Davidenko, A.A. Synthesis, structure, and analgesic activity of picolylamides of 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylic acids. Pharm. Chem. J. 2018, 52, 601–605. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.J.; Tovar, F.; Austin, N.; Tresadern, G.; Trabanco, A.A. Benzazaborinines as novel bioisosteric replacements of naphthalene: Propranolol as an example. J. Med. Chem. 2015, 58, 9287–9295. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Brindisi, M.; Butini, S.; Kshirsagar, G.U.; Maramai, S.; Chemi, G.; Gemma, S.; Campiani, G.; Novellino, E.; Fiorenzani, P.; et al. (S)-2-Amino-3-(5-methyl-3-hydroxyisoxazol-4-yl)propanoic acid (AMPA) and kainate receptor ligands: Further exploration of bioisosteric replacements and structural and biological investigation. J. Med. Chem. 2018, 61, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Patina, G.A.; LaVoie, E.J. Bioisosterism: A rational approach in drug design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Azotla-Cruz, L.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New synthesis, structure and analgesic properties of methyl 1-R-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates. Sci. Pharm. 2017, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Shishkina, S.V.; Voloshchuk, N.I.; Malchenko, O.V. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid. Peculiarities of preparation, structure, and biological properties. Sci. Pharm. 2018, 86, 9. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877570. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877571. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877572. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1865723. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 4 September 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877573. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877574. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877575. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877576. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877577. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Ukrainian Law No. 3447-IV. On Protection of Animals from Severe Treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 4 August 2017).
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1103–1106. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.G.O.; Berger, W.; Domschke, G.; Fanghänel, E.; Faust, J.; Fisher, M.; Gentz, F.; Gewald, K.; Gluch, R.; Mayer, R.; et al. Organikum: Organisch-Chemisches Grundpraktikum; 24 Auflage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 751–774. [Google Scholar]
- Terent’ev, P.B.; Stankiavichyus, A.P. Mass Spectrometry Bioactive Nitrogenous Bases; Mokslas: Vilnius, Lithuania, 1987; pp. 60–64. [Google Scholar]
- Shet, A.R.; Grant, D.J.W. Relationship between the structure and properties of pharmaceutical crystals. KONA Powder Part. J. 2005, 23, 36–48. [Google Scholar] [CrossRef]
- Galek, P.T.A.; Pidcock, E.; Wood, P.A.; Bruno, I.J.; Groom, C.R. One in half a million: A solid form informatics study of a pharmaceutical crystal structure. CrystEngComm 2012, 14, 2391–2403. [Google Scholar] [CrossRef]
- Larsen, E.M.; Wilson, M.R.; Taylor, R.E. Conformation–activity relationships of polyketide natural products. Nat. Prod. Rep. 2015, 32, 1183–1206. [Google Scholar] [CrossRef] [PubMed]
- Do, T.D.; Checco, J.W.; Tro, M.; Shea, J.-E.; Bowers, M.T.; Sweedler, J.V. Conformational investigation of the structure–activity relationship of GdFFD and its analogues on an achatin-like neuropeptide receptor of Aplysia californica involved in the feeding circuit. Phys. Chem. Chem. Phys. 2018, 20, 22047–22057. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Chen, Y.; Galvin, G.M.; Pabba, P.K. Conformation–activity relationships inpolyketide natural products. Towards the biologically active conformation of epothilone. Org. Biomol. Chem. 2004, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Stoica, C.; Verwer, P.; Meekes, H.; van Hoof, P.J.C.M.; Kaspersen, F.M.; Vlieg, E. Understanding the effect of a solvent on the crystal habit. Cryst. Growth Des. 2004, 4, 765–768. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef] [PubMed]
- Leonidov, N.B. History of the development of the concept of polymorphism of chemical substances (short essay). Russ. Chem. J. (J. Russ. Chem. Soc. DI Mendeleyev’s) 1997, XLI, 10–21. [Google Scholar]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72 Pt 5, 411–415. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).